Abstract
The increase in demand for crude oil and the depletion of light crude oil reserves have led to increase in the production of heavy crude oils. This has resulted in an interest in decreasing the pumping costs by techniques such as heating, dilution and/or mixing with water. Among the aforementioned methods, emulsification of heavy crude oil with water is the cheapest, and hence, optimizing its application has found increasing attention. This study uses one of the heavy crude oils in Oman to study the viscosity reduction achieved by emulsification with water at different oil fractions, temperatures, shear rates and salt content. Two chemicals were used to stabilize the emulsions, namely a nonionic surfactant and an anionic, high molecular weight polymer. It was found that viscosity reduction higher than 90% can be obtained through the creation of oil-in-water emulsions with oil content of less than 70%. Generally, it was found that there is no effect on the viscosity reduction obtainable from oil-in-water emulsions produced using the nonionic surfactant at shear rate up to 1620 s−1, temperature up to 80 °C and salt content up to 2.0 wt%. On the other hand, the viscosity reduction using water-in-oil emulsions at a temperature of 30 °C increases with surfactant concentration and shear rate. Also, the increase in salt content adversely affected the viscosity reduction obtained using water-in-oil emulsions. Moreover, it was possible to create oil-in-water emulsions with 60% oil content and viscosity reduction of around 100% in tap water using 100 and 1000 ppm polymer at all temperatures investigated. However, the use of 2.0 wt% salt resulted in lowering the viscosity reduction to 53 and 80% at 30 and 45 °C, respectively. This diverse effect of salt was almost neutralized at 60 and 80 °C resulting in viscosity reduction higher than 90%. The emulsions created using 3 wt% surfactant were generally stable for one week to a temperature of around 23 °C. The stability of oil-in-water emulsions with an oil content higher than 60% was not affected by the presence of salt. The emulsions prepared with an oil content of 60% in 100 ppm polymer were stable only for 1 h. However, the stability in water for these emulsions was greatly enhanced by increasing the viscosity of the continuous water phase by using 1000 ppm polymer, which resulted in stability higher than 70% after 24 h of incubation at 23 °C. The presence of 2.0 wt% salt in 1000 ppm polymer emulsions resulted in rendering these emulsions as instable even after 1 h of incubation. It can be concluded that high molecular weight polymers can find application in drag reduction for heavy crude oils after thoroughly investigating the effect of different polymer molecular parameters such as molecular weight and charge density.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The decline of light crude oil reserves and the increase in oil prices before 2014 have triggered the production of difficult oils, which has higher density and viscosity. According to their API, these crude oils are classified as heavy and extra-heavy oils. Pipeline transportation of these oils from the oilfields to ports and refineries is very a challenging task due to the high pumping cost to overcome their high viscosities. In order to make the production of these oils economically feasible especially during the current low oil prices, methods have been proposed ranging from heating (Layrisse 1999; Yaghi and Al-Bemani 2002; Saniere et al. 2004), dilution with light oils (Iona 1978; Yaghi and Al-Bemani 2002) and/or transportation of the crude oil as oil-in-water emulsions (Lappin and Saur 1989; Yaghi and Al-Bemani 2002; Gregoli et al. 2006; Ashrafizadeh and Kamran 2010) or as core annular flow (Bai et al. 1997). Dilution with gas condensates and/or light crude oils is an expensive choice due to the amounts of diluents needed to achieve significant viscosity reduction (Yaghi and Al-Bemani 2002; Martínez-Palou et al. 2011). Moreover, asphaltene precipitation due to mixing with incompatible crude oils (Zahan et al. 2004) might result in pipeline blockage. Pumping the heavy crude oils at high temperatures requires special designs to insulate the pipeline to preserve the oil temperature produced at the wellhead or the increase in temperature obtained through electrical heating (Martínez-Palou et al. 2011). This technique can be impractical for some crude oils due to low crude temperature at the wellhead and/or high energy requirements. Transporting heavy oil by core annular flow decreases the pressure drop across the pipeline by assuring a flow regime that allows oil isolation from the pipeline wall by a layer of water. However, the well-developed flow regime can collapse with flow cessation due to shutdowns (Simon and Poynter 1970).
Due to the abundance of water phase in oilfield operations as it is produced along with the oil in often large quantities, viscosity reduction in heavy crude oils and bitumens can be achieved by transporting these crude oils as oil-in-water emulsions (Langevin et al. 2004). This does not impose any operational problems during emergency shutdowns (Simon and Poynter 1970) since emulsions can be stabilized for long periods of time using nonionic surfactants because they are cheap and thermodynamically stable in saline water (Rivas et al. 1998). Emulsions are prepared by mixing the oil and water phases through stirring and/or flow through flow restrictions such as valves and chokes. The produced oil-in-water emulsions can have a viscosity more than two orders of magnitude lower than the oil (Yaghi and Al-Bemani 2002). An additional advantage of the transport of heavy oil via oil-in-water emulsions is the reduction in pipe corrosion sediment formation (Poynter and Tigrina 1970).
The requirements of an effective and economic transportation of heavy crude oils as oil-in-water emulsions are to have the highest oil content for the targeted viscosity reduction. The viscosity of the emulsion desired to reduce the pumping cost is around 400 cP at ambient temperature. The viscosity of the oil-in-water emulsions is generally very low below the phase inversion point (i.e. the change of the continuous phase from water to oil). Above phase inversion, water-in-oil emulsions result in sharp increase in emulsion viscosity. Hence, phase inversion point of a certain oil/water system should be avoided.
Drag-reducing polymers can be also injected in the oil/water flow to further enhance the reduction in pressure drop and decrease the pumping cost. Our research in using drag-reducing polymers in oil–water flow showed that injecting small amount of polymers (less than 20 ppm) results in a drag reduction of more than 50% (Al-Wahaibi and angeli 2007; Al-Wahaibi et al. 2007a, b, Yusuf et al. 2011, 2012a, b, c).
The work was conducted to test the different factors affecting the viscosity and stability of oil-in-water emulsions prepared using heavy Omani crude oil. These include surfactant concentration, oil content, temperature, salt concentration and polymer addition.
Experimental
Materials
The crude oil used in this study was Omani crude oil having an API of 18, viscosity of 800–1640 and a density of 940 kg m−3 at 30 °C. The surfactant used was iso-octylphenoxypolyethoxy ethanol with a chemical structure shown in Fig. 1. The commercial name for this surfactant is Triton X-100. This surfactant was obtained from BDH Middle East LLC, United Arab Emirates. Triton X-100 is a high-purity, water soluble, nonionic surfactant, with and HLB value of 13.5, which is typical for the production of O/W emulsions.
Sodium chloride (NaCl) was the salt used to investigate the effect of salt in stability and viscosity of the emulsions.
A commercial polymer supplied by SNF Floerger (France) and used in enhanced oil recovery under the name Flopaam 3630S was used to prepare polymer aqueous solutions with concentrations of 100 and 1000 ppm. This polymer is a partially hydrolyzed polyacrylamide composed of acrylamide and acrylate as shown in Fig. 2. The polymer has a molecular weight of 18 million Daltons and a 27% hydrolysis.
Emulsions were prepared using ultrasound mixer (Unidrive 1000 from CAT). Viscosity of oil and emulsions was measured using Haake coaxial cylinder viscometer (M10) connected to a TECHNE RB-12 heating–refrigerating bath. The viscosity behaviour of Flopaam 3630S aqueous solutions was measured using Cannon–Fenske capillary viscometers.
Procedure
Different samples of oil-in-water emulsions were prepared using the crude oil and tap water. Emulsions were prepared in 200-mL graduated beakers with different oil contents. The water/oil mixture was vigorously agitated using the ultrasound mixer at 8500 rpm for 1 h at room temperature of around 23 °C. Two samples of the emulsion produced were then taken: one to measure the viscosity and another to observe emulsion stability. The viscosity was measured at temperatures of 30, 45, 60 and 80 °C and in a shear rate range from 27 to 2700 s−1.
The viscosity measurements were validated against a standard specimen supplied by the manufacturer at three different temperatures. The difference between the exact and measured viscosity values at temperatures 20, 60 and 100 °C was (5, 7 and 10) cP, respectively. The measurements uncertainty in the viscosity and temperature were estimated as 3–5% of measured value and ±0.01 °C, respectively.
On the other hand, the stability of the emulsion was investigated by measuring the volume of water separated at the bottom of graduated glass container at 1, 4, 24 and 168 h.
The emulsion stability was indicated in percentage using the following equation:
To evaluate the extent of the viscosity reduction as a result of emulsification, viscosity reduction percentage (VR%) is calculated using the following equation:
A 10,000 ppm stock polymer solution was prepared by adding the required weight of the polymer to the water while stirring using a paddle mixer. The solution was stirred overnight to guarantee complete dissolution. The stock solution was then diluted to 100 and 1000 ppm before mixing with crude oil and surfactant to prepare the emulsions.
Results and discussion
Oil viscosity and phase inversion
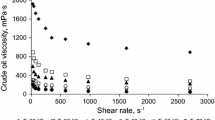
The viscosity of oil as a function of shear rate from 27 to 2700 s−1 at temperatures of 30, 45, 60 and 80 °C is shown in Fig. 3. The oil exhibits a shear thinning behaviour, which characterizes the flow behaviour of heavy oils. The viscosity of crude oil decreases with the temperature, and it maintains the exponential Arrhenius-type association:
From which
This is shown in Fig. 4. The activation energy and the pre-exponential constant are 5.54 × 10−20 J and 1.33 × 10−3 mPa s, respectively.
At the shear rate of 970 s−1, the viscosity of the oil decreased from 852 mPa s at 30 °C to 188 and 134 mP s at 60 and 80 °C, respectively. Hence, significant viscosity reductions of 79 and 84% can be obtained through heating at 60 and 80 °C, respectively. However, the heating option to decrease the viscosity of heavy oils is expensive (Yaghi and Al-Bemani 2002) since it demands high energy and expensive insulation along the pipeline.
The viscosity of emulsions prepared with 70% oil content and 3 wt% Triton X-100 at 30, 45, 60 and 80 °C is also shown in Fig. 3. Similar to the rheological behaviour of the oil, the emulsions also exhibit shear thinning at all temperatures with viscosities of the emulsion an order of magnitude less than those obtained with the crude oil. For example, the viscosity at 30 °C and 972 s−1 for the crude oil and the emulsion with 70% oil content is 852 and 46.2 mPa s, respectively. This is equivalent to around 95% viscosity reduction. However, the extent of this decrease in viscosity when the oil content increased to 80% is much lower than that of the 70% oil content at all temperatures investigated. For example, the viscosity reduction at 30 °C and 972 s−1 for the 80% oil content is calculated to be 67% (viscosity of the emulsion is 281 mPa s). This is due to phase inversion from O/W to W/O emulsion above critical oil content of around 70% as shown in Fig. 5. Phase inversion is indicated by sudden increase in viscosity due to the higher viscosity of the oil continuous phase. Figure 5 shows the viscosity values measured for emulsions prepared at oil contents from 40 to 100%. It can be seen that there are two distinctive behaviours of viscosity with respect to oil content; there is slight increase in emulsion viscosity at low oil contents followed by a sharp increase above critical oil content. This sudden change in viscosity indicates the inversion point from oil-in-water emulsion to water-in-oil emulsion which coincides with 70.5% oil content at 30 °C and 245 s−1 using 3 wt% Triton X-100.
Phase inversion of emulsions prepared using 1, 3 and 5 wt% Triton X-100 is listed at 30 °C at three shear rates in Table 1. The phase inversion increases by the increase in Triton X-100 dosage from 1 to 3 wt%, above which the increase in surfactant concentration to 5 wt% has effective phase inversion. It can be observed also that phase inversion occurs in the water oil content range of 66–72%, which is consistent with the results obtained by Abdurahman and co-investigators (Abdurahman et al. 2012). Hence, the optimum surfactant dosage based on phase inversion is 3 wt% based on the crude oil and water under investigation.
Effect of shear rate on phase inversion
The effect of shear rate in the range from 27 to 1620 s−1 on phase inversion is shown in Fig. 6 for emulsions prepared using 3 wt% Triton X-100 at different temperatures. Generally, phase inversion is in the range of oil content between 65 and 70.5%. At 30 °C, phase inversion increases with shear rate until a shear rate of 245 above which it decreased to a plateau value of 69.2%. For temperatures 45, 60 and 80 °C, phase inversion initially decreases with shear rate up to a critical value above which it increases showing plateau levels of 68.7 and 67.8% at 45 and 60 °C, respectively. The critical shear rates for the increase in phase inversion are 75, 245 and 350 s−1 at 45, 60 and 80 °C, respectively. Generally, phase inversion increases with the increase in temperature at low shear rates (below 100 s−1), whereas it decreases with temperature at high shear rates (above 350 s−1).
Effect of surfactant concentration on viscosity reduction and emulsion stability
Viscosity was measured for emulsions prepared with varying surfactant concentrations, 1, 3 and 5 wt%, and oil content (40–80%) to investigate the effect of surfactant dosage on viscosity reduction as shown in Fig. 7. All the measurements were conducted at 30º C. It can be seen that the viscosity reduction is almost 100% up to an oil content of 70% using 1 and 3 wt% surfactant. With the 5 wt% concentration of the Triton X-100, the viscosity reduction decreased above 60% oil content, however, maintaining viscosity reduction above 90% up to oil content of 70%. The viscosity reduction sharply decreases above the inversion point (66–72% oil content as shown in Table 2) to values ranging between 58 and 74%, with higher viscosity reduction for higher surfactant concentrations using an oil content of 80%. This indicates that reasonable viscosity reduction can be still obtained above the inversion point by using high surfactant concentrations.
Table 2 lists the stability of the emulsions prepared using Triton X-100 with surfactant concentrations of 1, 3 and 5% after 1, 4, 24 and 168 h. Three images taken after one week of the study using 70% oil content and surfactant concentrations of 1, 3 and 5 wt% are shown in Fig. 8. It can be observed that the stability of the emulsions after the inversion point (i.e. W/O emulsions) is 100%. This can be attributed to the high viscosity of the continuous oil phase. At an oil content of 70%, the emulsions prepared with 5% surfactant are 92% stable after 168 h, which decreases with the decrease in oil content to around 14% using 60% oil. Generally, the emulsions prepared using 1% surfactant demonstrated the best stability at all oil contents. However, the emulsions prepared using 3% surfactants are completely stable for oil contents higher than 60%.
Effect of shear rate on viscosity reduction
Figures 9 and 10 show viscosity reduction at different oil contents obtained at 30, 45, 60 and 80 °C using emulsions prepared with 3 wt% Triton X-100 at shear rates of 45 and 1620 s−1, respectively. At 45 s−1, viscosity reduction below oil content of 60% (i.e. O/W emulsions) is around 97% is independent of the temperature. The viscosity reduction decreases slightly to around 95% for the oil content of 70% for all temperatures investigated. On the other hand, the viscosity reduction decreases sharply to varying values from 49% at 45 °C to 67% at 80 °C for the W/O emulsions at an oil content of 80% (i.e. above inversion points). At the shear rate of 1620 s−1, viscosity reduction values are generally at 98% at all temperatures investigated at an oil content of 40%. The viscosity reduction starts to be dependent on the temperature with the increase in oil content from 50 to 80% showing higher values at lower temperatures.
At 30 °C, viscosity reduction is independent of the shear rate for O/W emulsions (i.e. oil content below 70%) as shown in Fig. 11. The W/O emulsions at an oil content of 80% show wide range of viscosity reduction obtained at 30 °C, which increases with shear rate. On the other hand, the dependence on shear rate for the viscosity reduction obtained at 80 °C at different shear rates as shown in Fig. 12 starts with emulsions prepared with an oil content of 60%, showing wider variation of viscosity reduction with respect to shear rate at higher oil contents. Generally, higher viscosity reduction was obtained at lower shear rates at 80 °C, which is a trend opposite to that at 30 °C.
Effect of salinity on viscosity reduction and emulsion stability
Emulsions were prepared with 3 wt% Triton X-100 and two NaCl concentrations, 0.2 and 2.0 wt%, to investigate the effect of salt content on viscosity reduction and emulsion stability. Figures 13 and 14 show viscosity reduction at different temperatures as a function of oil content at a shear rate of 972 s−1 using 0.2 and 2.0 wt% NaCl, respectively. Viscosity reduction measured using the two salt concentrations is above 90% for oil contents below 70% at all temperatures. Sharp decrease in viscosity reduction occurred at 80% oil content using both salt concentrations indicating inversion point above oil content of 70%. This is similar to the trend observed in emulsions prepared without salt addition. The temperature effect on the viscosity reduction at oil content of 80% is significant with viscosity reduction increasing with the increase in temperature from 30 to 80 °C for both salt concentrations. This effect of temperature on viscosity reduction above inversion point for the emulsions prepared using salt is opposite to that when no salt was used as described above. The viscosity reduction at an oil content of 80% for emulsions prepared using no salt is significantly higher than values obtained using salt as shown in Fig. 15 at 30 °C and 972 s−1.
The emulsion stabilities after 1 h of preparation using tap water, 0.2 and 2 wt% NaCl with different oil contents are listed in Table 3. It can be observed that salt addition decreases the stability of the emulsions prepared using 40 and 50% oil content. On the other hand, emulsion prepared at 60–80% oil content is almost stable independent of salt content. Hence, it can be concluded that the existence of salt negatively affects the stability of O/W emulsions with low oil content (i.e. less than 50%). This was also the case for the stability monitored after 24 h of emulsification.
Effect of polymer on viscosity reduction and emulsion stability
The most important property of a polymer is its ability to increase the solution’s viscosity. The viscosity the Flopaam 3630S in tap water was measured using Cannon–Fenske capillary viscometers at room temperature of around 23 °C as shown in Fig. 16. The viscosity of the 100 and 1000 ppm polymer solutions was 2.3 and 34 mPa s, respectively.
Figure 17 shows the viscosity reduction in emulsions prepared with 60% oil content and 100 and 1000 ppm polymer at temperatures from 30 to 80 °C. Surprisingly, viscosity reductions between 97 and 99% were obtained at all temperatures using the two polymer concentrations when the continuous phase (i.e. water) does not contain salt. These results are very significant since the viscosity of the crude oil can be significantly decreased using minute amounts of polymer in the emulsification process, which can be otherwise obtained using large amounts of surfactants. This will significantly reduce the amount of chemicals needed to achieve the desired viscosity reduction in field applications which is a crucial factor that can determine the viability of the application. Existence of the salt seems to adversely affect viscosity reduction as shown in Fig. 17 when 2 wt% NaCl was added in the emulsion prepared using 1000 ppm polymer. The viscosity reduction at 30 °C was around 53% increasing to a plateau value of around 90% above 60 °C.
The emulsion stability of samples prepared with 60% oil content and with 100 and 1000 ppm polymer is shown in Table 4. Emulsions with 100 ppm polymer with no salt inclusion are generally not stable after 4 h of incubation. However, the increase in polymer concentration seems to stabilize the emulsions prepared with reasonable stability of around 73% after one day of incubation. This is due to the higher viscosity of the 1000 ppm polymer solution being one order of magnitude higher than that of the 100 ppm solution as shown in Fig. 17. This high viscosity of the continuous phase of the emulsion resists any droplet migration due to gravity effect (Dalgleish 1996). On the other hand, emulsions prepared using 1000 ppm polymer and 2 wt% NaCl were generally not stable. This might be attributed to both the lower viscosity of the 1000 ppm polymer in the presence of the salt and the screening effect of the salt to any double-layer repulsion.
Conclusions
The use of stabilized O/W emulsions to reduce the pumping cost of heavy crude oils has been investigated using one of the Omani crude oils. The use of a nonionic surfactant with different concentrations to stabilize the emulsions has been studied using different oil contents. A viscosity reduction of around 100% was obtained up to a critical oil content between 66 and 70%, which indicates the phase inversion of the emulsions from O/W to W/O emulsions. Phase inversion has increased when the surfactant concentration was increased from 1 to 3%. However, further increase in surfactant concentration to 5 wt% did not affect the phase inversion point. The inversion point was found to depend on the shear rate. Generally, the phase inversion point decreases with shear rate up to a critical value above which it starts to increase to a plateau value at shear rates above 1000 s−1. This critical shear rate shifts to higher values with temperature. The viscosity reduction was not affected by surfactant concentration in the range of 1–5% for the oil-in-water emulsions. However, emulsion stability was negatively affected when 5% surfactant was used for oil contents below 60%. On the other hand, water-in-oil emulsions (oil content of 70 and 80%) are generally stable for one week independent of surfactant dosage.
The effect of temperature on the viscosity reduction was more pronounced at higher shear rates with viscosity reduction being decreased at higher temperatures especially for oil contents of 70 and 80%. At 30 °C and 80% oil content, the viscosity reduction is higher with higher shear rates. However, this trend was reversed at 80 °C. There was no clear effect of salt content at concentrations of 0.2 and 2.0 wt% on viscosity reduction below oil content of 70% at all temperatures investigated. An important effect was observed at an oil content of 80% with the presence of salt inducing higher viscosity reductions at higher temperatures, which is the inverse effect to that observed without salt. At oil content of 80% and 30 °C, the viscosity reduction was higher in the absence of salt by around 35% compared to both salt concentrations used in this study. The addition of salt affects negatively the emulsion stability of those prepared using oil contents of 40 and 50%. The stability of emulsions with higher oil contents was not affected by the presence of salt.
One of the important results of this study is the ability of minute amounts of high molecular weight polymer to induce high stability and viscosity reduction for the oil-in-water emulsions prepared using 60% oil. Viscosity reduction as high as 98% was obtained using as low as 100 ppm polymer without salt, which is comparable to those obtained using 3 wt% surfactant. The inclusion of salt in the emulsions prepared using 1000 ppm polymer resulted in low viscosity reductions, which increases with the increase in oil content. This has practical consequences since the water phase in the oilfields is saline. However, the polymer seems to induce viscosity reductions above 90% at the desired oil contents. The poor emulsion stability obtained using the polymer only can be enhanced using higher polymer concentrations that will increase the viscosity of the water phase to an optimum value since the polymer stabilizes the emulsions primarily by increasing the viscosity of the continuous water phase. This was indicated by the enhancement of emulsion stability at a polymer concentration of 1000 ppm compared to that at 100 ppm.
Abbreviations
- μ :
-
Dynamic viscosity (mPa s)
- µ crude :
-
Viscosity of crude oil at specific shear rate and temperature (mPa s)
- µ emulsion :
-
Corresponding viscosity of emulsion samples (mPa s)
- E μ :
-
Activation energy (J)
- k B :
-
Boltzman constant 1.3806488 × 10−23 (J K−1)
- O/W:
-
Oil-in-water
- T :
-
Temperature (K)
- VR%:
-
Viscosity reduction (%)
- W/O:
-
Water-in-oil
- γ :
-
Shear rate (s−1)
References
Abdurahman NH, Rosli YM, Azhari NH, Hayder BA (2012) Pipeline transportation of viscous crudes as concentrated oil-in-water emulsions. J Petrol Sci Eng 90–91:139–144
Al-Wahaibi T, Angeli P (2007) Transition between stratified and non-stratified horizontal oil–water flows: part i (stability analysis). Chem Eng Sci 62(11):2915–2928
Al-Wahaibi T, Smith M, Angeli P (2007a) Effect of drag-reducing polymers on horizontal oil–water flows. J Petrol Sci Eng 57(3–4):334–346
Al-Wahaibi T, Smith M, Angeli P (2007b) Transition between stratified and non-stratified horizontal oil–water flows: part ii (mechanism of drop formation). Chem Eng Sci 62(11):2929–2940
Ashrafizadeh SN, Kamran M (2010) Emulsification of heavy crude oil in water for pipeline transportation. J Petrol Sci Eng 71(3):205–211
Bai R, Joseph DD, Chen KP, Renardy YY (1997) Core-annular flows. Annu Rev Fluid Mech 29:65–90
Dalgleish D (1996) Food emulsions. Surfactant science series, vol 61. Marcel Dekker, New York
Gregoli AA, Hamshar JA, Olah AM, Riley CJ, Rimmer DP (2006) Preparation of stable crude oil transport emulsions. US Patent 4,725,287
Iona M (1978) Process for producing low-density low sulfur crude oil. US Patent 4,092,238
Langevin D, Poteau S, Hénaut I, Argillier JF (2004) Crude oil emulsion properties and their application to heavy oil transportation. Oil Gas Sci Technol 59:511–521
Lappin GR, Saur JD (1989) Alpha olefins applications handbook. CRC Press, New York
Layrisse I (1999) Heavy oil production in Venezuela: historical recap and scenarios for next century. In: SPE international symposium on oilfield chemistry, pp 749–761
Martínez-Palou R, Mosqueira M, Zapata-Rendón B, Mar-Juárez E, Bernal-Huicochea C, Clavel-López J, Aburto J (2011) Transportation of heavy and extra-heavy crude oil by pipeline: a review. J Petrol Sci Eng 75:274–282
Poynter G, Tigrina S (1970) Pipelining O/W mixtures. US Patent 3,519,006
Rivas H, Gutierrez X, Cardenas AE, Morales A (1998) Natural surfactant with amines and ethoxylated alcohol. US Patent 5792223
Saniere A, Henaut I, Argillier JF (2004) Pipeline transportation of heavy oils, a strategic, economic and technological challenge. Oil Gas Sci Technol Rev IFP 59(5):455–466
Simon R, Poynter WG (1970) Pipelining oil/water mixtures. US Patent 3519006
Yaghi M, Al-Bemani A (2002) Heavy crude oil reduction for pipeline transportation, energy sources, vol 24. Taylor and Francis, London, pp 93–102
Yusuf N, Al-Wahaibi T, Al-Wahaibi Y, Al-Ajmi A, Al-Hashmi A, Olawale A, Mohammed I (2011) Effect of pipe diameter on the efficiency of drag reducing polymer in horizontal oil–water flows. Presented at the 15th international conference on multiphase production technology, Cannes, France, 15–17 June, 2011
Yusuf N, Al-Wahaibi T, Al-Wahaibi Y, Al-Ajmi A, Al-Hashmi A, Olawale AS, Mohammed IA (2012a) Comparison on the efficiency of two drag reducing polymers in horizontal oil–water flow. Presented at international symposium on multiphase flow and transport phenomena, Agadir, Morocco, April 22–25, 2012a
Yusuf N, Al-Wahaibi T, Al-Wahaibi Y, Al-Ajmi A, Al-Hashmi AR, Olawale AS, Mohammed IA (2012b) Experimental study on the effect of drag reducing polymer on flow patterns and drag reduction in a horizontal oil–water flow. Int J Heat Fluid Flow 37:74–80
Yusuf N, Al-Wahaibi Y, Al-Wahaibi T, Al-Ajmi A, Olawale AS, Mohammed IA (2012c) Effect of oil viscosity on the flow structure and pressure gradient in horizontal oil–water flow. Chem Eng Res Des 90:1019–1030
Zahan M, Bjorndalen N, Islam MR (2004) Detection of precipitation in pipelines Petrol. Sci Technol 22:1119–1141
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al-Hashmi, A.R., Al-Wahaibi, T.K., Al-Wahaibi, Y.M. et al. Transportation of heavy oils using polymer-stabilized oil-in-water emulsions. J Petrol Explor Prod Technol 7, 881–890 (2017). https://doi.org/10.1007/s13202-016-0298-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-016-0298-7