Abstract
Wax deposition inside pipeline and process equipment is a major problem in oil industry. In this study, a multi-solid thermodynamic model was developed to predict wax disappearance temperature (WDT). Paraffinic–naphthenic–aromatic (PNA) analysis was performed and two correlations were introduced for fusion properties of these species. In addition, WDT of 12 Iranian oil and condensate samples were measured using viscometry and differential scanning calorimetry (DSC) techniques. Experimental data of multi-component and ternary systems were utilized for validation of the model. It was observed that measured WDT by viscometry method is higher compared to DSC. Statistics analysis shows that DSC technique has lower average absolute relative error (AARE) and standard uncertainty compared to viscometry. Results show that the AARE of the model for ternary systems is 0.52% which is much lower among the previous developed thermodynamic models. In addition, AARE of the new model for 68 data was calculated about 0.23%, and R-square of model prediction was calculated about 0.97. The cumulative distribution function also indicates that P50 values are almost the same for model and experimental data. These results show that the model has a good accuracy. In addition, the accuracy of model increases as the average carbon number of oil mixtures increases. Finally, it was found that PNA analysis and distribution of each component in its sub-fractions have a considerable effect on the model accuracy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding of wax formation potential in waxy oil samples is necessary for avoiding wax deposition during oil and condensate production. Wax deposition increases pressure drop and power requirements (Misra et al. 1995), pig stuck (Bai and Bai 2005) and decreases effective flow area of the pipeline which causes oil production reduction (Jalalnezhad and Kamali 2016). To prevent these problems, it is required to predict wax precipitation as a function of composition and thermodynamic conditions correctly. Various experimental studies were carried out for determining WDT and wax appearance temperature (WAT) of crude oils and condensate samples. Wang et al. (2003) used a light transmittance technique to measure the WAT and WDT of paraffinic mixtures of C24 and C36 in C10. Binary mixtures were prepared by dissolving 5 wt% of C24 or C36 in C10. Poly n-butyl methacrylate (nBuMA), poly n-nonyl methacrylate (NMA), poly n-octadecyl methacrylate (ODMA), polyalkyleneimine (D), and unknown (E) solvent were used as inhibitor. Their results show that solvents that decrease WAT also increase WDT of C24 and C36 mixtures. In addition, the ability of inhibitors for reducing the WAT of mixtures containing C24 was higher than those which comprised from C36. In addition, the order of inhibitor efficiency for WAT reduction was E > D > ODMA > NMA = nBuMA. Several experimental studies were performed to measure wax formation condition in multi-component systems. In another study, Nitin and Anil (2004) studied the liquid–solid-phase equilibrium of waxy mixtures containing 6–22 wt% wax. These samples consisted n-alkanes with carbon number in range of C20–C40. In addition, C16 and Norpar13 which comprised from mixture of C9–C16 were employed as solvents. They reported that measured WDT data were higher than the WAT values by < 3 °C. Results of these studies show that the highest and lowest WDT values which, respectively, obtained for 22 wt% wax in C16 and 6 wt% wax in Norpar13 are 41 and 26 °C. In addition, it was observed that wax-Norpar13 mixture had lower WDT compared to mixture that used C16 as solvent. Martos et al. (2008) characterized wax samples precipitated from two crude oils at different temperatures by optical microscopy, elemental analysis, HNMR spectroscopy, DSC, and mass spectroscopy. Two naphthenic and paraffinic oil samples and their precipitated fractions were utilized for investigation. An HNMR spectrometer was used to quantify different types of hydrogen atoms. The temperature profile for DSC tests varied in range of 353 to 153 at 3 K min−1 and vice versa. Two different techniques including gas chromatography and high-resolution mass spectroscopy were employed for n-paraffin distribution of each fraction. They reported that crude oils had lower WDT values compared to corresponding precipitated samples because precipitated fractions had higher wax concentration. In addition, they observed that fractions which precipitated at lower temperatures had lower WDT, because lighter components crystallize at lower temperatures. The effect of wax properties of different oil mixtures from Iran reservoirs on shape, WAT, and crystals morphology was investigated by Taheri-Shakib et al. (2018). The crude oil samples had asphaltene content in range of 0.67–16.19 wt% and wax content between 2.52 and 8.5 wt%. They used cross-polarized microscopy (CPM) and viscometer for WAT measurement. Their studies showed that naphthenic waxes comprised from polar compounds, while paraffinic waxes not contained from polar/naphthenic compounds. They reported that crystalline form affected and WAT increased by increasing asphaltene content. In addition, their results showed that WAT of semi-micro-crystalline samples increased by increasing sample polarity. In addition, the authors found that WAT values can be reduced by eliminating the oxygen (O), sulfur (S), and nitrogen (N) elements in the waxy mixtures. In addition, they observed that in waxy mixtures with high and low WAT values, wax carbon content was about 80 and 90%, respectively. Therefore, higher carbon content and lower SNO elements increase the non-polarity of wax and reduce the WAT. Using a micro-DSC, couple of CPM and image processing method and rheometer, Japper-Jaafar (2016) measured WAT of three crude oils from South East Asia. These oils were classified into mild, medium, and severe waxy crude oils. The effects of cooling rate on WAT and wax aggregation from various equipment under cooling rate of 1 °C min−1 are explored and discussed in detail. Their finding shows that the wax type, cooling rate, and shear rate affected the WAT values significantly. In addition, due to the presence of micro-crystalline waxes, they observed that micro-DSC technique detects wax crystals at higher temperatures compared to CPM and rheometry. In addition, their studies showed that wax crystal aggregation reduced by increasing cooling rate and this effect increased by increasing wax content of crude oil. Finally, rheological investigations indicated that WAT of oil samples is shear-dependent when paraffin content is higher than 23.89 wt%.
Development of suitable thermodynamic model is essential for wax investigation. Thermodynamic models are very useful and economical to predict the wax deposition conditions especially in places, where access to them is difficult and costly. In addition, experimental studies are time consuming and have considerable errors. Currently, two kinds of thermodynamic models for wax prediction exist that are based on flash calculations. These models are classified as multi-solid (MS) and solid solution (SS) thermodynamic models. In the SS approach, it is assumed that all components contribute in wax formation, and over-estimate the amount of the precipitated wax (Vafaie-Sefti et al. 2000). SS models use two different types of equation of state (EOS) and activity coefficients simultaneously for non-ideality description of the liquid state which makes these models, thermodynamically inconsistent (Rahimpour et al. 2013). However, MS model uses only EOS for non-ideality of phases in equilibrium. The MS model does not require any adjustable mixture parameters which makes it very simple and accurate in practice (Rahimpour et al. 2013).
Won (1986) developed an SS model and correlations for fusion properties as a function of molar mass. Snyder et al. (1993) performed spectroscopic studies for binary systems and indicated that formed phases comprised of pure components. They suggested that wax formation from multi-component oil mixtures produces pure immiscible phases. Lira-Galeana et al. (1996) stated that since the solubility of each component is highly dependent on temperature, it is expected that only part of components exist in solid–liquid equilibrium during cooling process. Dorset (1990) used calorimetry and investigated the solid formation from binary paraffinic systems. He observed that separated phases comprised from pure components. Lira-Galeana et al. (1996) suggested a new MS model for wax precipitation. They employed experimental data of naphthenic and aromatic hydrocarbons and derived new correlation for prediction of fusion temperature. In addition, they suggested that fusion enthalpy correlation of Won (1986) overestimates the amount of wax precipitated below WAT; therefore, they developed a new correlation. The fusion heat capacity was estimated by Pedersen et al. correlation (Pedersen 1995). Ericsson et al. (1993) modified the Won (1986) model and proposed a new SS model. They showed that fusion temperature drops significantly when a branch is added to normal paraffin. They measured the amount of normal paraffins directly by performing laboratory composition analysis of component with carbon number up to 40. Won (1986) considered that all components with the same carbon number have the same melting temperature. However, Ericsson et al. (1993) revealed that melting temperature of aromatics and naphthenes is different from normal paraffins, and they modified Won (1986) correlation for fusion temperature of these components. Aftab et al. (2015) developed two SS models and measured the WDT of two ternary normal alkane systems. They applied PC-SAFT for liquid non-ideality illustration. In addition, solid-phase non-ideality has been described indirectly by EOS using solid-to-liquid fugacity ratio. In the second model, solid and liquid non-idealities were determined by different activity coefficient methods. They reported that regular solution theory for liquid-state non-ideality and predictive Wilson approach for solid-state non-ideality present improved accuracy relative to other activity models. Nichita et al. (2001) proposed an MS model for wax formation prediction in gas condensate systems. They used Won (1986) correlation to estimate fusion temperature of normal paraffins. The authors introduced new correlations for fusion enthalpy and transition temperature. Solid–solid-phase transition term was added into solid-to-liquid fugacity ratio equation of pure components. In addition, PR (Peng and Robinson 1976) EOS was utilized in their model for VLE calculation. Ghanaei et al. (2007) proposed an MS model based on different activity coefficient approaches and used 56 experimental equilibrium data to validate their models.
In this study, an MS thermodynamic model was developed for WDT prediction at low pressures. Phase behavior of liquid was described by using PR EOS. Paraffinic–naphthenic–aromatic (PNA) analysis was applied to divide each component into its paraffinic–naphthenic and aromatic sub-fractions. In addition, two correlations for fusion properties of mentioned sub-fractions were developed to better estimation of solid-phase behavior. WDT of four ternary systems including C14–C15–C16 (system #1), C16–C17–C18 (system #2), C18–C19–C20 (system #3), and C19–C20–C21 (system #4) are investigated with a new thermodynamic model. In addition, WDT of 12 Iranian crude oils and condensates were measured using viscometry and DSC analysis and these data are used for validation of the model. In the following sections, experimental method is explained first. Then, thermodynamic model is developed and applied on selected dataset. Next, results are presented and discussed. Concluding remarks appear at the end of paper.
Materials and methods
Nine Iranian crude oils and three gas condensate samples were supplied from Kharg oil terminal and south pars gas refineries, Bushehr, Iran. Anton Paar SVM3000 viscometer and Mettler Toledo DSC823 analysis tool were used for WDT determination of oil samples. WDT measured by viscometry technique has inherent shortcomings and should not be used solely for wax characterization. Therefore, two methods are used for WDT determination. These two techniques were used for accuracy evaluation of the results. Composition of oil samples was determined by simulated distillation (SimDis) method according to ASTM D7169 (D7169-16, A 2016). GC device model 7890b manufactured by Agilent was used. The device is equipped with a 5-m column with 0.53-mm diameter. The oven has the ability to program the temperature so that its initial and final temperatures are − 50 and 430 °C, with heating rate of 15 °C min−1. The detector is also a flame ionization type. These experiments were carried out in Iranian Research Institute of Petroleum Industry. About 50 µl of the sample with 200 µl of CS2 as solvent was mixed at ambient temperature and then 1 µl of this mixture was injected into the column. The approximate duration of each test was 35 min. Results of SimDis analysis are shown in Table 1.
Plus fraction splitting of all collected samples was done using two-parameter gamma probability function (Whitson and Brulé 2000) and Ahmed (2007) methods. For each sample, components with carbon number greater than 7 were lumped in C7+ fraction and then splitted to components with carbon number up to 20. After that, estimated compositions of components with carbon number between 7 and 19 were compared to experimental values measured by SimDis technique. Figure 1a, b shows distribution of plus fraction (C7+) for 2 oil samples (oil #2 and #8) compared to experimental data.
AARE of other samples was calculated by Eq. (1) and given in Table 2:
where Nt is number of total data, and θexp and θpre are experimental and predicted properties, respectively.
Comparing results of these methods show that gamma distribution technique splits plus fraction accurately and has lower AARE than Ahmed method; therefore, gamma probability function was used to split plus fraction of oil and condensate samples.
Viscometry method
Each oil and condensate samples were heated to 80 °C and maintained at these conditions for 2 h to remove all the precipitated wax crystals and thermal history. The sample was then cooled to 45 °C by 5 °C h−1 cooling rate; thereafter, 2.5 ml of the homogenized samples was injected into viscometer and cooled to 0 °C by 3 °C min−1. Semi-log plotting of the dynamic viscosity vs. inverse of temperature was employed to determine the wax disappearance temperature. WDT can be determined using viscometer data and Arrhenius equation as follows:
In Eq. 2, C is constant; µ/Pa s, Ea/J mol−1, R, and T/K, respectively, are dynamic viscosity, activation energy, gas constant, and temperature. According to Arrhenius equation, when wax crystals form, viscosity increases suddenly and oil behavior changes to non-Newtonian.
DSC method
DSC method measures changes of samples enthalpy at different temperature. Enthalpy changes because of variation in physical and chemical properties of samples. Mettler Toledo DSC823 system was employed to measure WDT. 200 µl of oil samples was injected into DSC and then heated from 25 to 80 °C at 3 °C min−1 to completely dissolve the precipitated crystals and to remove any shear history. Then, sample was cooled down from 80 to − 10 °C by 3 °C min−1. In the enthalpy vs. temperature plot, the point on plot where the slope suddenly changes is WDT.
Experimental dataset
In this study, four ternary systems were utilized to validate the new developed thermodynamic model. These data were reported by Metivaud et al. (1999). A total of 56 experimental data including 11 different compositions for system #1, 11 data for system #2, and 18 data for system #3 and 16 data for system #4. Experimental data of ternary systems are given in Tables 3, 4, 5, and 6.
Model development
For thermodynamic equilibrium, chemical potential of the ith species in all phases should be equal. For solid–liquid equilibrium, this criterion is written as follows:
In addition, Eq. (3) can be written in terms of fugacity:
where n is the number of components, and µil,µis, fil, and fis are chemical potential and fugacity of the ith component in liquid and solid states, respectively.
There is no EOS to estimate the solid-phase behavior. In this regard, predictive thermodynamic models were developed using the fugacity of pure solid and liquid components at the same conditions. Figure 2 shows the general sketch of thermodynamic path for chemical potential of pure liquid and solid.
Diagram for relating chemical potential of pure liquid and solid (Firoozabadi 1999)
According to Fig. 2, difference between pure liquid and solid chemical potentials is written as follows (Firoozabadi 1999):
Equation (6) can be written in the form of Eq. (7) (Firoozabadi 1999):
Combining Eq. (3) and Eq. (9) gives Eq. (10):
In addition, fugacity of pure solid is related to liquid fugacity as follows (Firoozabadi 1999):
Substituting Eq. (10) into Eq. (11) gives
where Δh/cal gmol− 1, Δv/cm3 mol− 1, and ΔC/cal gmol− 1 K− 1 are difference in enthalpy, molar volume, and heat capacity of pure liquid and solid components. fpure,l and fpure,s are fugacity of pure components in liquid and solid states, respectively; Δhf/cal gmol− 1 is the change of fusion enthalpy; Tf/K is fusion temperature; and Pf/MPa is pressure corresponding to the fusion temperature. T/K, P/MPa, and R are temperature, pressure, and gas constant, respectively.
Stability analysis
Stability analysis was used in MS models to determine all components that form as solid phase. Components that meet the following criteria precipitate as pure solid phase:
In Eq. 13, f (T, P, x) is the fugacity of components in the original mixture with composition x, and thermodynamic conditions of P and T. fspure (T, P) is pure solid fugacity at the same T and P.
In this study, PR (Peng and Robinson 1976) EOS was considered for SLE. Fugacity of pure liquid was estimated by PR EOS, and pure solid fugacity was indirectly calculated from pure liquid fugacity (Eq. 12). The poynting term was ignored in this work, because it is effective only at high pressures.
Fusion properties
Using suitable correlation for fusion properties is an important step in thermodynamic modeling of wax prediction. In this paper, the PNA analysis was performed using Riazi (2005) method. PNA analysis separates each component into paraffinic (P), naphthenic (N), and aromatic (A) species. Finally, each property was estimated by Eq. (14):
where θ is property, and xP, xN, and xA are composition of the P, N, and A species, respectively.
A general correlation was developed for fusion temperature of all hydrocarbon species. Two sub-fractions are considered for each component including paraffinic–naphthenic (PN) species and aromatic species. In this study, experimental data of Himran et al. (1994) for fusion temperature of normal paraffins, and experimental fusion temperature data of naphthenic and aromatic hydrocarbons (American Petroleum Institute, R 1964) were utilized to derive a correlation in the following form:
where Mw is molecular weight and a, b, c, d, and e are constants for PN and A species which were optimized by Newton algorithm method and are tabulated in Table 7.
The new developed correlation has no adjustable parameter in estimating fusion enthalpy of PN species based on average properties of these sub-fractions. The correlation is as follows:
The fusion enthalpy of aromatic species was calculated from the following equation (Lira-galeana et al. 1996):
The correlation developed by Pedersen (1995) was employed for estimating heat capacity difference of all components, as follows:
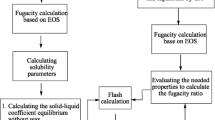
For physical properties, correlations proposed by Edmister (1958), Søreide (1989), Twu (1984), Chueh and Prausnitz (1967), and Cavett (1964) were employed to estimate the acentric factor, boiling point temperature, critical temperature, critical molar volume, and critical pressure, respectively. Figure 3 indicates the proposed algorithm for development of the MS model.
Results and discussion
New MS model was introduced for WDT estimation of crude oils and condensates. Then, it was validated with experimental data of four ternary systems and 12 crude oil mixtures. WDT of these crude oil mixtures was measured using DSC and viscometry techniques. Figures 4, 5, 6 and 7 show the predicted WDT for systems 1 through 4.
Comparison of experimental (Metivaud et al. 1999) and predicted WDT vs. mixture number for system #1
Comparison of experimental (Metivaud et al. 1999) and predicted WDT vs. mixture number for system #2
Comparison of experimental (Metivaud et al. 1999) and predicted WDT vs. mixture number for system #3
Comparison of experimental (Metivaud et al. 1999) and predicted WDT vs. mixture number for system #4
It is observed that the MS model is able to predict the WDT of ternary systems, successfully. Table 8 shows the average absolute deviation of this model for each system. In this table, WDTexp and WDTpre are experimental and predicted WDT values, respectively.
According to Table 8, AARE for systems #1 and #2 is lower than all the previous developed thermodynamic models except the MS models of Ghanaei et al. (2007) which is based on the predictive Wilson model for these systems. Considering the fact that wax deposits are mainly composed of components with a carbon number of 18–36 (for macro-crystalline waxes) or 30–60 (for micro-crystalline waxes), concentration of wax forming compounds decreased with corresponding increase in composition of non-waxing components in mixture no. 7 and beyond. Therefore, the ability of model to predict WDT decreased and the difference between experimental and predicted results increased. Unlike systems #1 and #2, average absolute deviation of the new developed MS model for systems #3 and #4 is lower than all other thermodynamic models. Results show that as carbon number of the systems increases, the AARE of developed model decreases. For 56 experimental data point of four ternary systems, this model has AARE of 0.52% which is the lowest among other models. Table 8 indicates that the error of thermodynamic models of Ghanaei et al. (2007) (five models), Nichita et al. (2001), and Lira-Galeana et al. (1996) have, respectively, 1.24, 1.24, 1.24, 0.99, 0.75, 1.24, and 12.3% AARE which is higher than the new developed MS model. In addition, results reveal that consideration of PNA analysis is essential for accurate estimation of equilibrium conditions.
Figure 8 shows viscosity vs. reciprocal of temperature for oil sample no. 2. According to this figure, at temperatures above wax formation temperature, viscosity has a linear trend and deviates slightly at lower temperatures. In this figure, cooling at temperatures before wax formation temperature leads to increase in viscosity by 3.7E-05 mPa s °C−1, while this rate increases to 108E-05 mPa s °C−1 immediately after the wax formation.
Energy of the oil samples reduces when wax crystal was formed, because formation of wax crystals is an exothermic process. When wax crystals are formed, the heat flow decreases suddenly and slope of heat flow vs. inverse of temperature changes. Figure 9 shows that at temperatures below 298.41 K, reduction rate of oil energy is constant, but immediately after this temperature energy was reduced with higher rate.
Table 9 summarizes viscosity and WDT values of all crude oil and condensate samples measured by viscometry, DSC, and predicted values of WDT by new developed model. It was observed that measured WDT by viscometry method is higher (+ 1 to + 3 °C) compared to DSC. This can be due to precipitation of non-crystalline particles in the oil sample (e.g., asphaltene) or appearance of liquid–liquid separation that cannot be detected by DSC equipment (KOK et al. 1996). Results show that the model has a good agreement with 12 experimental data and can predict WDT in situations where viscometry and DSC analysis are not available.
To investigate the prediction ability of developed thermodynamic model, statistical analysis was performed. In this regard, R-square, average relative error (ARE), average absolute relative error (AARE), standard deviation (STDEV), root-mean-square error (RMSE), and standard uncertainty were calculated to test the model performance. In addition, the same statistical analysis was carried out for validation of data measured by viscometry and DSC techniques. Results of statistics calculation are summarized in Table 10. Statistical results show that WDT measured by DSC technique has lower standard uncertainty compared to those of viscometry. Therefore, DSC has higher accuracy in comparison with viscometry. In addition, the new introduced model has 0.23% AARE, R-square about 0.97, and 1.11 standard uncertainty which show a well agreement between experimental data and the new thermodynamic model.
Using both predicted and measured WDT, the cumulative distribution function is shown in Fig. 10. Three parameters of P10 (10% probability), P50 (50% probability), and P90 (90% probability) were determined for measured and predicted WDT data. According to this figure, the difference between P10 and P90 of predicted and experimental data was 0.95, while this difference is 0.75 for P50. These differences between model and experiment are very low. According to the results, the thermodynamic model developed in this study has a good performance and can be used for WDT estimation.
Conclusion
In this study, an MS thermodynamic model was developed using PR EOS and tested with 56 data points of the ternary systems and 12 experimental data of crude oil mixtures. Results show that fusion temperature and fusion enthalpy of components are the most important parameters in WAT prediction. Comparing results of gamma probability function and Ahmed methods show that gamma distribution technique splits plus fraction accurately and has lower AARE than Ahmed method. PNA analysis and distribution of each component in its sub-fractions including paraffinic–naphthenic and aromatic species have a considerable effect on the model accuracy. It is shown that by increasing carbon number of components in oil mixtures, accuracy of model increases. In addition, predicted WDT by the developed model has a good match with 12 experimental data which were measured by viscometry and DSC methods. For 56 experimental data points of four ternary systems, this model has error of 0.52% in terms of AARE that is lower than the AARE of other thermodynamic models. R square was calculated about 0.97, which shows that the model is able to predict experimental data. The cumulative distribution function also indicates that P50 values for model and experimental data are almost the same which approved the model accuracy. In addition, statistical analysis show that DSC technique has lower AARE and standard uncertainty compared to viscometry. These findings show that the proposed thermodynamic model has a good ability to predict WDT of waxes in multi-component systems, as well as ternary mixtures.
References
Aftaba S, Javanmardia J, Nasrifarb K (2015) Experimental investigation and thermodynamic modeling of wax disappearance temperature for n-undecane + n-hexadecane + n-octadecane and n-tetradecane + n-hexadecane + n-octadecane ternary systems. Fluid Phase Equilib 403:70–77
Ahmed T (2007) Equations of state and PVT analysis, 1st edn. Gulf Publishing Company, Houston, p 553
American Petroleum Institute, R (1964) Selected values for physical and thermodynamic properties of hydrocarbons and related compounds, Proj. 44. Texas A&M University, College Station
Bai Y, Bai Q (2005) Subsea pipelines and risers, 1st edn. Elsevier Science, Amsterdam
Cavett RH (1964) Physical data for distillation calculations, vapor–liquid equilibria, vol 42(III). In: Paper presented to session on computer application, 27th midyear meeting of the Am. Petrol. Inst., Div. Refining, San Francisco, pp 351–366
Chueh PL, Prausriitz JM (1967) Vapor–liquid equilibria at high pressures: calculation of partial molar volume in non-polar liquid mixtures. AIChE J 13:1099–1107
D7169-16, A (2016) Standard test method for boiling point distribution of samples with residues such as crude oils and atmospheric and vacuum residues by high temperature gas chromatography. ASTM International, West Conshohocken
Dorset DL (1990) Chain length and the cosolubility of n-paraffins in the solid state. Macromolecules 23:623–633
Edminster WC (1958) Applied hydrocarbon thermodynamics part 4. Compressibility factors and equations of state. J Pet Refiner 37:173–179
Erickson DD, Niesen VG, Brown TS (1993) Thermodynamic measurement and prediction of paraffin precipitation in crude oil. In: Presented at the SPE annual technical conference and exhibition, Houston, 3–6 Oct. SPE-26604-MS
Firoozabadi A (1999) Thermodynamics of hydrocarbon reservoirs, chap. 5. McGraw-Hill, New York
Ghanaei E, Esmaeilzadeh F, Fathi Kaljahi J (2007) New multi-solid thermodynamic model for the prediction of wax formation. World Acad Sci Eng Technol 29:129–134
Himran S, Suwono A, Mansoori GA (1994) Characterization of alkanes and paraffin waxes for application as phase change energy storage medium. Energy Sources 16:117–128
Jalalnezhad MJ, Kamali V (2016) Development of an intelligent model for wax deposition in oil pipeline. J Pet Explor Prod Technol 6:129–133
Japper-Jaafar A (2016) A new perspective on the measurements of wax appearance temperature: comparison between DSC, thermomicroscopy and rheometry and the cooling rate effects. J Petrol Sci Eng 147:672–681
Kok VM, Letoffe JM, Claudy P, Martin D, Garcin M, Volle JL (1996) Comparison of wax appearance temperatures of crude oils by differential scanning calorimetry, thermomicroscopy and viscometry. Fuel 75:787–790
Lira-galeana C, Firoozabadi A, Prausnitz JM (1996) Thermodynamics of wax precipitation in petroleum mixtures. AIChE J 42:239–242
Martos C, Coto B, Espada JJ, Robustillo MD, Gómez S, Peña JL (2008) Experimental determination and characterization of wax fractions precipitated as a function of temperature. Energy Fuels 22:708–714
Metivaud V, Rajabalee F, Oonk HAJ, Mondieig D, Haget Y (1999) Complete determination of the solid (RI)-liquid equilibria of four consecutive n-alkane ternary systems in the range C14H30–C21H44 using only binary data. Can J Chem 77:332–339
Misra S, Baruah S, Singh K (1995) Paraffin problems in crude oil production and transportation: a review. SPE Prod Oper 10:50–54
Nichita DV, Goual L, Firoozabadi A (2001) Wax precipitation in gas condensate mixtures. SPE Prod Facil 16:250–259
Nitin VB, Anil KM (2004) Measurement and prediction of the phase behavior of wax–solvent mixtures: significance of the wax disappearance temperature. Ind Eng Chem Res 43:3451–3461
Pedersen KS (1995) Prediction of cloud point temperatures and amount of wax precipitation. SPE Prod Oper SPE-27629-PA 10:46–49
Peng DY, Robinson DB (1976) A new two-constant equation of state. Ind Eng Chem Fundam 15:59–64
Rahimpour MR, Davoudi M, Jokar SM, Khoramdel I, Shariati A, Dehnavi MR (2013) Wax formation assessment of condensate in South Pars gas processing plant sea pipeline (a case study). J Nat Gas Sci Eng 10:25–40
Riazi MR (2005) Characterization and properties of petroleum fractions, 1st edn. American Society For Testing and Materials, West Conshohocken, PA
Snyder RG, Conti G, Strauss HL, Dorset DL (1993) Thermally induced mixing in partially microphase segregated binary n-alkane crystals. J Phys Chem 97:7342–7350
Søreide I (1989) Improved phase behavior predictions of petroleum reservoir fluids from a cubic equation of state. Dr. Ing. dissertation, Department of Petroleum Technology and Applied Geophysics, Norwegian Inst. of Technology, Trondheim
Taheri-Shakib J, Rajabi-Kochi M, Kazemzadeh E, Naderi H, Shekarifard A (2018) A comprehensive study of the impact of wax compositions on the wax appearance temperature (WAT) of some Iranian crude oils: an experimental investigation. J Pet Sci Eng 165:67–80
Twu CH (1984) An internally consistent correlation for predicting the critical properties and molecular weight of petroleum and coal-tar liquids. J Fluid Phase Equilib 16:137–150
Vafaie-Sefti M, Mousavi Dehghani SA, Zadeh M, Bahar M (2000) Modification of multisolid phase model for prediction of wax precipitation: a new and effective solution method. Fluid Phase Equilib 173:65–80
Wang KS, Wu CH, Creek JL, Shuler PJ, Tang Y (2003) Evaluation of effects of selected wax inhibitors on wax appearance and disappearance temperatures. Pet Sci Technol 21:359–368
Whitson CH, Brulé MR (2000) Phase behavior. SPE Monograph Series, vol 20. Society of Petroleum Engineers Inc., Richardson, Texas
Won KW (1986) Thermodynamics for solid–liquid–vapor equilibria: wax phase formation from heavy hydrocarbon mixtures. Fluid Phase Equilib 30:265–279
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mansourpoor, M., Azin, R., Osfouri, S. et al. Study of wax disappearance temperature using multi-solid thermodynamic model. J Petrol Explor Prod Technol 9, 437–448 (2019). https://doi.org/10.1007/s13202-018-0480-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-018-0480-1