Abstract
To meet rising global demands for energy, the oil and gas industry continuously strives to develop innovative oilfield technologies. With the development of new enhanced oil recovery techniques, sandstone acidizing has been significantly developed to contribute to the petroleum industry. Different acid combinations have been applied to the formation, which result in minimizing the near wellbore damage and improving the well productivity. A combination of hydrofluoric acid and hydrochloric acid (HF:HCl) known as mud acid has gained attractiveness in improving the porosity and permeability of the reservoir formation. However, high-temperature matrix acidizing is now growing since most of the wells nowadays become deeper and hotter temperature reservoirs, with a temperature higher than 200 °F. As a result, mud acid becomes corrosive, forms precipitates and reacts rapidly, which causes early consumption of acid, hence becoming less efficient due to high pH value. However, different acids have been developed to combat these problems where studies on retarded mud acids, organic-HF acids, emulsified acids, chelating agents have shown their effectiveness at different conditions. These acids proved to be alternative to mud acid in sandstone acidizing, but the reaction mechanism and experimental analysis have not yet been investigated. The paper critically reviews the sandstone acidizing mechanism with different acids, problems occurred during the application of different acids and explores the reasons when matrix stimulation is successful over fracturing. This paper also explores the future developing requirement for matrix acidizing treatments and new experimental techniques that can be useful for further development, particularly in developing new acids and acidizing techniques, which would provide better results and information of topology, morphology and mineral dissolution and the challenges associated with implementing these “new” technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Well stimulation is a technique used to improve the flow of oil or gas from the reservoir by dissolving the rock or creating new channels around the wellbore (Schechter 1992; Schlumberger 2000; Crowe et al. 1992). The most commonly applied stimulation techniques are acidizing and hydraulic fracturing. In hydraulic fracturing, fluids are injected at a pressure greater than the formation pressure to create channels/fractures through the formation through which the production of oil or gas may increase (Halliburton 2000c). In acidizing, acids have been applied to the sandstone and carbonate formations to increase the formation permeability and porosity near the well bore (Economides and Nolte 2001; Halliburton 2000a, b). These acids can dissolve different minerals like quartz, carbonates and feldspar present in reservoir rocks, thus increasing the permeability which ultimately increases the flow rate of hydrocarbon fluid from to the wellbore. Both of these operations (fracturing and acidizing) have their own advantages and limitations in sandstone stimulation. The choice whether to go for fracture or acidize a sandstone reservoir depends on various factors which include the formation geology, production history and well intervention objectives (Al-Harthy et al. 2008/2009). A formation with high permeability and porosity usually does not require fracturing in comparison with a tight formation with relatively low porosity and permeability, which requires hydraulic fracturing. Usually, loosely bound formation can cause formation collapse due to the overburden pressure if posed to the hydraulic fracturing. Also, the damaged formation due to drilling and production process is not recommended to be stimulated with hydraulic fracturing instead of matrix acidizing. Acid fracturing is more successful to be applied in carbonate formations with high natural fractures and high permeability (Qiu et al. 2014).
Currently, the world demand for energy is increasing and (Aboud et al. 2007) predicted that 40% more energy will be required in 2020 than in the present. To fulfill this demand, high-temperature reservoir acidizing is of paramount importance because the exploration of new reserves targeting much deeper and hotter reservoir (Al-Harthy et al. 2008/2009). Most of the deep hot reservoirs require matrix acidizing at a certain stage in their life span where the recent technology is suitable for a temperature range of 100–400 °F. This trend is going to increase into much higher temperature of above 500 °F, which will demand improvements in all aspects of acidizing, from corrosion rates to treatment-fluid stability. Thus, using acidizing methods at such conditions require more improvements in treating agents and procedures to meet difficult conditions in the near future. In order to improve the matrix acidizing techniques at elevated temperatures, new acid combinations are being developed. Conventional mud acid used at high temperature of 200 °F can cause rapid reactions rates. Consequently, this would result in inefficiency of acidizing process as the acids are consumed too early (Al-Harthy et al. 2008/2009). The fast reaction of mud acid is due to the presence of minerals inside the sandstone formation which mostly consists of silica (quartz) and silicate minerals. It also includes quartz, clays, feldspars and zeolites which are commonly cemented by silica, calcite or iron oxides as described by Muecke (1982). Sandstone is a clastic sedimentary rock also known as arenite. Figure 1 represents the structure of different minerals present in the sandstone formation.
Sandstone matrix as described by Al-Harthy (2008/2009). “The framework of sandstone reservoirs is typically made up of grains of quartz cemented by overgrowth of carbonates (A), quartz (B), and feldspar (C). Porosity reduction occurs from pore-filling clays such as kaolinite (D) and pore-lining clays such as illite (E)”
Sandstone acidizing
The most imperative target of sandstone matrix acidizing is to dissolve/remove siliceous particles (clay, feldspar and quartz) that restrict the flow of hydrocarbons and reduce permeability around the wellbore (Crowe et al. 1992; Lindsay 1976). This can be achieved by the injection of hydrofluoric acid (HF acid) or its precursors (Kalfayan and Metcalf 2001; Kalfayan 2008). After the discovery of HF acid in 1935, it was extensively applied on sandstone formation to remove the damage and to solve problems related to the sandstone drilling and production damage. Initially, the main application of this acid was only limited to remove the mud filter cake, but now it is also being applied to solve many problems such as removal of siliceous particles and damage around wellbore. This acid proved to be very successful while treating the sandstone formations containing a small amount of calcium minerals. Sandstone formation particles such as sand grains, feldspar and clays react with HF acid because only fluoride ion (F−) has the capability to react with silica and clay. Smith and Hendrickson (1965) illustrated the reactive nature of HF acid with silica which makes it exceptional in the application of sandstone acidizing. Hydrochloric, sulfuric and nitric acids do not react with the sandstone formation effectively as showed by Smith and Hendrickson (1965). In 1940, Dowel hit the idea of mixing HCl acid with HF acid to reduce the possibility of reaction products precipitation. The mixture is called mud acid, and in sandstone acidizing, the common practice is to inject the mud acid with a concentration of 3% HF and 12% HCl as described by Smith and Hendrickson (1965) and Abdelmoneim and Nasr-El-Din (2015). Sandstone acidizing is extremely difficult and challenging task due to multiple stages of fluids and their reactions with the minerals in the porous media. The fluid–mineral interactions can cause precipitation reactions, which can potentially reduce the reservoir permeability. Due to multiple stages of fluids-formation reactions during sandstone acidizing, the success rate to remove the damage is generally not according to the requirement. During sandstone acidizing, precipitation reactions may occur, leading to the formation damage and reduction in permeability and porosity (Gomez 2006). When mud acid reacts with the formation to dissolve different minerals, the most important apprehensions are the reactions of carbonates with HCl and HF and the reactions of HF with silicates, quartz (Eq. 1) and feldspar.
“Silicon tetra fluoride (SiF4) is a soluble gas just like CO2 and is capable of undergoing further reactions when held in solution by pressure. Acids used to stimulate Sandstone formations contain Fluoride Ion (F−) in some form. It is a very reactive ion and is the only chemical that will react with sand and clay significantly” (Smith and Hendrickson 1965).
Acidizing mechanism
HF acid starts dissolution of minerals as soon as it enters a sandstone formation. The speed of reaction and dissolution of minerals depends on their reaction rate with acid and the exposed surface areas. The sandstone minerals are divided into two different categories: slow and fast reacting. “Quartz tends to act at a slower rate whereas feldspars and clays tend to react at a faster rate” (Ponce da Motta et al. 1992). Figure 2 shows the types of reactions occurred when sandstone formation is exposed to mud acid.
Sandstone acidizing reactions as described by Al-Harthy (2008/2009)
When the sandstone formation is treated with the mud acid, usually three groups of reactions take place which are explained by Al-Harthy (2008/2009). The primary reaction occurs close to the wellbore, which results in the formation of aluminum and silica fluorides. In these reactions, minerals are usually dissolved rapidly and without any precipitation. Away from the wellbore, the secondary reaction takes place in which these primary products further reacted to form silica gel (slow reaction), which is a precipitate. At a greater distance from the injection zone, additional silica gel precipitates due to tertiary reactions. The sandstone acidizing treatment may fail due to the rapid kinetics of the secondary and tertiary reactions at a higher temperatures. HF acid is the main reactant with formation rocks, while HCl acid is intentionally added into the mixture to reduce HF consumption and to maintain an acidic environment, which prevents precipitations of HF reaction by-products (Al-Harthy 2008/2009).
When sandstone formation is treated with mud acid, several types of minerals may precipitate described by Mahmoud et al. (2011). The following precipitation reactions are most common and may lead to the formation damage and reduction in the permeability and porosity (Smith and Hendrickson 1965).
-
Formation of potassium and sodium silicate precipitates
-
Formation of calcium fluoride precipitates
-
Formation of hydrated silica precipitates
Silicates These are formed due to the reaction of sodium and potassium ions with fluosilicic acid.
Fluorides These precipitates are formed due to the reaction between calcium and fluoride ions, i.e., CaF2. The solubility of this product is very low, and it has the ability to form a precipitate, but it can be removed if adequate HCl pre-flush is applied.
Colloidal silica The reaction of hydrofluoric acid (HF) with sandstone formation is very complex because of several interactions. Initially, HF acid reacts with silica to produce silica tetrafluoride (SiF4), which produce fluosilicic acid (H2SiF6) due to the further reaction between HF acid.
As soon as the concentration of reacting HF becomes very low, silica precipitates as explained by Shaughnessy and Kunze (1981). The reaction that dissociated with the high concentration of HF dissolves silicate minerals.
The reaction reverses itself at a low concentration to regenerate HF acid and precipitates silica (Al-Harbi et al. 2011):
To avoid the formation of silicates (Na2SiF6, K2SiF6) and fluorides (CaF2), ammonium chloride (NH4Cl) or hydrochloric acid (HCl) is used as a pre-flush ahead of main acid. To prevent the hydrated silica precipitation; HCl or organic acid is used in the main acid stage. However, this silica precipitates as hydrated colloid Al2Si2O5(OH)4 and causes the reduction in rock permeability. These precipitation reactions may be avoided by following three stages of acidizing described by several researchers Gidley (1971), Kalfayan and Metcalf (2001), Aboud et al. (2007) and Hill et al. (1981).
-
Pre-flush stage is employed to dissolve any Na, K and Ca ions that may produce insoluble silicates when reacted with the silica. Besides preventing the live HF acid to enter into a high pH region, pre-flush also provides a low pH region reducing the risk of precipitate formation.
-
Main acid stage is applied to dissolve the quartz, clay, feldspar and silicates. This acid may also dissolve the remains of carbonates present after the pre-flush stage.
-
An after-flush stage is used to keep the wettability of the formation to the original state and it cleans the formation rapidly by removing the spent acid. Mutual solvents, diesel oil, NH4Cl, acetic acid or HCl can be applied at this stage for the efficient displacement of the spent acids.
Problems associated with mud acid
Despite the reasonable success of mud acid application on sandstone formation in recent years, still some critical problems are associated with its use, which limit its effectiveness. Shuchart and Gdanski (1996), (Thomas et al. 2001, 2002) and Al-Dahlan et al. (2001) discovered that the most likely limitations of mud acid are rapid spending due to fast reaction, which results in consequent precipitations of reaction products followed by secondary and tertiary reactions (Li 2004). This limits the acid penetration in the formation especially at elevated temperatures. A combination of problems such as precipitations, matrix unconsolidation, high corrosion rate and incompatibility of hydrochloric (HCl) acid with sensitive clays (illite) resulted in the inconstant success rate or failure of stimulation treatments with mud acid reported by Shuchart and Gdanski (1996), Thomas et al. (2002).
To avoid the formation of silicates and fluorides precipitates described by McLeod et al. (1983), hydrochloric acid (HCl) was used as a pre-flush ahead of the main acid stage by Hill et al. (1994), while Thomas et al. (2002) added ammonium chloride in organic acid and used it as a pre-flush. Shafiq et al. (2013) added acetic acid in hydrochloric acid and used as a pre-flush acid and found it more effective as compared to HCl acid. Shafiq at al. (2016) applied nuclear magnetic resonance analysis on core samples obtained after core flooding using HCl and CH3COOH:HCl and found the later one more effective, hence validated the results of previous analysis using this combination. Furthermore, to prevent the hydrated silica precipitation, HCl or an organic acid like citric acid, formic acid or acetic acid are added in the main acid stage with HF acid as reported by Yang et al. (2012). McLeod presented the basic guidelines for sandstone acidizing mentioned in Table 1.
But due to the problems that associated with the use of mud acid, these guidelines are not effective in certain cases. The original McLeod guidelines were modified by himself McLeod and Norman (2000). The new guidelines mentioned in Table 2 have three sets: high, medium and low permeability. McLeod and Norman also recommended replacing HCl with an organic acid (acetic acid) for formations with chlorite and zeolite. These new guidelines covered two main limitations of McLeod previous guidelines. First of all, recommendations have been added for medium permeability reservoirs and second they considered sensitivity of HCl acid toward chlorite and zeolite. Abdelmoneim and Nasr-El-Din (2015) mentioned the drawback of these recommendations that they are restricted to only three concentrations per permeability range.
Kalfayan and Metcalf (2001) modified the basic McLeod’s guidelines presented in 1984 and 2000 to fill certain gaps. These guidelines are mentioned in Table 3. These guidelines recommended more than three concentrations per permeability range which was the drawback of McLeod and Norman’s guidelines presented in 2001.
Mud acid and organic mud acid are the main focus in the presented guidelines, while the guidelines of other acids have not been discussed due to less or minimal experimental investigation and analysis. For pre-flush stage, hydrochloric acid and ammonium chloride were discussed in guidelines. Some of the acids developed during last decades for sandstone acidizing are discussed in coming sections with benefits and limitations of each acid are presented.
Development of different acids
Researchers have applied different combinations of acids with varying concentrations to get the best results for acidizing of sandstone formation in terms of permeability, porosity and precipitation. Various approaches have been implemented to overcome the problems that are associated with the mud acid and conventional hydrochloric acid. Listed below are some of these.
Mud acid (HF:HCl)
Gomaa et al. (2013) had carried out core flood test at 180 °F to investigate the effect of different ratio of HF:HCl on the permeability of the sandstone core sample. Based on the results, all tested ratios of HF:HCl acids successfully enhanced the permeability of the core samples. However, he observed that better permeability enhancement can be achieved with increasing HF:HCl ratio.
Al-Harthy et al. (2008/2009) stated that combination of HF:HCl acid was consumed too early during acidizing process due to rapid reaction rates at temperatures above 200 °F. Although mud acid was proved to be efficient and had been widely used, inefficiency of acidizing process at high-temperature limits its success. It was an indisputable fact that mud acid is very hazardous and can cause corrosion to wellbore equipment. Therefore, in the view of the shortcomings related to mud acid and by considering all of these disadvantages, new acid combinations (HF:H3PO4 and HBF4:HCOOH) seem to be a better choice. These combinations used previously by Shafiq et al. (2015), Shafiq and Mahmud (2016) inferred to not only be having better permeability improvement, but also less corrosive.
Retarded mud acids
Gdanski (1985), Thomas et al. (2001) and Gomaa et al. (2013) applied retarded mud acids during the main acid stage, which are supposed to decrease the reaction rate between acids and minerals. Three retarded hydrofluoric acids (RHF acids) based on boric acid (H3BO3), aluminum chloride (AlCl3) and phosphonic acid were tested. However, these methods also posed similar problems at high temperatures. For example, when RHF acid was applied: some minerals form precipitates that were not formed when normal mud acid was used. For example, Thomas et al. (2001) investigated the formation of potassium tetrafluoroboron (KBF4) precipitate when the fluoboric acid reacted with feldspar. Fluoboric acid has been produced when boric acid reacts with HF acid, presented in Eq. 4 and 5, respectively.
Fast reaction
Slow reaction
The second system aluminum retarded hydrofluoric acid (ALRHF) consists of aluminum chloride (AlCl3). Aluminum fluoride species formed when aluminum chloride reacts with HF (Eq. 6). Zhou and Nasr-El-Din (2014) added AlCl3 as a retarding agent for regular mud acid and found it suitable in controlling AlF3 precipitation.
As HF spends on siliceous minerals, AlF4 hydrolyzes to regenerate HF (Eq. 7).
The third system phosphonic retarded hydrofluoric acid (PRHF) is based on a phosphonic acid complex which contains five hydrogen atoms. Zhou and Nasr-El-Din (2014) described “This acid reacts with ammonium bifluoride to produce an ammonium phosphonate salt and HF. The fluoride ions are provided by the ionization of dissolved ammonium bifluoride.” In comparison with mud acid, phosphonic-based HF acid system shows significantly better performance of permeability enhancement of 177.86% at 300 °F.
Shafiq et al. (2015), Shafiq and Mahmud (2016) and Shafiq et al. (2014) performed experimental study with different acid combinations, replacing conventional mud acid. These studies focused on the combinations of HF:H3PO4 and HBF4:HCOOH. The Berea sandstone core samples were reacted with these acid combinations and analyzed in terms of the porosity, permeability, mineralogy, pH change and strength. According to the findings, although all these acid combinations can be used as the main acid in sandstone acidizing, yet the best acid combination is 3%HF:9%H3PO4. The permeability increment using this acid combination is 135.32%, even better than standard mud acid, which showed permeability increase of 101.76%. However, these experiments were performed at ambient temperature conditions, which is not representative at real field condition. Initial experiments revealed that these combinations are less corrosive and stable and allow deep penetration due to slow hydrolytic reaction. Then, these results can be compared with the results of mud acid at the same temperature. Therefore, it can be concluded that the experimental procedures and outcomes from the studied literature have provided some productive approaches for the selection of acid and experimental design. Tables 4, 5 and 6 represent some of the findings and comparison between these acids and mud acid.
Chelating agents
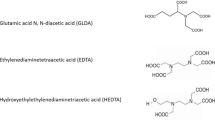
Chelating agents may be used to stimulate sandstone formations entirely using without using any HF-containing chemical. Different chelating agents have been used at high-temperature conditions. Ethylenediaminetetraacetic acid (EDTA) and hydroxyethylenediaminetetraacetic acid (HEDTA) used at a high temperature resulted in the increase in gas production without the use of HF-containing fluids. Wormholes can be formed by EDTA and HEDTA at temperatures up to 400 °F. Frenier and Hill (2002) and Mahmoud et al. (2011) used chelating agent Na3HEDTA and found it more effective in sandstone acidizing as compared to mud acid. Using trisodium HEDTA (Na3HEDTA) has given better results in stimulating sandstone as compared to HCl. Various stimulating studies on sandstone formation using HEDTA chelating agent have been conducted by Frenier et al. (2004), Ali et al. (2008) and Mahmoud et al. (2011) and showed that it gave better results in increasing permeability compared to mud acid especially at high temperatures. Hydroxethylaminocarboxylic acid (HACA) group of chelating agents can be used as an alternative to the mud acid. Mahmoud et al. (2011) also showed that HEDTA, diethylenetriaminepentaacetic acid (DTPA) and disodium EDTA (Na2EDTA) are effective in acidizing sandstone formations. Single-stage sandstone acid has been developed by Gomaa et al. (2013), which consists of boric acid (H3BO3), ammonium bifluoride (NH4H.HF) and HCl to generate fluoboric acid. This system eliminates the use of pre-flush and after-flush stages. GLDA has been applied by Reyes et al. (2015), Rignol et al. (2015) and found it effective in increasing sandstone permeability at high temperatures.
It is highly admirable that many researches had focused on the development of acids which can be applied in high-temperature reservoirs. Although these acids proved to be very useful and appropriate, however, chelating agents are usually more suitable for heterogeneous carbonates and clay-rich sandstones. The success of these acids is limited for clean homogeneous sandstones due to the precipitation of silica. It is also very costly in comparison with mud acid, retarded and organic acids.
Organic-HF system
Studies on sandstone acidizing using mud acid have shown that the presence of HCl acid is effective in maintaining the acidic environment and solubility of reaction products. However, use of HCl can cause damage such as corrosion, crude oil sludging and unpredictability of formation minerals. These problems become more severe at high temperatures. Therefore, Shuchart and Gdanski (1996), Shuchart (1997), Shuchart and Buster (1995), Al-Harbi et al. (2011) and Yang et al. (2012) applied organic-HF in sandstone matrix acidizing to overcome the problems. Due to less corrosion rate and retarded nature of organic acids, these acids provide better results especially at elevated temperatures and considered as an excellent alternative compared to mud acid in sandstone acidizing. These acids proved to be very useful when HCl sensitive clay (can cause damage to the formation when reacts with HCl), such as illite, is present in the formation. The two organic acids used commonly are acetic acid and formic acid, combination of HF with HCOOH also known as organic mud acid (Blake and Walter 1999). These acids also possessed some problems similar to mud acid like fast reaction rates and formation of precipitates.
Shafiq and Shuker (2013), Shafiq et al. (2014) used organic mud acid to acidize sandstone formation in comparison with mud acid and presented their results in terms of acid pH value change. The results revealed that the pH value change after the acidizing is not much when organic mud acid is used as compared to standard mud acid, and this change is even less when fluoboric acid was added in place of hydrofluoric acid. It shows effective buffer effect and slow reaction rate which can be useful for deep penetration of acid into the formation.
Emulsified acid system
Emulsified acids are basically retarded acids, which are extensively used in acid fracturing and matrix stimulation. Several studies (De Rozieres et al. 1994; Navarrete et al. 1998; Conway et al. 1999; Kasza et al. 2006; Al-Mutairi et al. 2009) examined the reaction rate of emulsified acid with carbonates. Navarrete et al. (1998) indicated that the reaction rate of 28 wt% HCl emulsified acid with limestone was 8.5 times slower than that of regular acid that contained 28 wt% HCl with limestone.
Xiong (2010) applied a novel emulsified acid in the Chinese oil fields. The significance of this emulsified acid is that increases the permeability of oil-saturated cores by 96.1% while by only 10.1% for water-saturated cores. Pandya (2013) applied emulsified acids on high-temperature reservoirs ranging from 275 to 375 °F compared to non-retarded and gelled acids. He studied the stability of emulsified acids when corrosion inhibitor is added in. It has been found that only emulsified acid system sustained this high-temperature range as compared to other acids. Claims (2016), studied deeper well stimulation using improved emulsified acid systems at a high temperature up to 300 °F. The CT images for the treated core samples show that the stabilized acid system had less face dissolution and had the desired wormhole characteristics, i.e., narrow and directional propagation behavior with deeper penetration into the core sample. He concluded that stabilized system achieve up to three times increment in core permeability as compared to conventional acid system.
Other acids
Hartman et al. (2003) applied 10% acetic acid which proved to be useful in sandstone acidizing at a higher temperature and showed better results compared to 10% HCl. This acid is only effective at low temperature. Martin (2004) insisted on using the non-HF-based system because of the damaging nature of this acid and complex reaction mechanism. Fluosilicic acid (H2SiF6) plays an important role in removing the formation damage. It was applied successfully to stimulate sandstone in two injector wells in off-shore Brazil in 1999 by Da Motta and Dos Santos (1999), Kalfayan and Metcalf (2001) achieved 200% increment in permeability by applying the same acid. During sodium fluoride manufacturing, H2SiF6 formed as a by-product, which is also considered as a viable option for sandstone acidizing operations because of its low cost. This acid can cause precipitation reactions at high temperatures. Table 7 shows the summary of acids development with time.
Why acidizing is preferred over hydraulic fracturing?
Acidizing may, in fact, be the oldest stimulation technique and still in modern use. Still researchers are trying to develop acids that can be used at different temperatures and pressures because of the advantages of acidizing over hydraulic fracturing (Abdelmoneim and Nasr-El-Din 2015). Acidizing can be used instead of hydraulic fracturing in many cases like high permeability formation with loose packing, naturally fractured reservoirs and removing damage around wellbore. Moreover, sandstone acidizing can be utilized in depleted sandstone reservoirs for carbon capture storage (CCS), where hydraulic fracturing cannot be implemented.
The increase in well stimulation activity (acid and frac jobs) has been increased in recent years with the double number of treatments performed more than through the 1990s. In 1994, 79% of the stimulation jobs were acid treatments, but since they are a low cost, low volume operation than hydraulic fracturing treatment, they only consumed 20% of the money spent for well stimulation. For acid jobs, the observed failure rate was 32%. The failure rate is less frequent but more expensive hydraulic fracturing treatments were much lower, only 5% (Collier 2013). There are a limited number of reasons why sandstone acidizing treatments do not succeed. The six-step process to succeed sandstone acidizing is as follows:
-
Determine the presence of acid-removal skin damage;
-
Determine appropriate fluids, acid types, concentrations and treatment volumes;
-
Determine a proper treatment additive program;
-
Determine a treatment placement method;
-
Ensure a proper treatment execution and quality control;
-
Evaluate the treatment.
Some oil companies have found acidizing more effective in the Monterey Shale than fracking (Segee 2013). Conventional fracking, in which water and other chemicals are pumped at a high pressure to create fissures in the rocks, reportedly it does not work well in many parts of the Monterey Shale—a rock formation known for its complexity and low permeability, which makes fracking less effective. In drought-prone California, acidizing with HF works much better than fracking in the Golden State because the oil-bearing shale is already naturally fractured and buckled from tectonic activity (Collier 2013).
Aspect of additives and other factors
Apart from different complex reactions which taking place in sandstone acidizing, there are some other factors which have a certain impact on sandstone acidizing. Some of them are: concentration of acids, temperature, pressure, permeability and porosity of the formations. The choice of acid concentration is a very difficult practice. It has been revealed that the acid reaction rate is directly proportional to the concentration, i.e., double the acid concentration doubles the reaction rate (Gidley 1971). Acid spending also affected by two more factors, i.e., temperature and pressure. Increasing temperature causes an increase in the reactivity between the acid and sandstone formations, while pressure has two different effects. The increase in pressure increases the solubility of by-product gases, carbon dioxide and silicon tetra fluoride. The reaction of hydrochloric acid with carbonate in sandstone is retarded by carbon dioxide trapped in the solution at a high pressure. The increase in the silicon tetra fluoride solubility improves the reaction rate of silicate minerals with Fluosilicic acid. “Thus, the response of sandstone reservoir to matrix acidizing is very temperature–pressure dependent” (Farley et al. 1970).
Moreover, it has been described by McCune et al. (1975) that “rock sample with permeability less than 100 mD could be acidized more successfully compare to the sands having more permeability. Study on porosity showed that sandstone formation with the porosity less than 20% was treated much more adequately than those with porosity 25% or more.” Other than acids, some additives are also added during sandstone acidizing procedure for different purposes. Frenier and Hill (2002) showed that these additives have a minimal effect on the performance of the acids. For example, stimulation process is not affected by the addition of certain additives such as surfactants and corrosion inhibitors. These additives are added to minimize other problems like corrosion and incompatibility (Rabie and Nasr-El-Din 2015). Incompatibility problems can be minimized using surfactant additives in the main acid and after-flush stages. Corrosion inhibitors are used at concentrations of 0.1% uniformly throughout the acid stages to protect the metal integrity (McLeod et al. 1983). Iron sequestrants are added in the HCl stage to stabilize any dissolved iron and to minimize the precipitation of iron compounds. A mutual solvent ethylene glycol monobutyl ether (EGMBE) was included in the after-flush stage to restore the formation wettability and to reduce the surface tension of the returning spent acids Sutton and Lasater (1972).
Effect of mineral dissolution
Dissolution of calcium carbonate and anorthite minerals releases free calcium ions which can be used for CO2 storage later on described by Kumar et al. (2005). Hence, acidizing can be helpful in a way for CO2 storage also. Taron and Elsworth (2009) studied chemical precipitation/dissolution using TOUGHREACT simulator and indicate that mineral precipitation plays a large role in reservoir evolution. Calcite can be dissolved by the addition of chelates. Chemical changes and the effectiveness of chemical treatments depend on hydromechanical fracture flow properties. Precipitation and dissolution of calcite and amorphous silica are responsible for initial permeability change. Other likely minerals, such as potassium feldspar and quartz, are also followed in order to see the effect of their precipitates.
As minerals precipitate due to reaction between and injected fluid and the rock, changes are experienced in the porosity and permeability of the fracture system. In currently operating geothermal systems, calcite and amorphous silica precipitation have posed problems at recovery and injection wells, respectively. Calcite precipitation can successfully be inhibited using acidic injection. Other problems due to acidizing include the potential for corrosion of casings, although corrosion inhibitors can often be added to avoid this problem. Another alternative for calcium dissolution is chelating agents, which reduce the activity of metal ions (through binding), whereas inhibiting the precipitation of amorphous silica has proven to be more difficult. Acidizing using HF acid is currently the preferred method, but some have suggested that amorphous silica can be dissolved using chelating agents at high pH to prevent calcite deposition (which would be favored at high pH). This research is, however, relatively recent, and uncertainty remains as to the effects of these treatments on the host rock (Taron and Elsworth 2009). Potential for porosity loss due to the formation of anhydrite will also need to be assessed.
Pore-level imaging is very important to know the change in mineralogy due to dissolution (Khishvand et al. 2016). Mapping the pore space of synthetic and natural porous media can be analyzed significantly by recent advances in pore-level imaging. Three-dimensional snapshots of fluid occupancy can be obtained by researchers/scientists during flow experiments, which enables the investigations of rock/fluid interactions on a pore-by-pore basis. During last decade, a large number of studies have been performed concentrating on the use of pore-level imaging techniques to study complications associated with a wide range of flow through porous media problems.
High-resolution X-ray images have been used for characterization of relative permeability changes due to gel injection, capillary pressure calculation, and quantification of porosity changes during mineral dissolution, pore-scale contact angle measurement, mapping of pore-scale fluid distribution during drainage, and investigation of trapped non-wetting phase distribution in the pore space. Wildenschild and Sheppard (2013), Khishvand et al. (2013) have provided a detailed discussion on the applications of micro-CT imaging in this area of research.
Conclusion and future challenges
Acidizing of sandstone reservoirs is an essential step to ensure high production by removal of damage or by introducing new pathways. Many studies have been performed so far highlighting the importance of acidizing in sandstone formations. Many researchers developed different acid combinations, applied different chelating agents to get the best results related to permeability, porosity and precipitation, but still there are some limitations like fast spending of acid, precipitation reactions, less penetration of acids and corrosion of pipelines. New acid combinations are required for future sandstone acidizing aspect, and further study is needed on current technology because of the limitations of present acid combinations at high-temperature wells and limited study of these combinations on different sandstone formations. New combinations proposed or used at high temperatures are expensive and not regularly applied at field operations due to their limitations. Some combinations are not developed completely as their mechanisms are not yet known and poorly understood. All the developed guidelines are representative of mud acid only, whereas no guidelines has been established or proposed for other acids used during sandstone acidizing. Therefore, a comprehensive knowledge is required for the relevant reactants and their chemistry and also extensive research is required using these combinations. In future, acid combinations should be developed which can be applied economically at high-temperature operations and to mitigate the precipitation reaction issue at elevated temperatures. In future, pore-scale imaging will be very beneficial to find the change in topology, morphology and wettability of the rock sample due to acidizing.
References
Abdelmoneim SS, Nasr-El-Din HA (2015) Determining the optimum HF concentration for stimulation of high temperature sandstone formations. Society of Petroleum Engineers, SPE-174203-MS
Aboud RS, Smith KL, Forero Pachon L, Kalfayan LJ (2007) Effective matrix acidizing in high-temperature environments. Society of Petroleum Engineers, SPE 109818-MS
Al-Dahlan MN, Nasr-El-Din HA, Al-Qahtani AA (2001) Evaluation of retarded HF acid systems. Society of Petroleum Engineers, SPE-65032-MS
Al-Harbi BG, Al-Khaldi MH, AlDossary KA (2011) Interactions of organic-HF systems with aluminosilicates: lab testing and field recommendations. Society of Petroleum Engineers, SPE-144100-MS
Al-Harthy S (2008/2009) Options for high-temperature well stimulation. Oil Field Rev 20(4):52–62
Al-Mutairi SH, Nasr-El-Din HA, Hill AD (2009) Effect of droplet size on the reaction kinetics of emulsified acid with calcite. SPE J 14(4): 606–616. SPE-112454-PA
Ali SA et al (2008) Stimulation of high-temperature sandstone formations from West Africa with chelating agent-based fluids, SPE-93805-PA
Claims AJ (2016) Targeting enhanced production through deep carbonate stimulation: stabilized acid emulsions, presented at SPE international conference and exhibition on formation damage and control, Louisiana, 24–26 February, SPE 178967
Blake RE, Walter LM (1999) Kinetics of feldspar and quartz dissolution at 70–80 and near-neutral pH: effects of organic acid and NaCl. Geochim Cosmochim 63:2043–2059
Conway MW, Asadi M, Penny G et al (1999) A comparative study of straight/gelled/emulsified hydrochloric acid diffusivity coefficient using diaphragm cell and rotating disk. Presented at the SPE annual technical conference and exhibition, Houston, 3–6 October, SPE-56532-MS
Collier R (2013) Fracking’s more dangerous bedflow: acidizing. The Next Generation, Ernst vs EnCana Corporation
Crowe C, Masmonteil J, Touboul E, Thomas R (1992) Trends in matrix acidizng. Oil Field Rev 4(4):24–40
Da Motta EP, Dos Santos JA (1999) New fluosilicic acid system removes deep clay damage. Society of Petroleum Engineers, SPE-54729-MS
De Rozieres J, Chang FF, Sullivan RB (1994) Measuring diffusion coefficients in acid fracturing fluids and their application to gelled and emulsified acids. Presented at the SPE annual technical conference and exhibition, New Orleans, 25–28 September. SPE-28552-MS
Economides MJ, Nolte KG (2001) Reservoir stimulation, 3rd edn. Prentice Hall, Englewood Cliffs
Farley JT, Miller BM, Schoettle V (1970) Design criteria for matrix stimulation with hydrofluoric–hydrochloric acid. JPT 22: 433–440, SPE 2621
Frenier W et al (2004) Hot oil and gas wells can be stimulated without acids, SPE-86522-PA
Frenier WW, Hill DG (2002) Effect of acidizing additives on formation permeability during matrix treatments. Society of Petroleum Engineers, SPE-73705-MS
Gdanski RD (1985) AlCl3 retards HF acid for more effective stimulation. Oil Gas J 83:111–115
Gidley JL (1971) Stimulation of sandstone formations with the acid-mutual solvent method, SPE-3007-PA
Gomaa AM, Cutler J, Qu Q, Boles J, Wang X (2013) An effective single-stage acid system for sandstone formations. Society of Petroleum Engineers, SPE-165147-MS
Gomez JN (2006) Design, set-up and testing of a matrix acidizing apparatus. Texas A&M University, Texas
Halliburton (2000a) Carbonate matrix acidizing treatment. Best practice series. Halliburton, Houston
Halliburton (2000b) Effective sandstone acidizing. Best practice series. Halliburton, Houston
Halliburton (2000c) Hydraulic fracturing. Best practice series. Halliburton, Houston
Hartman RL, Lecerf B, Frenier W, Ziauddin M, Fogler HS (2003) Acid sensitive aluminosilicates: dissolution kinetics and fluid selection for matrix stimulation treatments. University of Michigan
Hill AD, Lindsay DM, Silberberg IH, Schechter RS (1981) Theoretical and experimental studies of sandstone acidizing, SPE-6607-PA
Hill AD, Sepehrnoori K, Wu PY (1994) Design of the HCl preflush in sandstone acidizing, SPE-21720-PA
Kalfayan LJ (2008) Production enhancement with acid stimulation. PennWell, Tulsa
Kalfayan LJ, Metcalf AS (2001) Successful sandstone acid design case histories: exceptions to conventional wisdom. Society of Petroleum Engineers, SPE-63178-MS
Kasza P, Dziadkiewicz M, Czupski M (2006) From laboratory research to successful practice: a case study of carbonate formation emulsified acid treatments. Presented at the international symposium and exhibition on formation damage control, Lafayette, Louisiana, USA, 15–17 February, SPE-98261-MS
Khishvand M, Akbarabadi M, Piri M (2013) Trapped non-wetting phase clusters: an experimental investigation of dynamic effects at the pore scale using a micro-CT scanner, SCA 24 (2013)
Khishvand M, Akbarabadi M, Piri M (2016) Micro-scale experimental investigation of the effect of flow rate on trapping in sandstone and carbonate rock samples. Adv Water Resour 94:379–399
Kumar A et al (2005) Reservoir simulation of CO2 storage in aquifers. Spe J 10(03):336–348
Li C (2004) Fine scale sandstone acidizing coreflood simulation. University of Texas at Austin, Austin
Lindsay DM (1976) An experimental study of sandstone acidization. The University of Texas at Austin, Austin
Mahmoud MA, Nasr-El-Din HA, De Wolf C, Alex A (2011) Sandstone acidizing using a new class of chelating agents. Society of Petroleum Engineers, SPE-139815-MS
Martin AN (2004) Stimulating sandstone formations with non-HF treatment systems. Society of Petroleum Engineers, SPE_90774-MS
McCune CC, Ault JW, Dunlap RG (1975) Reservoir properties affecting matrix acid stimulation of sandstones, SPE-4552-PA
McLeod HO Jr, Ledlow LB, Till MV (1983) The planning, execution, and evaluation of acid treatments in sandstone formations. Society of Petroleum Engineers, SPE-11931-MS
McLeod HO Jr, Norman WD (2000) Sandstone acidizing. In: Reservoir stimulation. Wiley, Chichester
Muecke TW (1982) Principles of acid stimulation. Society of Petroleum Engineers, SPE-10038-MS
Navarrete RC, Holms BA, McConnell SB et al (1998) Emulsified acid enhances well production in high-temperature carbonate formations presented at the European petroleum conference, The Hague
Pandya N (2013) A novel emulsified acid system for stimulation of very high-temperature carbonate reservoirs, presented at the IPTC Beijing, China, 26–28 March 2013, IPTC 16452
Ponce da Motta E, Plavnik B, Schechter RS (1992) Optimizing sandstone acidization, SPE-19426-PA
Qiu XW, Zhao W, Dyer SJ, Al Dossary A, Khan S, Sultan AS (2014) Revisiting reaction kinetics and wormholing phenomena during carbonate acidising. International Petroleum Technology Conference, Doha, Qatar, pp 15
Rabie AI, Nasr-El-Din HA (2015) Effect of acid additives on the reaction of stimulating fluids during acidizing treatments. Society of Petroleum Engineers, SPE-175827-MS
Reyes EA, Smith AL, Beuterbaugh A, Calabrese T (2015) GLDA/HF facilitates high temperature acidizing and coiled tubing corrosion inhibition. Society of Petroleum Engineers, SPE-174264-MS
Rignol J et al (2015) Improved fluid technology for stimulation of ultrahigh-temperature sandstone formation. Society of Petroleum Engineers, SPE-173755-MS
Schechter RS (1992) Oil well stimulation. Prentice Hall, Englewood Cliifs
Schlumberger (2000) Reservoir stimulation. Wiley, Chichester
Segee B (2013) Dirty water: fracking offshore California. In: Environmental Defence Center, pp 258–268
Shafiq MU, Shuker MT (2013) Finding suitable acid for acidizing of low permeable sandstone formation: a research. Society of Petroleum Engineers, SPE-169671-MS
Shafiq MU, Mahmud HKB (2016) An effective acid combination for enhanced properties and corrosion control of acidizing sandstone formation, IOP conference series: materials science and engineering, vol 121
Shafiq MU, Kyaw A, Shuker MT (2013) A comprehensive research to find suitable acid for sandstone acidizing. Adv Mater Res 787:274–280
Shafiq MU, Shuker MT, Kyaw A (2014) Performance comparison of new combinations of acids with mud acid in sandstone acidizing. RJASET 7(2):323–328
Shafiq MU, Mahmud HKB, Hamid MA (2015) Comparison of buffer effect of different acids during sandstone acidizing, IOP conference series: materials science and engineering, vol 78
Shafiq et al (2016) New acid combination for a successful sandstone acidizing. Presented in 29th symposium of Malaysian chemical engineers (SOMChE), Miri, Sarawak, Malaysia
Shaughnessy CM, Kunze KR (1981) Understanding sandstone acidizing leads to improved field practices, SPE-9388-PA
Shuchart CE (1997) Chemical study of organic-HF blends leads to improved fluids. Society of Petroleum Engineers, SPE-31281-MS
Shuchart CE, Buster DC (1995) Determination of the chemistry of HF acidizing with the use of F NMR spectroscopy. Society of Petroleum Engineers, SPE-28975-MS
Shuchart CE, Gdanski RD (1996) Improved success in acid stimulations with a new organic-HF system. Society of Petroleum Engineers, SPE-36907-MS
Smith CF, Hendrickson AR (1965) Hydrofluoric acid stimulation of sandstone reservoirs, SPE-980-PA
Taron J, Elsworth D (2009) Thermal-hydrologic-mechanical-chemical processes in the evolution of engineered geothermal reservoirs. Int J Rock Mech Min Sci 46(5):855–864
Thomas RL, Nasr-El-Din HA, Lynn JD, Mehta S, Zaidi SR (2001) Precipitation during the acidizing of a HT/HP illitic sandstone reservoir in eastern Saudi Arabia: a laboratory study. Society of Petroleum Engineers, SPE-71690-MS
Thomas RL, Nasr-El-Din HA, Mehta S, Hilab V, Lynn JD (2002) The impact of HCl to HF ratio on hydrated silica formation during the acidizing of a high temperature sandstone gas reservoir in Saudi Arabia. Society of Petroleum Engineers, SPE-77370-MS
Wildenschild D, Sheppard AP (2013) X-ray imaging and analysis techniques for quantifying pore-scale structure and processes in subsurface porous medium systems. Adv Water Resour 51:217–246
Xiong C (2010) Application and study of acid techniques using novel selective emulsified acid system, presented at CPS, SPE international oil and gas conference and exhibition in China, Beijing 8–10 June, 2010, SPE 131216
Yang F, Nasr-El-Din HA, Al-Harbi BM (2012) Acidizing sandstone reservoirs using HF and formic acids. Society of Petroleum Engineers, SPE-150899-MS
Zhou L, Nasr-El-Din HA (2014) Acidizing sandstone formations using a sandstone acid system for high temperatures. Society of Petroleum Engineers, SPE-165084-MS
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shafiq, M.U., Mahmud, H.B. Sandstone matrix acidizing knowledge and future development. J Petrol Explor Prod Technol 7, 1205–1216 (2017). https://doi.org/10.1007/s13202-017-0314-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-017-0314-6