Abstract
A water-soluble copolymer of maleic acid (MA) and sodium methallyl disulfonate (SMADS) were synthesized by aqueous solution free radical polymerization and evaluated as scale inhibitor for barium sulfate. The copolymer was characterized by FTIR, HNMR, GPC and HPLC results verified the structure of MA-SMADS copolymer and molecular weight was about 1050 g/mol. The experimental results indicates that the optimal conditions for copolymer synthesis are 105 °C, monomer dosage 30% (wt) and sodium persulfate (NaPS) dosage 7%. The inhibition performance of polymer on barium sulfate was evaluated through static bottle test and the static inhibition rate on barium sulfate can reach up to 98%. Consequently, the evaluation criteria was carried out by dynamic scale loop test.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oil exploration industries are currently facing the issue of scales of barium sulfate and other sulfates which form a strong layer on the inner surface of equipment, pipelines and systems. This particular issue may severely affect the optimum working of equipment and sometimes enduce breakdown. These scales are due to the precipitation and accumulation and cause severe blockage to the pipelines further declining oil recovery. This results in serious impact on oil field exploitation and economic gains.
The scale deposition in the oil field is mainly of calcium carbonate, calcium sulfate, barium sulfate, strontium sulfate, iron, silica and other insoluble sediments (Senthilmurugan et al. 2011; Dickinson et al. 2012). Due to the insolubility in acids and very less solubility in water these scales are considered to be the toughest scales to handle. Adding a scale inhibitor is considered to be the economical and simple yet effective solution to prevent scaling (Dickson et al. 2011). Various polymer scale inhibitors with good scale inhibition performance have been reported in the literature, of which, maleic polymers are widely used as inhibitor like carboxylic acid product homopolymerized or copolymerized with acrylic acid, maleic acid and other unsaturated carboxylic acids and sulfonates (Yue Qiansheng et al. 1999; Senthilmurugan et al. 2010). The high content of carboxylic acid provides the chelating nature and dispersing performance to the polymer, it can chelate with the scaling cations through the activated carboxylic in the molecular chains, thus inhibiting crystal nucleation and causing lattice deformation. It also forms a layer on the pipelines or walls of container, thus ensuring the long-term effect of these inhibitors (Lihui et al. 2004; Lijuan et al. 2015). These inhibitors work at very low dosages (substoichiometric or threshold levels) resulting to minimum sludge formation (Jensen et al. 2012). Also, the experimental findings show that the multi carboxyl polymers are considered poor in solubility and low in Ca+2 tolerance in medium with high minerals in it, whereas the sulfonic-based polymers having high-temperature tolerance and salt resistance can be used as a good inhibitor in this application (Yong et al. 2014).
In this research, our main aim was to synthesize and characterize new water-soluble copolymer (MA-SMADS) that can be used as a barium sulfate scale inhibitor and to study the effects of temperature and NaCl concentration on inhibition performance also, the influence caused by synthesized copolymer on the barium sulfate crystal structure were investigated. Finally, the scale inhibition performance of MA-SMADS copolymer was evaluated and compared with existing commercial dispersants through dynamic scale loop test.
Experimental work
Materials
Maleic anhydride (MA), sodium methallyl disulfonate (SMADS), sodium persulfate (NaPS) and sodium hydroxide (NaOH) were of commercial grades. All reagent chemicals used for solution preparation were of analytical grades, and DI water was used throughout the experiments.
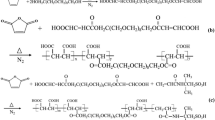
Synthesis of MA-SMADS copolymer
The MA-SMADS copolymer was synthesized through aqueous solution free radical polymerization using sodium persulfate as an initiator. 69.3 g of maleic anhydride was added into 515 g of DI water, heated to 55 °C and dissolved, then 194 g of SMADS was added into the above solution. Then the total solution was transferred into a five-neck 1.5 kg glass reactor prior to setting the stirrer in motion. Then the solution is heated to 105 °C (Chen et al. U1991). Sodium persulfate aqueous solution (19.1 g of NaPS dissolved in 96.9 g of DI water) was added drop wise into the reaction mixture, total reaction time was 180 min, after reaction completion, the material was aged at the same temperature for 30 min then the pH was adjusted to 7.0 with 113 g of sodium hydroxide solution after cooling to 60 °C (Jensen et al. 2012) (Fig. 1).
Characterization of copolymer
The polymer was purified through precipitation using methanol and dried with rotary vacuum evaporator (0.04 bar at 100 °C for 8 h) for characterization. The copolymer structure was confirmed by FTIR (Spectrum-100 PerkinElmer USA KBR pellets region of 400–4000 cm−2) and 1HNMR (AVANCE III HD 400 MHz with sample case 24 auto sampler). The molecular weight and residual monomer of polymer was done with GPC (Waters USA: E-2695 with RI detector), HPLC (Waters USA) and SEM (ZEISS-GEMINISEM), X-ray diffraction (XRD, CAD-SDPMH) used to check the copolymer effect on crystal morphology.
Static bottle test
The copolymer ability to inhibit barium sulfate scale inhibition was investigated and compared through a series of static bottle test following NACE Standard TM0197-2010 and Chinese Petroleum Industry Standard SY/T 5673-1993 test method. The brine solution for evaluation of scale inhibition performance of polymer was prepared. In the first step, a particular quantity of scale inhibitor was weighed in an Erlenmeyer flask; in the second step, 50 mL of cation solution was added and shaken for better mixing of solutions; and in the third step, 50 mL of anion solution was added to the mixture and shaken well. Then the flask was closed with a rubber cork and sealed with plastic sellotape. Finally, Erlenmeyer flasks were kept in oven for 24 h at constant temperature (Luo et al. 2015). Inhibitor efficiency was determined based on residual Ba+2 ions in solutions at different dosage along with the blank. Tests above 100 °C were carried out inside an autoclave, barium and sulfate concentrations are shown in the Table 1 (Liu et al. 2012).
Inhibitor efficiency was calculated based on residual Ba+2 ions in solution according to the formula below.
where m0 is the mass concentration of Ba+2 ions without inhibitor, m1 is the mass concentration of Ba+2 ions in solution without anion addition in the third step, m2 is the mass concentration of Ba+2 ions after inhibitor functions.
Dynamic scale loop test (tube blocking test)
A Dynamic scale loop test was employed to examine the precipitation and deposition of scale and other salt crystals in pipe work system like water or oil pipelines and reservoir conditions. The equipment determines the efficiency of scale inhibitor against inorganic scale minerals formed through minimum inhibitor concentration (MIC). Hence MIC is essential to perform any comparative analysis of various scale inhibitors. This test is used to determine the MIC under dynamic reservoir conditions as an industrial standard. The inhibitor efficiency can be calculated as the ratio between the time needed for tube blocking in the presence of inhibitor divided by the time needed for blocking in absence of inhibitor. The tests were performed with equal amount of cation and anionic brines at 90 °C, 1740 psi and both solutions were filtered before pumping separately through SS coils at 1 mL/min each (Ei-Sayed et al. 2016).
Effect on inhibition performance of inhibitor
Concentration of NaCl
Produced water in oilfields always has high salinity feature. In most cases, sodium chloride accounts for more than 90% among sodium chloride, potassium chloride and magnesium chloride salts. It is known that salts have an impact on scaling tendency by affecting the ionic strength of oilfield produced water. Hence, this ionic strength also influences the inhibitor’s performance. The polymer inhibition rate was studied at different sodium chloride concentrations, i.e., at 2.5, 5.0, 7.5, 10, 12.5, 15.0, 30, 60 g/L (Luo et al. 2015; Jiang et al. 1996).
Temperature
Scale formation is temperature dependent, this is the case also for the inhibitor. In general, inhibitor performance degrades at high temperatures. Thus, the inhibitor efficiency was also studied at 80–120 °C in this work (Dyer and Graham 2002).
Scale inhibition mechanism
Scale inhibition was studied through complexing effect, crystal modification and dispersing effect. Barium sulfate deposition was obtained with and without inhibitor dosages; it was collected and dried carefully for characterization. The SEM was used to observe the surface morphology of scale crystal and X-ray diffraction was used to study the crystal structure of the scale (Shen et al. 2012).
Results and discussion
Structural characterization of copolymer
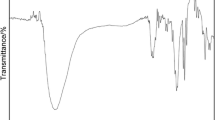
The structure of MA-SMADS copolymer was confirmed through FTIR and 1HNMR spectra’s. The FTIR spectrum shows the following absorption bands: 2933 cm−1 (Methylene stretching and vibration peak), 1718.39 cm−1 (Absorption peak at 1718.39 cm−1 is attributed to stretching vibration of carboxyls), 1174 and 1041 cm−1 (both peaks belong to sulfonic acid group), 623.47 cm−1 (stretching and vibration of sulfur oxide appears), 1HNMR spectra—2.6–2.2 ppm attributed –CH (MA), CH2 in alpha of SO3H is often around 2.5–3.0 ppm, CH2 is around 1.0 ppm of polymer backbone. All these data confirms the expected structure of the copolymer (Figs. 2, 3).
GPC
In GPC plot Fig. 4 MA-SMADS copolymer exhibited a very narrow molecular weight distribution, less than 10.5 × 102. Low molecular weight & polydispersity is a vital parameter for efficient scale inhibition which is achieved through uniform reaction rate. This optimum molecular weight & polydispersity allows for higher rate of inhibition efficiency.
HPLC
High-performance liquid chromatography was used to determine residual monomer levels in final MA-SMADS copolymer, residual maleic acid—7.3 ppm at 7.25 min and SMADS—15 ppm at 16.9 min. The analysis below confirmed that the monomers were totally polymerized (Fig. 5).
Evaluation of barium scale inhibition performance (static bottle test)
The effect of MA-SMADS copolymer dosage on BaSO4 inhibition performance at 70 °C is presented in Fig. 6. The scale inhibition rate increases with increase in inhibitor dosage, when the dosage reaches to critical value (30 ppm or 30 mg/L), the inhibition rate is 98%. After the increase in inhibitor dosage, the scale inhibition rate does not increase significantly: Hence, 30 mg/L is the threshold value of copolymer (Guo et al. 2012).
Effect on inhibition performance of copolymer
Concentration of NaCl
Figure 7 shows the copolymer inhibition efficiency function of sodium chloride concentration. Inhibition efficiency is increases with sodium chloride concentration. At 2.5 g/L NaCl concentration the scale inhibitor exhibits poor inhibition performance for barium sulfate scales. At salt content of 7.5 g/L or more, the rate of scale inhibition is observed to be more than 90%. It is seen that barium sulfate scale inhibition efficiency is better at the higher concentration of NaCl, i.e., concentration higher than 7.5 g/L or more is suitable for scale inhibition (Luo et al. 2015; Gallardo et al. 2000).
Temperature
Figure 8 shows the test results of inhibition efficiency at temperature 70–120 °C, at higher temperature of 90 °C minimum inhibitor dosages increases. However, 30 ppm is found to be maximum requirement of inhibitor dosage for 98% scale inhibition through the test temperature. At a high temperature of 120 °C, MA-SMADS copolymer exhibited 98% inhibition at 70 ppm dosage is shown in Table 2. The nature of flocculation behavior of copolymers reduces the scale inhibition efficiency (Shakkthivel and Vasudevan 2007) (Table 3).
Scale inhibition mechanism
The BaSO4 crystallization process is relatively complicated. It is challenging to study interaction between the inhibitor and forming nuclei. However, it is noticeable that complexing effect, crystal modification and dispersing effect all depend on the adsorption of the anionic-charged inhibitor on the surface of developing nuclei and on the positively charged growth sites of growing crystals.
Threshold inhibition
The term threshold inhibition can be elucidated as the adsorption of antiscalant on the crystal growth during the crystallization process. The adsorption on these ion clusters creates an unbalance and they redissolve rather than grow into visible size. This process can either prevent crystal growth or delay it for a period of time. Scale inhibition of threshold inhibitor depends on kinetic but not on thermodynamic effects. Hence, threshold inhibition is basically the capability of an inhibitor to lengthen induction time, between the process of supersaturated state and detection of first crystals.
Complexing effect
Maleic acid—sodium methallyl disulfonate copolymer molecular chains contain functional group of carboxyl and sulfonic acid that can form stable chelates with Ba+2, thus decreasing the concentration of Ba+2 in the solution and significantly reducing the probability of collision between Ba+2 and SO −24 . Hence, BaSO4 crystals or precipitates are not likely to form, thus prevents scaling.
Effect of MA-SMADS copolymer on the crystal modification of BaSO4 or lattice effect on BaSO4
Characterization of scale crystals were carried out by SEM and XRD to understand the scale inhibition mechanism. From Fig. 9, we observe that during the growth process of BaSO4 crystal nucleus which leads to formation of BaSO4 scales, MA-SMADS copolymer either adheres to the surface of crystal particles or penetrates into crystals, thus weakening its crystallizing power by complexing with scaling ions and hence the lattice of BaSO4 crystal deforms and can be broken easily (Senthilmurugan et al. 2010; Shen et al. 2012).
Figure 9 presents the BaSO4 images with and without treatment of inhibitor MA-SMADS copolymer, as shown in Fig. 9, absence of inhibitor on BaSO4 crystals are dense flake like crystals, tightly arranged with large blocked structure. But there is a significant change in the structure of BaSO4 crystals after undergoing the inhibitor treatment, the corners and edges are flattened and edges are in shape of an arc. Crystals are arranged in an unsystematic, irregular manner and fractured into smaller particles. This specifies that MA-SMADS copolymers have reacted with BaSO4 crystals, the solubility symmetry increases, suspending the BaSO4 scales disintegrating into tiny particles. MA-SMADS molecules have also attached to the surface of the barium sulfate tiny particles which prevents the precipitation and scale formation (Mavredaki et al. 2011).
Figure 10 shows the XRD spectrum of BaSO4 scales with and without treatment with MA-SMADS copolymer and the characteristic peaks of treated BaSO4 scales are weakened with emergence of other impurity peaks, this indicates that after reacting with BaSO4 particles, MA-SMADS copolymer has caused change in crystal form, decrease in degree of crystallinity of particles, increase in solubility and degree of dispersion of BaSO4 scale in the solution.
Dispersion effect
MA-SMADS deionize the negatively charged ions from sodium hydroxide solution, which was added during the neutralization, easily penetrates and adsorbs on the BaSO4 particles. This results in the repulsion between BaSO4 particles due to negative charge on the particle surface and hence BaSO4 particles effectively disperse in water solution forming a charged particle diffusion layer. The positive-charged particles in turn settle on the outer layer and an electric double layer is formed as shown in Fig. 11.
From Fig. 11, the anionic polymer MA-SMADS is adsorbed to the crystal particles of BaSO4 scale so that the crystal particles carrying anionic charges, causing increased contact between the complexing agent, inhibiting agent and the scale surface which enhances the dissolution performance. Simultaneously, copolymer of MA-SMADS envelop BaSO4 micro particles that have already dispersed so that BaSO4 crystal particles are stably dispersed, preventing aggregation, precipitation of BaSO4 crystal particles which results in formation of compact solid scale layer. This indicates that MA-SMADS can both disperse and loosen the scale, showing good scale control performance.
Performance comparison with other industrial scale inhibitors: Flosperse MAS and Flosperse 3000SM
Flosperse MAS has an excellent inhibition effect on both barium and strontium scales, which can not be controlled with existing scale inhibitors in the market. It is widely employed as a scale inhibiting dispersant for oilfield water injection system and industrially circulating cooling towers. Flosperse 3000SM is an acrylic/sulfonate-based copolymer used in oil field injection systems and industrial circulating cooling as a scale inhibiting dispersant. DSL equipment was used to evaluate and compare the performance of these three scale inhibitors in dynamic conditions.
The average blank time was determined to be approximately 5 min so, the scale inhibitor must prevent deposition (determined to be a rise in ΔP of 1 psi) of scale for a period of 15 min according to evaluation criteria (Fig. 12).
Inhibitor evaluation was done in two stages, in the first stage each inhibitor is kept at a dosage of 100 ppm if no tube blocking is observed during 15 min then the dosage is decreased to 75 ppm if the tube is blocked at 75 ppm, i.e., the MIC of the inhibitor is between 100 and 75 ppm the same process is continued for the three inhibitors until the MIC range of the inhibitors are determined. For Flosperse 3000SM, tube blocking is observed between 100 and 75 ppm. For Flosperse MAS, tube blocking is observed at 75–50 ppm and for MA-SMADS copolymer, tube blocking is observed at 50–25 ppm.
Second stage; to determine the exact MIC of each inhibitors, dosage is set at 5 ppm in the respective inhibitor MIC range, At this dosage the tube blocking for Flosperse 3000SM is observed at 95 ppm so, the MIC for this inhibitor can be regarded as 100 ppm to resist brine water scale deposition for a period of 7 min. For Flosperse MAS it is observed at 60 ppm so, MIC can be regarded as 65 ppm to resist brine water scale deposition for a period of 55 min and for new MA-SMADS copolymer, tube blocking was observed at 50 ppm so, the MIC can be regarded as 55 ppm to resist the brine water scale deposition for a period of 77 min. So MA-SMADS copolymer is superior to commercial Flosperse MAS and Flosperse 3000SM polymer in terms of scale inhibition performance.
Conclusions
-
1.
In this research, a potential new copolymer of maleic acid-sodium methallyl disulfonate was synthesized through aqueous solution free radical polymerization using sodium persulfate as an initiator.
-
2.
Copolymer characterization was done with FTIR, HNMR spectrums and it is confirmed that the copolymer was formed. The average molecular weight of copolymer was determined with GPC 1050 g/mol and a free (residual) monomer level in final copolymer was measured with HPLC.
-
3.
30 ppm is the threshold value of MA-SMADS copolymer, when the dosage is 30 ppm or 30 mg/L the inhibition efficiency of barium sulfate reaches 98% and it is found that an excess dosage of copolymer is not required.
-
4.
Barium sulfate scale inhibition efficiency, NaCl, temperature and effect on inhibition efficiency were studied through static bottle test. Sodium chloride concentration affects the inhibition efficiency of the inhibitor on barium sulfate scales but the polymer shows good performance under high salinity conditions.
-
5.
Scale inhibition mechanism was studied through complexing, lattice and dispersion effect. As per SEM and XRD images, the diameters of barium sulfate scales formed at low dosage inhibitor is higher than under blank conditions. Therefore, adequate inhibitor dosage is required to prevent barium sulfate scale formation.
-
6.
DSL test indicates: MA-SMADS copolymer is superior to the commercial scale inhibitor of Flosperse MAS and Flosperse 3000SM (AA/AMPS copolymer) in terms of scale inhibition performance under dynamic conditions.
References
Chen S-RT, Coraopolis, Vaughan CW (1991) U.S. Pat no 5,000,856
Dickinson W, Sanders L, Kemira (2012) Novel barium sulfate scale inhibitor for use in high iron environments. In: SPE Latin American and Caribbean Petroleum engineering conference, Mexico City, 16–18 Apr, 2012
Dickson W, Griffin R, Sanders L, Lowen C, Kemira (2011) Development and performance of biodegradable antiscalants for oilfield applications. In: Offshore technology conference, Houston, 2–5 May, 2011
Dyer SJ, Graham GM (2002) The effect of temperature and pressure on oilfield scale formation. J Petrol Sci Eng 35:95–107
El-Sayed M, Ramzi M (2016) Evaluation of scale inhibitors performance under simulated flowing field conditions using dynamic tube blocking test. Egypt Int J Chem Sci 14(1):16–28
Gallardo V, Zurita L, Ontiveros A, Durán JDG (2000) Interfacial properties of barium sulfate suspensions implications in their stability. J Pharm Sci 89(9):1134–1142
Guo XR, Qiu FX, Dong K, Zhou X, Qi J, Zhou Y, Yang DY (2012) Preparation, characterization and scale performance of scale inhibitor copolymer modification with chitosan. J Ind Eng Chem 18:2177–2183
Jensen MK, Kelland MA (2012) A new class of hyper branched polymeric scale inhibitors. J Petrol Sci Eng 94–95:66–72
Jiang C (1996) Solubility and Solubility Constant of Barium Sulfate in Aqueous Sodium Sulfate Solutions Between 0 and 80°C. J Solut Chem 25(1):1996
Lihui L, Jingamo Z, Yu Z (2004) Syntheiss of barrium-sulfate-scale inhibitor AA/MA/HPA polymer. Fine Chem 21(1):58–60
Lijuan L, Xilin Z, Jilin L (2015) Progress in the research on the mechanicm of scale inhibitor for water treatment. Guangdong Chem Ind 42(291):68–69
Liu X, Chen T, Chen P, Montgomerie H, Hagen T, Wang B, Yang X (2012) Understanding the co-deposition of calcium sulphate and barium sulphate and developing environmental acceptable scale inhibitors applied in HTHP wells. In: SPE international conference and exhibition on oilfield Scale, Aberdeen, 30–31 May, 2012
Luo H, Chen D, Yang X (2015) Synthesis and perforamnce of a polymeric scale inhibitor for oilfield application. J Petrol Explor Prod Technol 5:177–187
Mavredaki E, Neville A, Sorbie KS (2011) Initial stages of Barium Sulfate formation at surfaces in the presence of inhibitors. Cryst Growth Des 11:4751–4758
Qiansheng Y, Xingjin X, Wenfa X (1999) Overview of the application of antiscaling agent in oilfield water injection (J). Jiangsu Chem Ind 27(4):10–13
Senthilmurugan B, Ghosh B, Kundu SS, Haroun M, Kameshwari B (2010) Maleic acid based scale inhibitors for calcium sulfate scale inhibition in high temperature application. J Petrol Sci Eng 75:189–195
Senthilmurugan B, Ghosh B, Sanker S (2011) High performance maleic acid based on oil well scale inhibitors-development and comparative evaluation. J Ind Eng Chem 17:415–420
Shakkthivel P, Vasudevan T (2007) Newly developed itaconic acid copolymers for gypsum and calcium carbonate scale control. J Appl Polym Sci 103:3026–3213
Shen ZH, Li JS, Xu K, Ding LL, Ren HQ (2012) The effect of synthesized hydrolyzed polymaleic anhydride (HPMA) on the crystal of calcium carbonate. Desalination 284:238–244
Yong X, Weizhong W, Fengbo G et al (2014) Progress in the research on the scale inhibitor for water injection in oil fields. Gunagzhou Chem Ind 42(18):42–44
Acknowledgements
The authors thank Research Director, SNF SPCM and SNF INDIA for their constant encouragement and permission to communicate the manuscript for publication. Dr. Guillaume Moreia, Dr. PLyot and Dr. PCheucle, thank members of Dispersants Scientist Group and Industrial Research and Development teams, France, India, for this Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kommanapalli, K.K., Lyot, P., Sunkara, J.R. et al. Synthesis and characterization of maleic acid and sodium methallyl disulfonate new copolymer: application as a barium sulfate scale inhibitor. J Petrol Explor Prod Technol 9, 223–232 (2019). https://doi.org/10.1007/s13202-018-0450-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-018-0450-7