Abstract
CO2 enhanced oil recovery by using newly synthesized CO2 philic surfactant as CO2 mobility control agent is reported. Nonyl phenol ethoxylate sulphonate (NPES) was prepared by the esterification of alcohol with maleic anhydride followed by its sulphonation. Effect of interfacial tension (IFT) between CO2 and brine using NPES was investigated to verify the CO2 philicity of the surfactant. The detailed account of effects of pressure and temperature on IFT between CO2/brine in the presence and absence of surfactant on specified pressure and temperature conditions is studied and discussed. NPES effectively lowered the IFT between CO2/brine (30 mN/m) to less than 5.23 mN/m. Foam quantity was improved by using betaine as foam booster. The static foam endurance properties in the presence and absence of oil were also examined prior to the actual performance test of surfactants using a reservoir core plug sample. It was noted that the foam stability was 900 s in the presence of oil at 90 °C in CO2 environment. The effect of foam by using synthesized NPES on mobility of CO2 is also presented. The CO2 mobility reduction factor was found to be 3.1. It is concluded that NPES has great potential for CO2-EOR applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the fact the CO2-EOR is gaining dominance compared to other EOR methods, the existence of an unfavourable mobility ratio has hampered the efficiency of the CO2 usage as a favourable displacement fluid. The vast differences in viscosity and density between CO2 (injected fluid) and the reservoir fluids are the causes of the unfavourable mobility ratio. This mobility ratio challenge results in viscous fingering, early CO2 breakthrough, gas channelling and, consequently, the CO2 inability to effectively contact much of the reservoir and the oil it contains (Irawan et al. 2012; Mclendon et al. 2012; Enick et al. 2012; Motealleh et al. 2013; Bagci 2007; Le and Nguyen 2008) and overall a poor oil recovery is obtained.
Injection and migration of CO2 in deep reservoir depends upon the drainage of brine by CO2 and the migration of CO2 slug, and the imbibition of CO2. Both of these processes are characteristics of the relative permeability and capillary pressure. These characteristics are governed by the interfacial tension (IFT) of brine and CO2 (Bachu and Bennion 2009). A low IFT between carbon dioxide and brine is required for the generation of stable foam for CO2-enhanced oil (Li et al. 2012a, b). In that context, surfactants can reduce IFT between CO2 and reservoir fluids and also increase the CO2 apparent viscosity by foam generation. Therefore, surfactants can provide in-depth CO2 mobility control at an affordable price (Gandomkar and Kharrat 2012) and foam can effectively be used as mobility reducing agent to overcome the CO2 mobility issues.
The generation of foam itself, is not an important challenge; the main ones are foam quality, structure of foam, and its stability in CO2 environment at high temperature especially when in contact with oil (Khalil and Asghari 2006; Farajzadeh et al. 2012; Simjoo et al. 2011; Masalmeh et al. 2011). Until now the surfactants used for foam generation have had a poor tolerance with salinity and have suffered from excessive adsorption on carbonate rocks (Lawson 1978; Tsauet al. 2000; Sagir et al. 2015). The CO2 philic surfactants are a new type of surfactants employed for the generation of stable foams for CO2 mobility control applications. CO2-philic surfactants have been given importance in recent years due to their superior performance in CO2 gas injection (Enick et al. 2012). Very little work has been reported on the possibility of their use in the EOR process, as this class of surfactants is new to the surfactant industry especially for EOR applications. There are only a few reference works available for this particular class of surfactants.
In order to produce stable foam, the CO2 philic surfactants play a fundamental role. These surfactants act on the water-CO2 interface to lower down the IFT between CO2 and water to generate stable foam. As a result, the mobility reduction factor (MRF) of CO2 increases as the IFT reduction factor between CO2 and aqueous surfactant solution increases. This incredible capability of foam stability reduces the CO2 flow thereby controlling the CO2 mobility to mobilize the trapped oil with improved efficiency (Gandomkar and Kharrat 2012; Sagir et al. 2013). NPES can be used as CO2 philic surfactant as the structure of this surfactant contains a number of functional groups which favour CO2 philicity such as branches and methyl groups, which are usually missing in conventional surfactants like alpha olefin sulphonates.
The current study reports the effect of newly synthesized CO2 philic surfactant on the IFT of CO2/brine systems over a wide range of temperature, pressure and salinity conditions. In addition, foam stability and CO2 mobility reduction with and without surfactant is also reported.
Materials and methods
Raw material and chemicals
Maleic anhydride, sodium bisulphite, Hyamine 1622 (standard 0.004 M solution) were purchased from Fisher Scientific Malaysia. 2-butyl-1-octanol was obtained from Merck Germany. Sodium bicarbonate, p-Toluene Sulphonic acid (PTSA), iso-propanol, anhydrous sodium sulphate and diethyl ether were obtained from Sigma-Aldrich Belgium. Nonyl phenol ethoxylate alcohol was obtained from the PETRONAS chemical group of companies.
Synthesis of surfactant
The surfactant was synthesized by following the method discussed in one of our previous articles (Sagir et al. 2014a, b). The esterification reaction for the synthesis was executed by mixing maleic anhydride (50 mmol) and ethoxylate alcohol (250 mmol) in three-neck flask (1000 mL). The flask was placed in an oil bath and the desired temperature of esterification reaction was achieved and the catalyst PTSA was loaded. Initial acid number was measured and the reaction was continued until the completion of reaction as indicated by the lowest acid number. The product was washed with NaHCO3 saturated solution (Grzesik et al. 2000; Yadav and Thathagar 2002; Kulawska et al. 2011). The obtained product was sulphonated by using sodium bisulphite as the sulphonating agent (Sagir et al. 2014a, c). The diester to sodium bisulphite mole ratio of 1:1.2 in 100 mL water and equal amounts of iso-propanol was refluxed for 24 h for the sulphonation of the produced product. The sulphonation process was monitored by measuring anionic contents (%) by the titration of product against a standard Hyamine (0.004 N) solution.
IFT experiments
The IFT between CO2 and surfactant solution was measured in order to determine the CO2 philicity of developed surfactant by using pendent drop method by employing the Vinci IFT 700 system. The CO2 and aqueous solutions were loaded in their respective accumulators and left for sometime to attain equilibrium at specified conditions of temperature and pressure. A fine pendent drop was created and an image was recorded by a camera to calculate the IFT.
Foam endurance test
In the first stage, the foamability and foam stability of the synthesized surfactant was tested using the DFA 100 instrument at room temperature by using air as the gas source. The tests were conducted in the absence of crude oil. Betaine (Betadet S20) in 40 % weight ratio was incorporated in order to boost up the foam quality and quantity. All of the foam stability measurements were conducted by using mixture of NPES and Betaine. In this foam, betaine was used to increase the quantity of foam, while NPES for quality.
The foam stability was also measured in the presence of crude oil by using a 0.5 % surfactant solution in a 1000-mL graduated cylinder. A volume of 200 mL of the surfactant solution and 20 mL of crude oil were mixed in the cylinder. The mixture was heated until the desired temperature was reached. A gas dispersion tube was immersed inside the solution and the CO2 flow was started. The flow rate was kept constant for all the experiments in order to perform the initial screening of the foam quality and stability. The gas flow was continued until the foam height reached 1000 mL. The decay time of the foam was noted when 20 % of the foam height was left standing in the measuring cylinder. The same procedure was repeated for surfactant concentrations.
Mobility in porous media
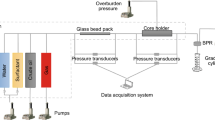
Porous media and core flood system
Berea sandstone (10 mD, 6.00 in. length, 1 in. diameter, porosity 17 %) core samples were used as the porous medium for mobility control experiments using CO2. The pressure drop data were recorded. For the CO2 mobility control determination, the RPS-800 core flood system was used for pressure drop data collection of neat CO2 flow in brine-saturated porous media and CO2 flow in the presence of foam in brine-saturated core (Sagir et al. 2014d).
Core flood experiment
The core was loaded in a core holder. An overburden pressure ~2300 psi was maintained. The solutions, CO2, brine and surfactant were loaded in accumulators. The CO2 was pressurized. The desired temperature 90 °C and pressure 1800 psi were attained and equilibrium was achieved.
The core was saturated with brine. A base case for CO2 mobility was obtained by the CO2 flow through a brine-saturated core at the flow rate of 0.22 cc/min. The core was saturated with 0.5 % surfactant. For CO2 mobility resistance by using the foam, the CO2 was made to flow after the Berea core was saturated with surfactant. The CO2 flow was achieved using the same conditions as in the baseline case. The pressure drop data is shown in Fig. 5.
The pressure drop data involving CO2 flow through the porous media in the base case and after the incorporation of foam were recorded to calculate mobility resistance factor (MRF) by using Eq. 1.
Core flood procedure for the displacement experiments
Core flood process is a multistep procedure which included the initial brine injection, oil saturation, brine injection, and surfactant alternating gas injection (SAG). The core flood experiments were conducted at 90 °C. The following section describes the details of each and every step of the core flood experiments.
Water (brine) injection The brine was injected at variable flow rates to determine the absolute brine permeability. The injection was continued until the steady state conditions were reached at the flow rate of 1–5 mL min−1.
Oil injection The core was saturated with the prefiltered crude oil. The injection rate was 1–5 mL min−1; the same as in the water (brine) injection. The oil injection was continued until steady state conditions of the pressure drop were achieved. The oil saturation was calculated from the amount of brine being displaced by oil. The initial oil saturation and residual water saturation were calculated.
Initial oil saturation: Initial oil saturation was estimated by the material balance during the oil flooding as the amount of water produced during the oil flooding was equal to the amount of oil remaining inside the core. The calculations were made by using the following expression 2.
where V w was the volume of the produced water, V p was the pore volume of the core and S oi was the initial oil saturation.
Water (brine) injection Water was injected after the oil flooding. The flow rate for the water injection was 0.22–0.45 mL min−1. This water injection was continued until there was less than a 1 % of oil cut. The residual oil saturation was calculated by the mass balance of the oil recovered by the water injection and the total initial oil saturation.
Residual oil saturation: The water injection data under steady state conditions after the core saturation with oil were used for the calculation of the residual oil saturation. Equation 3 was used for these calculations.
where S orw was the residual oil saturation after water flooding, V w was the volume of water produced from the oil flooding, V o was the oil produced and V p pore volume.
Foam injection SAG process was used for the foam injection for the actual oil recovery. A small slug of surfactant 0.2 pore volume (PV) was followed by 0.2 PV of CO2 gas injection. This alternating injection process was repeated until it reaches 1 PV of the surfactant solution.
The residual oil saturation after the SAG injection was calculated by using the expression.
where S or.sag was the residual oil saturation after the SAG process.
Oil recovery The actual oil recovery by using the synthesized surfactant NPES was calculated after the SAG injection by using the volume of oil recovery by SAG flooding divided by the residual oil saturation after the water injection.
where V op was the total produced oil volume and V or was the residual oil volume.
Resuts and discussions
IFT measurements
IFT values between CO2/water, CO2/brine and CO2/NPES/brine solution by using the pendent drop method are given in Figs. 1 and 2. Sodium chloride (3.5 wt. %) solution was used as brine. The IFT between CO2 and water was observed as 60 mN/m, which decreased to 30 mN/m by applying a pressure of 1070 psi. After this point the change in IFT was not so pronounced. The IFT between CO2 and water determined for base case in this study matched with literature values as reported in various studies (Sagir et al. 2014d; e). It was noted that IFT between CO2 and water increased by the addition of sodium chloride. The effect of pressure on IFT between CO2/brine is shown in Fig. 1. The reverse effect of pressure on IFT between CO2 gas with brine and surfactant was noted. An abrupt change of IFT decrease with an increase in pressure until the pressure of 1070 psi (CO2 critical pressure) was noticeable in Fig. 1.
The IFT value was successfully decreased to a very low value of 5.24 mN/m with the addition of only 0.5 % NPES as shown in Fig. 2. The IFT lowering effect of NPES at high pressure and low temperature reveals that the surfactant interfacial activity at CO2/water interface is quite significant and the developed surfactant has a strong CO2 philicity.
Foam stability
Newly developed surfactant was found to generate foam in the foam analyser using air as gas at ambient conditions as shown in Fig. 3. The foam analysis results are shown in Fig. 4. Betaine (Betadet S20) in 40 % weight ratio was incorporated in order to boost up the foam quality and quantity (Ganchev et al. 2000). The foam endurance results obtained from foam analysis can be described by four periods of foam evolution. First, is foam injection (a slanting vertical line up to 160 mm height), the second period is the retention (horizontal line going downwards slowly parallel to the x-axis) up to the foam height of 150 mm and drop line (the line drops quickly to the x-axis). It took about 400 s for the foam to disintegrate. The foam decay, the process of bubble coalescence is negligible in the second period. However, in the third period the rapid drainage of foam height from 150 to 100 mm in only 60 s was observed. Afterward, a slow drainage of the foam height and bubble coalescence was observed in the fourth stage. The drop portion was stable and was not very steep. This process continued until the foam height of 50 mm in more than 2000 s is reached.
The surfactant generated excellent foam with CO2 gas as shown in Fig. 5. The foam stability time up to 20 % remaining height was 12 min, which was excellent. The unique surfactant structure with more number of branching and the methyl groups, tert-butyl tip, the presence of minimum number of methylene groups were some of the reasons for the excellent foaming properties of the newly developed surfactant. All these structural factors are known to enhance the CO2 affinity with water/brine and lower down the IFT between CO2/water which results in stable foam generation (Sagir et al. 2014d).
CO2 mobility control
The MRF was measured in order to observe the actual resistance to the CO2 flow delivered by the NPES in the porous media. Berea sandstone core sample was used as the porous medium for the mobility control experiment of the CO2. The pressure drop data were collected by the flow through the porous medium.
The pore volume injected (PVI) versus the pressure drop data for mobility control test was determined using core flood experiments. From the pressure drop data it was observed that the pressure drop was 3–4 time increased by the addition of 0.5 % NPES solution as compared to the pressure drop in the control (base case) experiment without using the surfactant. The increment in pressure drop by employing surfactant shows that the mobility of CO2 was lowered down as compared to neat CO2. The surfactant provided the needful resistance to CO2 flow in porous media and reduced the mobility of CO2 in porous media. The results are shown in Fig. 5. The MRF value was also calculated and was found to be 3.1.
Oil recovery
The cumulative oil recovery achieved by using surfactant was 94 %. The surfactant had different tails, side chains, carbonyl and other associated groups as compared to a conventional foaming surfactant. The results are shown in Fig. 6. From the oil recovery results, it was found that the surfactants which produce more stable foam were also effective in the oil recovery performance. It is, therefore, concluded that the oil recovery performance of a surfactant is highly dependent on its hydrocarbon structure, and its head group is important to overcome solution interaction and sensitivity issues, such as salinity and temperature tolerances.
The CO2 attraction of the hydrocarbon chains of NPES improved the foam generation and stability, which resulted in the intensification of the oil recovery (Xing et al. 2012). The sulphonate group was stable at more than 100 °C making this surfactant a strong candidate for enhanced oil recovery formulations in elevated temperature conditions. The betaine was helpful in foam quantity boost.
Conclusions
A new surfactant NPES was developed by using maleic anhydride and nonyl phenol ethoxylate alcohol by the esterification reaction followed by the sulphonation reaction. The attraction of the surfactant towards CO2 was tested by measuring the IFT between CO2/brine and CO2/surfactant. NPES has successfully reduced the IFT values between CO2/brine to a very low value of less than 5.24 mN/m. The foam endurance tests were also conducted in the presence of oil and CO2 at high temperature. It was found that the mixture of NPES and betaine generated good stable foam with CO2 gas which stayed for 900 s in a challenging reservoir condition involving high temperature, high salinity and in the presence of oil. The mobility reduction studies of the developed surfactant were also conducted. By employing 0.5 % of surfactant the pressure drop was increased 3–4 times. The MRF value obtained by employing the developed surfactant was 3.1 and overall oil recovery was found as 94 % OOIP.
The generation of a stable foam as well as mobility reduction accomplished when NPES is employed suggests that it has a great potential as a CO2 EOR additive.
References
Bachu S, Bennion DB (2009) Dependence of CO2-brine interfacial tension on aquifer pressure, temperature and water salinity. Energy Procedia 1:3157–3164
Bagci AS (2007) Immiscible CO2 flooding through horizontal wells. Energy Sources Part A Recovery Util Environ Eff 29:85–95
Enick RM, Olsen DK, Ammer JR, Schuller W (2012) Mobility and conformance control for CO2 EOR via thickeners, foams, and gels—a literature review of 40 years of research and pilot tests. Presented at the SPE improved oil recovery symposium. Tulsa
Farajzadeh R, Andrianov A, Krastev R, Hirasaki GJ, Rossen WR (2012) Foam-oil interaction in porous media: implications for foam assisted enhanced oil recovery. Adv Colloid Interface Sci 183–184:1–13
Ganchev D, Basheva ES, Denkov ND, Kasuga K, Satoh N, Tsujii K (2000) Role of betaine as foam booster in the presence of silicone oil drops. Langmuir 16(3)
Gandomkar A, Kharrat R (2012) The tertiary FAWAG process on gas and water invaded zones: an experimental study. Energy Sources Part A Recovery Util Environ Eff 34:1913–1922
Grzesik M, Skrzypek J, Gumuła T (2000) The effect of the catalyst used on the kinetics of di-2-ethylhexyl maleate synthesis. React Kinet Catal Lett 71:13–18
Irawan F, Irawan S, Awang M (2012) A unique opportunity for liquid carbon dioxide as an enhanced oil recovery method. Energy Sources Part A Recovery Util Environ Eff 34:654–661
Khalil F, Asghari K (2006) Application of CO2-foam as a means of reducing carbon dioxide mobility. J Can Pet Technol 45:37–41
Kulawska M, Moroz H, Kasprzyk A (2011) Kinetic investigations on the esterification of phthalic anhydride with n-heptyl, n-nonyl or n-undecyl alcohol over sulfuric acid catalyst. React Kinet Mech Catal 104:9–15
Lawson JB (1978) The adsorption of non-ionic and anionic surfactants on sandstone and carbonate. Presented at the SPE symposium on improved methods of oil recovery. Tulsa
Le SVQ, Nguyen QP, SPE, The University of Texas at Austin, and Aaron W. Sanders, The Dow Chemical Company (2008) A novel foam concept with CO2 dissolved surfactants. Presented at the SPE/DOE Symposium on improved oil recovery. Tulsa
Li X, Boek ES, Maitland GC, Trusler JPM (2012a) Interfacial tension of (Brines + CO2): CaCl2(aq), MgCl2(aq), and Na2SO4(aq) at temperatures between (343 and 423) K, pressures between (2 and 50) MPa, and molalities of (0.5 to 5) mol·kg−1. J Chem Eng Data 57:1369–1375
Li H, Yang D, Tontiwachwuthikul P (2012b) Experimental and theoretical determination of equilibrium interfacial tension for the solvent(s)–CO2–heavy oil systems. Energy Fuels 26:1776–1786
Masalmeh SK, Wei L, Blom CPA (2011) Mobility control for gas injection in heterogeneous carbonate reservoirs: comparison of foams versus polymers. In: SPE Middle East Oil and Gas Show and Conference. Manama
Mclendon WJ, Koronaios P, McNulty S, Enick RM, Biesmans G, Miller AN, et al (2012) Assessment of CO2-soluble surfactants for mobility reduction using mobility measurements and CT imaging. Presented at the SPE improved oil recovery symposium. Tulsa
Motealleh M, Kharrat R, Hashemi A (2013) An experimental investigation of water-alternating-CO2 coreflooding in a carbonate oil reservoir in different initial core conditions. Energy Sources Part A Recovery Util Environ Eff 35:1187–1196
Sagir M, Tan IM, Mushtaq M, Ismail L, Nadeem M, Azam MR et al (2013) A novel surfactant for the reduction of CO2/brine interfacial tension. J Dispers Sci Technol 35:463–470
Sagir M, Tan IM, Mushtaq M, Ismail L, Nadeem M, Azam MR et al (2014a) Novel Surfactant for the Reduction of CO2/Brine Interfacial Tension. J Dispers Sci Technol 35:463–470
Sagir M, Tan IM, Mushtaq M, Ismail L, Nadeem M, Azam MR (2014b) Synthesis of a new CO2 philic surfactant for enhanced oil recovery applications. J Dispers Sci Technol 35:647–654
Sagir M, Tan IM, Mushtaq M, Nadeem M (2014c) CO2 mobility and CO2/brine interfacial tension reduction by using a new surfactant for EOR applications. J Dispers Sci Technol 35:1512–1519
Sagir M, Tan IM, Mushtaq M (2014d) CO2 philic surfactant as possible mobility control agent in EOR applications. AIP Conf Proc 1621:699–704
Sagir M, Tan IM, Mushtaq M, Talebian SH (2014) FAWAG using CO2 philic surfactants for CO2 mobility control for enhanced oil recovery applications. Society of Petroleum Engineers 172189-MS
Sagir M, Tan I, Mushtaq M, Talebian S (2015) Static adsorption of new CO2 philic surfactant onto Berea sandstone. In: Awang M, Negash BM, Md Akhir NA, Lubis LA (eds) ICIPEG 2014. Springer, Singapore, pp 129–135
Simjoo M, Dong Y, Andrianov A, Talanana M, Zitha PLJ (2011) novel insight into foam mobility control. Presented at the international petroleum technology conference. Bangkok
Tsau J-S, Syahputra AE, Grigg RB (2000) Economic evaluation of surfactant adsorption in CO2 foam application. Society of Petroleum Engineers 59365-MS
Xing D, Wei B, McLendon WJ, Enick RM, McNulty S, Trickett K et al (2012) CO2-soluble, nonionic, water-soluble surfactants that stabilize CO2-in-brine foams
Yadav GD, Thathagar MB (2002) Esterification of maleic acid with ethanol over cation-exchange resin catalysts. React Funct Polym 52:99–110
Acknowledgments
Authors acknowledge the financial support of UTP and PETRONAS Research Sdn. Bhd. (PRSB) and the usage of EOR Centre of Excellence facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sagir, M., Tan, I.M., Mushtaq, M. et al. CO2 mobility control using CO2 philic surfactant for enhanced oil recovery. J Petrol Explor Prod Technol 6, 401–407 (2016). https://doi.org/10.1007/s13202-015-0192-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-015-0192-8