Abstract
Shale formation is represented as one of the challenge formations during drilling wells because it is a strong potential for wellbore instability. Zubair formation in Iraqi oil fields (East Baghdad) is located at a depth from 3044.3 to 3444 m. It is considered as one of the most problematic formations through drilling wells in East Baghdad. Most problems of Zubair shale are swelling, sloughing, caving, cementing problem and casing landing problem caused by the interaction of drilling fluid with the formation. An attempt to solve the cause of these problems has been adapted in this paper by enhancing the shale stability through adding additives to the drilling fluid. The study includes experiments by using two types of drilling fluids, API and polymer type, with five types of additives (KCl, NaCl, CaCl2, Na2SiO3 and Flodrill PAM 1040) in different concentrations (0.5, 1, 5 and 10) wt% and different immersion period (1, 24 and 72 h) hours. The effect of drilling fluids and additive salts on shale has been studied by using different techniques: (XRD, XRF, reflected and transmitted microscope) as well shale recovery. The results show that adding 10 wt% of Na2SiO3 to API drilling fluid results in a high percentage of shale recovery (78.22%), while the maximum shale recovery was (80.57%) in polymer drilling fluid type gained by adding 10 wt% of Na2SiO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shale is a fine-grained, argillaceous, sedimentary rock. It is represented as one of the most complicated rock types in engineering applications (Adesoye 2009). It consists of clay minerals and small amounts of other non-clay minerals such as quartz, feldspar and calcite (Charles 1989). The mineral content of shale is very important when dealing with wellbore instability. Clay minerals are therefore highly studied to comprehend shale behavior. Its behavior is both complicated and delicate. The chemical properties of any fluid (pore fluid, drilling fluid, etc.) can affect the strength and stiffness of shale rock. Shale behavior is delicate because its transition to an unstable situation may occur rather quickly and easily. The interaction of shale with drilling fluid, or movement of drilling fluid into the shale matrix may happen within few hours leading to tremendous problems (Farrokhrouz and Asef 2013), such as swelling, an increase in pore pressure, bit balling, caving, stuck pipe and increase in torque and drag.
Swelling is a direct result of the volume expansion when the exchangeable cations are hydrated in aqueous solution or when the water enters between platelets of clay which causes expanding of platelets of shale. The magnitude of the hydration or swelling stress between clay platelets depends on the type of shale/fluid interaction and clay minerals. (Grim 1968) suggested that swelling can be grouped into three main parts: (1) crystalline swelling (ionic hydration or surface hydration), (2) osmotic hydration and (3) dissolution mechanism.
Many researchers worked on shale stability problem to develop a suitable solution to solve this problem by using different drilling fluids with different additives like: oil-based emulsion, simulated pore fluid, CaCl2 brine soluble potassium or sodium silicate, NaCl/KCL, NaCl/KCL/Amine, K acetate/polymer fluid, oil base fluid, base polymer system, lignite/lignosulfonates, gel/polymer, potassium chloride (KCl)/polymer, polyanionic cellulose (PAC)/starch and partially hydrolyzed polyacrylamide/polyacrylate (PHPA) and series of different nano-silicate substances with water-based mud (Nesbitt et al. 1985; Zevnalv-Andabilv et al. 1996; Chee et al. 1996; Sandra and Wenwu 2012; Simpson and Dearing 2000; Nediljka et al. 2004; Friedheim et al. 2011; Brady and Michael 2012; Wenwu et al. 2014; Peter et al. 2016).
Zubair formation is one of the formations of the Iraqi oil field located in the east of Baghdad. It is one of the formations that is represented by the Late Berriasian–Albian cycle and can be considered as an important reservoir in the south of Iraq (Rami et al. 2014). This composition consists of sandstone, shale, clay, limestone and marl Table 1. The depth of formation ranges from 3044.3 to 3544 m with thickness 499.7 m. This formation severs from shale stability problems, which are caving and sloughing. These problems will grow into tremendous cost due to lost in-productive time. Also, there are difficulties in running casing and poor cementing jobs. Hence, drilling fluids need to be formulated and adjusted with some special additive to reduce the effect of these problems. According to that, the aim of this paper is formulating drilling fluid with some special additive to reduce these problems. This is done through studying the characterization of Zubair shale as well as studying the effect of chemical interaction between the shale and salts additives in drilling fluid.
Experimental work
Experimental work includes two sets of experiments: first, shale characterization experiments, second, shale–drilling fluid interaction experiments. Characterization tests provide the knowledge on the composition and properties of the shale, while the shale–drilling fluid interaction tests give information about the recovery for shale samples and swelling test.
Samples preparation
Zubair samples have been taken from east Baghdad oil field at 3440 m depth. Its description is mentioned in Table 1. Two methods were used to remove the hydrocarbons and drilling additives from samples: first, Soxhlet method to remove hydrocarbons from shale by using toluene, methanol and benzene, then heated in Soxhlet device for 3 h, and the second method was to use wet sieving to remove additive salts.
The shale samples have been studied by different techniques. The petrography and the effect of drilling fluid additives of shale samples were examined by transmitted and reflected microscope type BX51M/Olympus. X-Ray diffraction (XRD-6100/7000) was used to analyze the mineralogical composition of the shale sample. Chemical composition of shale sample was examined by X-ray fluorescence (XRF-1800).
Native moisture content represents the total molecules of water in the shale samples. It is defined as the ratio of the mass of water founded in the samples to the total weight of the samples (Adesoye 2009). The native moisture content has been determined by weighting shale samples before and after drying in an oven with 100 °C for 24 h (Eq. 1)
MC moisture content (%), \( W_{\text{w}} \) weight of water removed by drying (g), \( W_{\text{s}} \) weight of shale samples before drying (g).
Drilling fluid preparation
Two types of drilling fluids were used water-based mud (WBM) (API Manual) and polymer mud (PM). WBM was prepared by mixing 350 ml water with 22.5 g bentonite by using Hamilton Beach mixer for 20 min. The suspension is aged in a sealed container for 24 h to ensure good hydration of bentonite. Then, salts with different concentrations 0.5, 1, 5 and 10 wt% were added to WBM and mixed for 10 min. Polymer-based mud was prepared by adding fixed quantities of KCl, KOH, polyacrylamide (PAC polymer) and XC polymer to the hydrated mixture of bentonite. Each additive was mixed for 2 min to ensure the dispersion of particles into the drilling fluid matrix. Finally, the mixture of PM was mixed for 10 min after that the salts with concentrations of (0.5, 1, 5 and 10) wt% were added and mixed for 10 min.
Experimental procedure
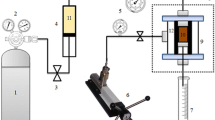
Zubair samples were immersed in two types of drilling fluids (WBM and PM) with different additives. Five types of salts additives (potassium chloride, sodium chloride, calcium chloride, sodium silicate and Flodrill) with different concentrations (0.5, 1, 5 and 10) wt% and different immersion period (1, 24 and 72) hours were used at room temperature.
After immersion, different tests were applied including shale characterization, immersion, swelling, dispersion and native moisture content test. The dispersion test was used to measure the recovery percentage with 100 °C and dynamic motion using OFITE roller oven.
Native moisture content represents the total molecules of water in the shale samples. It is defined as the ratio of the mass of water founded in the samples to the total weight of the samples (Adesoye 2009). The native moisture content has been determined by weighting shale samples before and after drying in an oven with 100 °C for 24 h, Eq. (2)
where MC is moisture content (%), \( W_{\text{w}} \) is weight of water removed by drying (g), and \( W_{\text{s}} \) is weight of shale samples before drying (g).
Results and discussion
Shale characterization
XRD analysis shows that Zubair shale composed mainly of kaolin with a minor amount of non-clay minerals: quartz and pyrite (Fig. 1). Table 2 shows the chemical composition of samples before and after cleaning from drilling fluid. SiO2, Fe2O3, Al2O3 and MgO represent the main components of shale. The concentration of CaO, Na2O and K2O was decreased after cleaning due to removing additive salts. The microscope image of shale samples before cleaning shows a high concentration of salts distributed on the surface of the clay, accumulated in sheets edge, and filled pores and microfractures, Fig. 2. After cleaning, a little distribution of salts is still on the edge of shale samples as shown in Fig. 3. Gypsum veinlets were appeared on the surface of shale, the veinlets twisted with another veinlet like a network, Fig. 4. The grains of clay were covered by salts due to the reaction of salts with clay minerals.
The native moisture content of Zubair shale is 0.11. The attractiveness of Zubair shale to moisture is very low because of the nature of kaolin mineral which has low tendency to the water.
Effect of drilling fluids on the Zubair shale
Immersion test
The recovery percentage values were calculated after immersion the samples in drilling fluid. These values were varied depending on the activity between the additive and shale samples (Gomez 2006). Figure 5 shows the effect of different salts additives with water-based mud on the shale samples. It can be seen that 0.5% of KCl salt with WBM gives the best recovery after 1 h with a percentage of recovery of 69.57%. After 72 h recovery decreases to 59.41% because water got enough time to interact with shale samples and 0.5% of KCl was not enough to reduce the effect of water on shale samples. Increasing KCl concentration to 1, 5 and 10 wt% causes a higher percentage of recovery after 1 h to 75.93, 77.52 and 81.28%, respectively.
The adding of 0.5% NaCl to WBM gives the recovery after 1 h with percentage of 67.9%, while the recovery reduced to 57.34% after 72 h. The recovery percentage of shale increased to 71.38%, 74.19% and 77.38% after 1 h when the concentration of NaCl increased to 1, 5 and 10%, respectively. As noticed in the results, the flow of water into the formation reduces with increase in salt concentration. The recovery percentage is very affected also by immersion time, and the results appeared the effect of increasing time interaction of drilling fluids with the shale. The recovery reduced even when the concentration of salts increased due to increase in immersion time. Comparing NaCl with KCl, NaCl has a higher preliminary viscosity relative to KCl in the mud and it has lower water activity which gives rise to higher osmotic pressures. NaCl is represented a better equipped for reducing filtrate invasion for drilling fluids to the shale (Farrokhrouz and Asef 2013).
The recovery of 0.5% CaCl2 with WBM was 67.11% after 1 h, while the recovery reduced to 56.92% after 72 h. The addition of 10% CaCl2 to WBM gives 78.59% percentage of recovery after 1 h. This is because of that the effect of 0.5% CaCl2 was not enough to cover the shale samples and the fracture was growing up due to the interaction of the sample with drilling mud as shown in Fig. 6. The accumulation of salts on the surface of clay and crystals of salts are created and coated the sample as shown in Fig. 7.
Figure 5 shows that the recovery of 0.5% of sodium silicate with WBM was 66.27% after 1 h, while the recovery reduced to 51.38% after 72 h. Increasing the Si2O3 concentration to 1, 5 and 10% with WBM causes a higher percentage of recovery after 1 h to 67.55, 79.25 and 83.71 wt%, respectively. Figure 8 shows that the recovery percentage of 0.5% Si2O3 with PM was 64.21% after 1 h, while the recovery reduced to 52.75% after 72 h. Increasing the Si2O3 concentration to 1, 5 and 10% with PM leads to increase the recovery to 67.83, 82.74 and 84.82 wt%, respectively. Soluble sodium silicate has the ability to invade the shale and react with available ions in the shale to consist insoluble precipitation. Figure 9 shows the sodium silicate accumulation on the shale samples and sodium silicate invaded into the fracture and built a bridge between two sides then covered the shales to prevent any interaction with other fluids.
The effect of salts additives with polymer mud on the shale samples is shown in Fig. 8. Comparing the effect of the additives between WBM and PM, it can be seen that these additives cause a significant influence on shale recovery in PM than that in WBM. The shale recovery values increased by using PM for all additive types and concentration. For example, a 0.5% KCl with PM gives the recovery after 1 h with percentage of recovery of 73.29%, while the recovery reduced to 62.54% after 72 h. Increasing the salt concentration to 1, 5 and 10% leads to increase the recovery percentage of shale samples after 1 h to 79.81, 82.12 and 83.49 wt%, respectively. The good effect of KCl can be explained by the ability of K+ to adsorb on the surface of shale and react with the ions on the surface of shale sheets. Due to the replacement of Si+4 with Al+3 in octahedral sheets and Al+3 changed with Mg+2 in tetrahedral sheets, this will increase the negative charge and causes the attractiveness of the positive charge on the surface of shale. Therefore, the positive ions like K+, Ca2+, Na+ and Si+4 were adsorbed on the surface of sheets (M-I Swaco 1989).
Flodrill PAM 1040 is other type of material that has been used to study its effect on shale stability as well as comparing it with other salt additives. It is a special additive used with polymer mud and tested with Zubair shales. Figure 10 shows the result of immersion test. Two concentrations were used 0.55% and 1.10% with PM. The recovery percentage of 0.55% Flodrill was reduced from 96.99% after 1 h to 94.71% after 72 h, and the recovery result of 1.1% concentration was reduced from 98.037% after 1 h to 95.409% after 72 h.
Swelling test
The results of the swelling test, Fig. 11, show a linear relationship between swelling with time of shale samples when soaked in different drilling fluids. The addition of KCl with different concentrations (0.5, 1, 5 and 10 wt%) to WBM as well as PM has increased swelling of Zubair shale.
The salts addition to PM caused a decrease in swelling compared to WBM due to the composition of polymers which is long chain, and it has the ability to seal the fracture as well as covering the plat of shale (Caenn et al. 2011).
Zubair shale consisted mainly of kaolin, and the swelling of kaolin is very little due to the structure of kaolin that consists of one octahedral unit and one tetrahedral unit and the d-spacing between units is very little; therefore, the ability of shale to absorb the water is very little (Grim 1968). The changes in the dimensions of kaolin are due to the separation of fissility, which is affected by either entering molecular of water or the growth of crystals of salts between the fissility and accumulation of salts on the kaolin grains (coated) as shown in Fig. 12. The results show that increasing the concentration of salts leads to cover the fissility of shale and prevents the solution from entering between the fissility, Fig. 13.
The swelling test of Zubair shale samples using Flodrill material is shown in Fig. 14. The swelling percentage for 0.55% Flodrill was increased from 0.08811% after 1 h to 0.1489% after 72 h and the swelling for 1.1% Flodrill was increased from 0.0383 to 0.1149%.
Dispersion test
Dispersion analyzes for shale, tested by using different drilling fluids at 100 °C. Figure 15 shows that the recovery percentage of shale was calculated after dispersion test. The maximum recoveries were obtained with polymer mud + 10 wt% Na2Sio3 (84.96%) and polymer mud + 10 wt% KCl (78.27%). The results of the dispersion test show that the temperature has an influential factor on dispersion values compared with dispersion results at room temperature.
The viscosity of drilling fluid decreases with increase in temperature (Ahmad 2017) and increases the molecules vibration, and this may lead to increase water movement into the fissility of shale. Thus, more samples of shale were crashed into fines and the percent recovery is decreased. The PM has recovery percentage better than WBM due to the effect of the polymer encapsulation and hydrate ability during exposure leading to increase in its surface area and that will lead to effectively coat the surface of shale and delay dispersion. This phenomenon is well known as an encapsulation of polymer (Adesoye 2009).
The recovery of dispersion test using Flodrill material is shown in Fig. 16; it can be seen that at 0.55% concentration the recovery was 93.894%, while 1.1% concentration the recovery was 95.763%. Flodrill PAM 1040 represents a better inhibitor for Zubair shale because the Flodrill has the ability to form gelatin, that is, will cover (coated) the shale and prevent the interaction with water.
Conclusion
-
1.
The problems of shale formation are improved by using additives that have the ability to react with shale and reduce the reaction between water and shale formation.
-
2.
The recovery percentages of samples reduced when exposure time of shale with drilling fluids increased because of the invasion of water between sheets and separate it.
-
3.
Increasing the concentration of salts leads to increase the recovery percentages; this improves that the concentration of salt has a strong effect on the recovery of samples by covering the sample of shale and deposited on the edges of the fissility and blocking it.
-
4.
The attractiveness of Zubair shale to moisture is very little because of the nature of kaolin mineral that has little attraction to water.
-
5.
Sodium silicate gives highest recovery percentages at 5% and 10% with WBM and PM, respectively.
-
6.
Flodrill PAM 1040 represents a better inhibitor for Zubair shale because the Flodrill has the ability to form gelatin, that is, will cover (coated) the shale and prevent the interaction with water.
References
Adesoye K (2009) Shale characterization for evaluating shale-drilling fluid interaction. University of Oklahoma, Norman
Ahmad KM (2017) The effect of high temperatures and pressures on the properties of water-based drilling mud. Ph.D. thesis, Institution of Petroleum and Natural Gas, University of Miskolc
Brady F, Michael J (2012) Potassium silicate-treated water-based fluid: an effective barrier to instability in the Fayetteville shale. In: IADC/SPE drilling conference and exhibition, IADC/SPE Program committee, San Diego, California, pp. 1–6
Caenn R, Darley H, Gray G (2011) Composition and properties of drilling and completion fluids, vol 6. Elsevier, Waltham
Charles WE (1989) Clays, muds, and shales. Elsevier, Amsterdam
Farrokhrouz M, Asef MR (2013) Shale engineering, Suite, Taylor & Francis
Friedheim J, Guo Q, Young S, Gomez S (2011) Testing and evaluation techniques for drilling fluids-shale interaction and shale stability. In: 45th US rock mechanics/geomechanics symposium, ARMA Technical Program Committee, San Francisco, pp 1–7
Gomez SA (2006) Laboratory method to evaluate the fracture development in hard shale formation exposed to drilling fluids. In: AADE fluid conference
Gomez S, He W (2012) Fighting wellbore instability: customizing drilling fluids based on laboratory studies of shale-fluid interactions. In: IADC/SPE Asia pacific drilling technology conference and exhibition, p 4
Grim RE (1968) Clay mineralogy. Ralph Early, New York
MDOC (2013) Final well report well No. EB-92. Midland Oil Company, Baghdad
M-I Swaco M-I Manual [Book] (1998)
Nediljka GM, Katarina S, Davorin M (2004) Laboratory investigations of silicate mud contamination with calcium. Laboratory investigations
Nesbitt LE, King GP, Thurber NE (1985) Shale stabilization principles. In: Annual technical conference and exhibition of the society of petroleum engineers, Society of Petroleum Engineers, Las Vegas, pp 1–3
Peter JB, Reddy BR, Matt H, Pete O’Connell T (2016) Functionalized nanosilicas as shale inhibitors in water-based drilling fluids. In: Offshore technology conference, OTC Program Committee, Houston, pp 1–17
Rami MI, Faisal RF, Nasser ME, Al-Ameri TK, Al-Rawi D (2014) Hydrocarbon potential of Zubair Formation in the south of Iraq. Arab J Geosci. https://doi.org/10.1007/s12517-014-1569-6
Simpson J, Dearing H (2000) Diffusion osmosis-an unrecognized cause of shale instability. In: IADC/SPE drilling conference, Society of Petroleum Engineers, New Orleans, pp 1–8
Tan CP, Richards BG, Rahman SS (1996) Managing physico-chemical wellbore instability in shales with the chemical potential mechanism. In: Asia pacific oil and gas conference, Society of Petroleum Engineers, Adelaide, pp 107–110
Wenwu H, Sandra LG, Russell SL, David T (2014) Shale-fluid interactions and drilling fluid designs. In: International petroleum technology conference, IPTC Programme committee, Doha, pp 1–12
Zevnalv-Andabilv EM, Chen H, Rahman SS (1996) Management of wellbore instability in shales by controlling the physical-chemical properties of Muds. In: SPE Asia pacific drilling technology, Society of Petroleum Engineers, Kuala Lumpur, pp 253–258
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alwassiti, A.A., AL-Bidry, M.A. & Mohammed, K. Experimental study of Zubair shale stability of east Baghdad oil field using different additives in water based mud. J Petrol Explor Prod Technol 10, 1215–1225 (2020). https://doi.org/10.1007/s13202-019-00820-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-019-00820-4