Abstract
Conventional treatment of drill cuttings, as drying and thermal desorption, is failing to meet environmental and economic standards; therefore, new alternatives for the treatment of this waste must be developed. The purpose of this study was to remove n-paraffin from drill cuttings using microemulsion systems (MES). The extraction percentage (%) of n-paraffin was quantified by gas chromatography with a flame ionization detector. The optimization of extraction parameters showed that the extraction percentage (%) is directly proportional to the stirring speed and contact time and inversely proportional to the HLB of the surfactant used in the microemulsion system. Results for MES using Alkonat® L90 and Renex® 95 were similar, but Alkonat® L90 was chosen as the best system considering the environmental issue. The MES/cuttings ratio did not influence the percentage of n-paraffin extracted, reaching 55.03% and 56.32% for the ratios of 0.5 and 2.0, respectively. The reuse of MES in multiple extractions showed that MES can be reused in up to two extractions, obtaining up to 86% extraction. The optimal parameters for Alkonat® L90 microemulsion systems were MES/cuttings ratio of 1.0, stirring speed of 132 strokes, and contact time of 80 min, achieving 86.27% extraction. Results obtained in this study may help to better understand n-paraffin removal from drill cuttings by MES, considering the future use of this technology in the design of an industrial treatment plant for both onshore and offshore operations.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Drill cuttings are rock fragments cut from the formation by the drill bit and carried to the surface by the drilling fluid. When drilling is made using paraffin-based non-aqueous fluids, this n-paraffin adsorbs on drill cuttings, promoting contamination, and, consequently, the treatment and disposal control of this waste are mandatory (Ball et al. 2012; Caenn et al. 2016).
In Brazil, there is no specific legislation on the disposal of drill cuttings; therefore, the limits established by the US Environmental Protection Agency (US EPA) are assumed. The US EPA standard requires that the drilling fluid content retained in the cuttings (ROC–retention on cuttings), defined as the mass of fluid/contaminated cuttings mass, should not exceed 6.9% for n-paraffin and olefin-based fluid and 9.4% for ester-based fluids (U.S. EPA 2000a, b).
Industrially, contaminated drill cuttings are generally treated in two different ways: by drying the cuttings or by performing thermal desorption. Drying uses a cuttings dryer (vertical centrifuge) to promote the separation of n-paraffin (which can be reused) and drill cuttings, which are generally disposed of in landfills. Thermal desorption utilizes high temperatures to promote contaminant evaporation, which can be condensed for reuse, and the solid waste can be discarded (OGP 2003; Jacques Whitford Stantec 2009; Ball et al. 2012).
Other technologies for the treatment or remediation of drill cuttings have been studied (Dejam et al. 2014, 2016). The use of microwave technology is well advanced since there are already treatment plants with a capacity to process 750 kg/h of waste (Petri Jr et al. 2019). A study by Robinson et al. (2009a, b) obtained results of less than 0.1% residual contaminants. Another study presents the use of microorganisms to promote the degradation of these contaminants, a process known as bioremediation (Jacques Whitford Stantec 2009; Ball et al. 2012). Chemical washing also appears as a treatment option, which uses an organic reagent, such as surfactant, to promote the release of the contaminants (Muherei and Junin 2007).
Surfactants are amphiphilic substances that are chemicals capable of interacting with polar and nonpolar substances, changing surface and interface free energies, even at low concentrations (Schramm 2000; Rosen and Kunjappu 2012; de Castro Dantas et al. 2017; Olayiwola and Dejam 2019). Microemulsions are transparent and thermodynamically stable systems formed spontaneously by an appropriate mixture of an aqueous phase, an oil phase, a surfactant, and, sometimes, a co-surfactant, which present very low surface tension (De Castro Dantas et al. 2001; Fanun 2009; Rosen and Kunjappu 2012; Tadros 2005).
The use of surfactants for the treatment of contaminated solids has been studied, demonstrating good results. Urum et al. (2004) studied the performance of commercial and biological surfactants in the remediation of soils contaminated by crude oil and obtained a contaminant extraction of 90%.
Childs et al. (2005) studied drill cuttings remediation using solutions of surfactant at low concentrations (< 0.1% w/w) achieving extraction percentages between 95 and 98%. Deshpande et al. (1999) developed a methodology for washing contaminated soils using ionic and nonionic surfactants. Yan et al. (2011), in their study, proposed the remediation of drill cuttings using a cleaning biosurfactant (rhamnolipid) combined with bioremediation. They obtained results of up to 83% extraction of organic compounds while studying extraction parameters like biosurfactant concentration and solution/cuttings ratio. Also using rhamnolipid for treatment of drill cuttings and oil contaminated solids, Olasanmi and Thring (2019) studied the extraction parameters and achieved around 85.4% extraction percentages.
The use of surfactants is well established, but studies concerning to the application of MES as a solvent in the treatment of drill cuttings are scarce, which may enable the design of pilot equipments or patents. The treatment used in this study will provide new ways for disposal of drill cuttings, giving the possibility of using this material, for example, in roads and civil construction, reducing the use of landfills and the environmental impact. In this context, this study reports the use of new microemulsion systems in the treatment of drill cuttings contaminated with n-paraffin, as well as the study of the influence of extraction parameters (solvent, microemulsion/cuttings ratio, stirring speed, and contact time) on the percentage of extraction. First, the materials and methods are described. Then, the results obtained in this study are presented and discussed. Finally, the main findings are summarized, considering the advantages of the developed methodology. Figure 1 shows the general sketch of the problem.
Materials and methods
Materials
Contaminated drill cuttings, collected in the shale shaker, and n-paraffin (used in drilling fluids) were supplied by Petrobras S.A. The reagents used in the experiments, supplied by Synth®, were n-butanol, anhydrous Na2SO4, and n-hexane. Two classes of nonionic surfactants were used: Alkonat® and Ultranex®. Six surfactants were tested: Alkonat® L60 (HLB = 11.5), Alkonat® L90 (HLB = 13.4), Alkonat® L230 (HLB = 16.9), Ultranex® NP 95 (HLB = 13), Ultranex® NP 110 (HLB = 13.7), and Ultranex® NP 230 (HLB = 16.4). These surfactants were supplied by Oxiteno®. Alkonat® has a linear chain and Ultranex® an aromatic one. The choice of these surfactants was based on the HLB (hydrophilic-lipophilic balance) value, i.e., surfactants with ability to emulsify oil in water (HLB values between 8 and 16), and the formation of a microemulsion in the water-rich side of the pseudoternary phase diagram.
Methods
Obtaining simulated drill cuttings
Contaminated drill cuttings were dried at 150 °C for 4 h. After drying, they were contaminated with n-paraffin at a 1:10 ratio. Dry and contaminated cuttings were stored in a refrigerator for at least 48 h to ensure that the n-paraffin was adsorbed on cuttings. The cutting samples submitted to this procedure were named “simulated cuttings.”
Contaminated and simulated cuttings samples were submitted to thermal gravimetric analysis (TGA), on a Netzsch® TG 209 F1 Libra® equipment. The TGA analysis sought to compare samples and to verify if the drying conditions used (150 °C for 4 h) were satisfactory.
Methodology for quantification of n-paraffin in the drill cuttings
The ultrasound extraction methodology, adapted from EPA 3550c, was used to determine the amount of n-paraffin in both, simulated and treated, cuttings and, then, to determine extraction percentage (US EPA 2007).
In this methodology, 25 g of drill cuttings were mixed with 50 ml of n-hexane and submitted to an ultrasound bath (Elma® Transsonic 460) for 15 min. After this time, the mixture was separated and a sample of the extract was centrifuged in a test tube at 2000 rpm for 4 min. After centrifugation, the sample was filtered in the presence of anhydrous Na2SO4. The extract obtained in this step was subjected to gas chromatography analysis with flame ionization detector (GC-FID), Thermo Scientific® Trace 1300 equipment, Agilent Technologies® DB-5 ms column, injection volume of 2 μl, carrier gas flow rate (N2) of 2.5 ml/min, and injector and detector temperatures of 220 and 340 °C, respectively.
The influence of solvent (MES)
The microemulsion systems were based on the Winsor IV point (microemulsion region) in the pseudoternary diagram shown in Fig. 2. For the delimitation of the microemulsion region inside the pseudoternary phase diagram, the sequential addition method was used. Varied amounts of active matter (n-butanol and the used surfactant, at a constant C/S ratio = 0.5) and n-hexane were poured into a 10-mL centrifuge tube at room temperature (27 °C). Water supply (WS) was then added and mixed to obtain the critical points, where the systems turned from a clear to a cloudy appearance. The system was then centrifuged during 5 min (2000 rpm) to verify phase separation and then weighed. The procedure was repeated with seven mixtures (2 g) with different proportions of active matter + n-hexane phase. The pseudoternary phase diagram was thus constructed by plotting the amounts of water supply, oil phase, and co-surfactant/surfactant phase used in each experimental run. It was used a MES composed by water supply (WS) = 83%, n-butanol/surfactant (C/S = 0.5) = 16%, and n-hexane = 1%. This point was chosen so that it originated a microemulsion using each surfactant studied, without the need to show all six pseudoternary diagrams obtained. Six nonionic surfactants were studied, three of which were ethoxylated lauryl alcohols (Alkonat® L60, L90, and L230) and the others were ethoxylated nonylphenols (Ultranex® NP 95, NP 110, and NP 230).
Following the formation of the six microemulsion systems, simulated cutting extractions were performed. This study used 25 g of simulated cuttings with 25 g of microemulsion (microemulsion/cuttings ratio = 1.0). It had a contact time of 10 min and used a simple extraction, without stirring. After the treatment, mixtures were separated. The refined ones (cuttings treated and the retained microemulsion) were submitted to ultrasound extraction to determine the n-paraffin content after extraction with MES.
Extractions to determine the best MES were performed in triplicate. The extraction percentage was determined by comparing the areas of the chromatograms obtained, for the simulated cuttings and the cuttings after treatment, using Eq. (1).
where Asimulated.c is the area of the chromatogram obtained from simulated cuttings samples subjected to ultrasound extraction; Atreated.c is the area of the chromatogram obtained from cutting samples treated with MES and subjected to ultrasound extraction.
The influence of MES/cuttings ratio
The study of the MES/cuttings ratio was based on the best microemulsion system determined by the methodology cited previously. The ratios chosen in this study were 0.25, 0.5, 1.0, 1.5, and 2.0 (mass/mass).
The methodology used consists of treating 25 g of simulated cuttings using the best MES result from the studied ratios. The experiments were performed in triplicate, and the mixtures were subjected to simple contact extraction, without stirring, and a contact time of 10 min. After treating and separating extracted and refined mixtures, the latter one was submitted to ultrasound extraction.
The extraction percentage was also found using Eq. (1), analyzing the chromatogram results of the treated samples at each studied ratio.
Stirring speed influence
For the study of the influence of the stirring speed, it was used as a Dubnoff water bath (Tecnal® TE-053) with a horizontal stirring platform, in which the number of stirring cycles was measured (strokes—stks). The study looked at how many times per minute the Erlenmeyer would exit and return to its original position after horizontal movement. The agitation speeds studied were 48, 84, and 132 stks.
The experimental methodology was similar to the one used in previous studies. Here, 25 g of simulated cuttings were mixed with the best MES, in the best ratio MES/cuttings, with 10 min of contact time, using a simple extraction, and at the mentioned stirring speeds. After extraction, the extracted mixture was separated from the refined one, and the refined was submitted to ultrasound extraction. Using the chromatography data gathered for both simulated and treated cuttings at each stirring speed studied, it was possible to determine the obtained extraction percentage by Eq. (1).
Influence of contact time
All studies performed so far used a contact time of 10 min. The influence of extraction time on extraction percentage was also studied. The contact times studied in this experiment were 1, 10, 20, 40, 80, and 160 min.
For this study, 25 g of simulated cuttings were mixed with the best MES, in the best MSE/cuttings ratio found. The mixture was subjected to simple extraction using the ideal stirring speed found and varying the contact time. After treatments, samples were separated and the refined sample was submitted to ultrasound extraction.
Using the chromatogram areas of simulated and treated cuttings samples, it was possible to determine the percentage of n-paraffin extracted for each extraction time.
Reuse of MES
To reduce the amount of MES used, the study of the reuse of MES was performed in three consecutive extractions, without promoting the treatment of the MES used. Figure 3 shows an illustrative scheme of how the study was conducted.
In this study, 50 g of simulated cuttings were mixed with the best MES, previously determined. This sample of MES was called unused microemulsion. Other extraction parameters, MES/cuttings ratio, stirring speed, and contact time had been optimized and determined previously. Extracted and refined samples were separated and an ultrasound extraction was performed on the refined sample, which was called refined 1. The microemulsion already used (microemulsion used 1x) was mixed in a new simulated cuttings sample repeating the procedure above mentioned.
Three simulated cuttings samples were treated. The MES was used three times, as shown in Fig. 2. All refined samples (1, 2, and 3), as well as the simulated cuttings samples treated, were subjected to ultrasound extraction. Using Eq. (1), it was possible to determine the extraction percentage for each experimental run.
Results and discussions
Thermal study of contaminated and simulated cuttings
The thermogravimetric curves for contaminated (a) and simulated (b) cutting samples are shown in Fig. 4.
Analyzing Fig. 4, one can observe that there is greater mass loss around 100 °C for the contaminated cuttings and around 150 °C for simulated cuttings. One can also notice a greater mass loss as a function of time, looking at the thermogravimetric curve (TGA) in simulated cuttings, which shows a n-paraffin content higher than that present in contaminated cuttings.
The simulated cuttings TGA graph, Fig. 4b, also displays better cutting stability over time, whereas no significant drop in mass is found in the temperature range below 50 °C (approximately 0.2%). Contaminated cuttings, Fig. 4a, show different results as significant mass losses can already be observed in this temperature range (approximately 2%). Based on these observations, it was concluded that 150 °C and 4 h of drying time are enough to remove the most volatile contaminants, as well as the n-paraffin adsorbed on contaminated cuttings.
Study of microemulsion system type influence
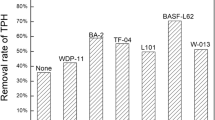
The extraction percentage for each MES using the six surfactants studied can be seen in Fig. 5. From these results, one can observe there is a tendency of reducing the extraction percentage when increasing the surfactant’s HLB (hydrophilic-lipophilic balance). The HLB measures the ratio between hydrophilic and lipophilic groups. HLB values for nonionic surfactants range from 0 to 20. Surfactants with HLB numbers > 10 have an affinity for water (hydrophilic), and the ones with values < 10 have an affinity for oil (lipophilic). Lower HLB surfactants tend to interact more with n-paraffin than the ones with higher HLB surfactants.
The two classes of surfactants used are nonionic and displayed very similar results. Nevertheless, it was preferred to use Alkonat® because, when comparing the molecular structures of the two-surfactant classes–Fig. 6, there is an aromatic ring in the structure of the Renex® surfactant, which generally characterizes greater toxicity to man and the environment. The Alkonat® L90 was chosen as the best MES because it is noted that the Alkonat® L60 has excessive foam formation, which makes it difficult to use in field operations.
Study of the influence of MES/cuttings ratio
The influence of the MES/cuttings ratio on the extraction percentage using the most suitable surfactant, Alkonat® L90, was studied. The results obtained can be observed in Fig. 7.
Analyzing the extraction percentages presented in Fig. 7, one can observe a slight increase in the extraction percentage with increasing MES/cuttings ratio, but this increase was not significant. Using a ratio of 2.0 would not be feasible since a ratio of 1.0 would be enough to get a similar extraction percentage.
The most logical thing to do is to use as little volume of MES as possible, aiming at the lowest cost in the process, but due to the difficulty of separating the phases, it was chosen 1.0 as an intermediate ratio.
Study of the influence of stirring speed
The results obtained in this study can be seen in Fig. 8. The extraction parameters used were Alkonat® L90 as the surfactant in MES and the MES/cuttings ratio of 1.0. Other conditions have already been mentioned in the methodology section of this work.
Analyzing the results presented in Fig. 8, one can observe there is an increase in the extraction percentage as the stirring speed increases. However, a percentage reduction behavior is also observed when comparing the results without stirring and with 48 stks stirring. This behavior is probably due to the type of stirring used: horizontal displacement. Therefore, at low stirring speeds, the formation of a film on top of the cuttings probably occurs, avoiding better contact between the microemulsion system and the cuttings. This film must be formed by the very fine particles of the cuttings to produce a poorly permeable layer, which restricts the interaction between the MES and the solid. With the continuous increase of the stirring speed, the extraction percentage increases, probably due to the stirring of the solid particles, preventing the formation of the impermeable film and, consequently, increasing the interaction between the MES and the cuttings.
The increase of the stirring speed cannot be very large because it can promote the deterioration of the solid sample. Therefore, it was chosen the highest stirring speed studied, 132 stks, as the optimal point.
Study of the influence of contact time
After determining the optimized parameters for the surfactant used in the MES, the MES/cuttings ratio, and the stirring speed, the contact time would be reviewed. The results obtained for the contact time are shown in Fig. 9.
Analyzing the results, one can see that the percentage of extraction increases with the increase of contact time, reaching 80.37%. It was also observed that the extraction is fast but not efficient. In just 1 min of contact time, the experiment had already reached an extraction percentage of 45.85%.
It was also clear the extraction percentage obtained in 160 min of extraction, 80.37%, is not feasible, because with only half of this time it was possible to reach 77% of extraction. With this in mind, it was chosen 80 min of contact time as the optimal duration.
With the obtained results, it was possible to determine the optimal parameters of solid–liquid extraction that provided the best result of extraction percentage (86.26%) of n-paraffin using MES. The following are the optimal process conditions obtained:
Alkonat® L90 as the surfactant of MES.
MES/drilling cuttings ratio equal to 1.0.
Stirring speed of 132 stks.
Contact time of 80 min.
Study of reuse of MES in multiple extractions
With the extraction parameters optimized, the study of the reuse of MES in multiple extractions was performed, without promoting the treatment of MES between the extractions. The results obtained can be seen in Fig. 10.
Analyzing the obtained results, one can observe the first and second extractions obtained high extraction percentages, 86.27% and 80.83%, respectively. However, a significant reduction in the extraction percentage, 28.52%, could be observed in the third extraction, which probably occurred due to MES saturation.
Each MES surface tension analysis, SensaDyne®, was performed to evaluate if the MES still presented a low surface tension. The results obtained can be seen in Table 1. Analyzing the results, one can see there is no significant change in this tension, maintaining the tension characteristic of MES. After the third extraction, the MES presented a phase separation displaying a two-phased region or Winsor I. This refers to the equilibrium proposed by Winsor (1948), which is characterized by a microemulsion phase in equilibrium with excess oil phase (Fig. 2). The presence of Winsor I justifies its saturation and significant reduction of the extraction percentage.
Analyzing these results, one can conclude that MES can be reused efficiently in up to two consecutive extractions. In addition, an MES treatment should be performed to remove n-paraffin.
Final considerations
After all the results presented, it can be determined that the use of MES in the treatment of drill cuttings has some advantages, such as:
Allow treatment of contaminated drill cuttings to acceptable contamination levels;
The use of a few types of equipment for its implementation compared to the microwave, heat desorption, and drying treatments;
The ability to allow more than one use with the same MES load without treatment;
Ease recovery of n-paraffin from the MES by only changing from the Winsor IV region to the biphasic one (Winsor I).
Some disadvantages may also be noted, such as:
The use of chemicals in its composition;
Despite treating drill cuttings at acceptable levels, the use of MES did not reach extraction rates as high as the use of microwaves.
Conclusions
Analyzing the results obtained, it was concluded that:
Drying the cuttings at 150 ℃ for 4 h is effective and promoted the removal of n-paraffin and the most volatile contaminants, which could impair chromatographic analysis.
The extraction percentage is inversely proportional to the surfactant HLB used in the microemulsion system.
The microemulsion system using Alkonat® L90 performed good results. Although it did not obtain the highest extraction percentage, it was the best option considering environmental issues.
The microemulsion system/cuttings ratio did not influence the extraction percentage significantly. Therefore, an intermediate ratio of 1.0 was chosen.
The extraction percentage is directly proportional to the stirring speed. The highest speed studied, 132 stks, was chosen as the optimum.
The extraction percentage is directly proportional to the contact time. The optimal extraction results were achieved with 80 min contact time.
The study of the reuse of the microemulsion system showed that microemulsions can be used efficiently in two extractions, obtaining extraction percentages higher than 86%.
The limitations of this technology are the relatively high contact time (80 min) and the need of stirring.
Comparing the results of this work with the ones found in literature for the use of biosurfactants, the MES is more efficient (Yan et al. 2011; Olasanmi and Thring 2019) and has the possibility of reuse. Although microwave processes have higher extraction percentages (Robinson et al. 2009a; Petri Jr et al. 2017, 2019), they require a more sophisticated apparatus and higher energy consumption.
Finally, with the extraction parameters optimized and presented, it can be concluded that the use of microemulsion systems is an efficient and technically feasible alternative for treating drill cuttings contaminated with drilling fluid paraffin.
References
Ball AS, Stewart RJ, Schliephake K (2012) A review of the current options for the treatment and safe disposal of drill cuttings. Waste Manag Res 30:457–473. https://doi.org/10.1177/0734242X11419892
Caenn R, Darley HCH, Gray GR (2016) Composition and properties of drilling and completion fluids, 7th edn. Gulf Professional Publishing, Cambridge
Childs JD, Acosta E, Scamehorn JF, Sabatini DA (2005) Surfactant-enhanced treatment of oil-based drill cuttings. J Energy Resour Technol 127:153. https://doi.org/10.1115/1.1879044
De Castro Dantas TN, Neto AAD, De Moura AMCP (2001) Removal of chromium from aqueous solutions by diatomite treated with microemulsion. Water Res 35:2219–2224. https://doi.org/10.1016/S0043-1354(00)00507-8
de Castro Dantas TN, de Souza TTC, Dantas Neto AA et al (2017) Experimental study of nanofluids applied in EOR processes. J Surfactants Deterg 20:1095–1104. https://doi.org/10.1007/s11743-017-1992-2
Dejam M, Hassanzadeh H, Chen Z (2014) Shear dispersion in a fracture with porous walls. Adv Water Resour 74:14–25. https://doi.org/10.1016/j.advwatres.2014.08.005
Dejam M, Hassanzadeh H, Chen Z (2016) Shear dispersion in a capillary tube with a porous wall. J Contam Hydrol 185–186:87–104. https://doi.org/10.1016/j.jconhyd.2016.01.007
Deshpande S, Shiau BJ, Wade D et al (1999) Surfactant selection for enhancing ex situ soil washing. Water Res 33:351–360. https://doi.org/10.1016/S0043-1354(98)00234-6
Fanun M (2009) Microemulsions: properties and applications, 1st edn. CRC Press, Boca Raton
Jacques Whitford Stantec (2009) Cuttings treatment technology evaluation. Report No. 166., Toronto
Muherei M, Junin V (2007) Potential of surfactant washing to solve drilling waste environmental problems offshore. Emirates J Eng Res 12:10
OGP (2003) Environmental aspects of the use and disposal of non aqueous drilling fluids associated with offshore oil and gas operations
Olasanmi IO, Thring RW (2019) Evaluating rhamnolipid-enhanced washing as a first step in remediation of petroleum-contaminated soils and drill cuttings. J Adv Res. https://doi.org/10.1016/j.jare.2019.07.003
Olayiwola SO, Dejam M (2019) A comprehensive review on interaction of nanoparticles with low salinity water and surfactant for enhanced oil recovery in sandstone and carbonate reservoirs. Fuel 241:1045–1057
Petri I Jr, Martins AL, Ataíde CH, Duarte CR (2017) Microwave drying remediation of petroleum-contaminated drill cuttings. J Environ Manage 196:659–665. https://doi.org/10.1016/J.JENVMAN.2017.03.068
Petri I Jr, Martins AL, Duarte CR, Ataíde CH (2019) Development and performance of a continuous industrial microwave dryer for remediation of drill cuttings. J Pet Sci Eng 176:362–368. https://doi.org/10.1016/J.PETROL.2019.01.075
Robinson J, Kingman S, Snape CE et al (2009a) Microwave treatment of oil-contaminated drill cuttings at pilot scale. SPE Drill Complet 24:430–435. https://doi.org/10.2118/111637-PA
Robinson JP, Kingman SW, Snape CE et al (2009b) Remediation of oil-contaminated drill cuttings using continuous microwave heating. Chem Eng J 152:458–463. https://doi.org/10.1016/j.cej.2009.05.008
Rosen MJ, Kunjappu JT (2012) Surfactants and interfacial phenomena, 4th edn. Wiley, Hoboken
Schramm LL (2000) Surfactants: fundamentals and applications in the petroleum industry. Cambridge University Press, Cambridge
Tadros TF (2005) Applied surfactants: principles and applications, 1st edn. Wiley-VCH, Weinheim
Urum K, Pekdemir T, Çopur M (2004) Surfactants treatment of crude oil contaminated soils. J Colloid Interface Sci 276:456–464. https://doi.org/10.1016/j.jcis.2004.03.057
U.S. EPA (2000a) EPA 821B-00-013 - development document for final effluent limitations guidelines and standards for synthetic-based drilling fluids and other non-aqueous drilling fluids in the oil and gas extraction point source category
U.S. EPA (2000b) EPA-821-B-00-015 - statistical analysis supporting final effluent limitations guidelines and standards for synthetic-based drilling fluids and other non-aqueous drilling fluids in the oil and gas extraction point source category
US EPA (2007) Method 3550C: ultrasonic extraction
Winsor PA (1948) Hydrotropy, solubilisation and related emulsification processes. Trans Faraday Soc 44:376. https://doi.org/10.1039/tf9484400376
Yan P, Lu M, Guan Y et al (2011) Remediation of oil-based drill cuttings through a biosurfactant-based washing followed by a biodegradation treatment. Bioresour Technol 102:10252–10259. https://doi.org/10.1016/j.biortech.2011.08.074
Acknowledgements
The authors of this paper would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES)–Finance Code 001 for financial support. The authors would also like to thank the Surfactants Technology Lab (LTT) and the Primary Processing Core and Reuse of Produced Water and Waste (NUPPRAR), both from the Federal University of Rio Grande do Norte (UFRN), for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dantas, T.N.C., da Silva, D.N.N., Dantas Neto, A.A. et al. Treatment of drill cuttings using microemulsion. J Petrol Explor Prod Technol 10, 1243–1251 (2020). https://doi.org/10.1007/s13202-019-00813-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-019-00813-3