Abstract
The quantitative assessment of n-alkanes, asphaltenes and resins in five different crude oil samples: Escravos, Bonny Export, Penningston, Bodo (Nigerian crudes) and Bassrah (from Iraq) was successfully carried out using a new approach coded NAASAR, (n-alkanes, asphaltenes, aromatics and resins) comprising urea adduction followed by gas chromatographic analyses (for n-alkanes), n-heptane precipitation (for asphaltenes) and column chromatography (for resins). The results established the occurrence of n-alkanes ranging from n-C8H18 to n-C40H82 with total weight percentage n-alkane yields in the order: Bodo 47.41 > Bonny Export 32.47 > Penningston 30.75 > Bassrah 11.22 > Escravos 5.58. N-heptane precipitation showed that Bodo crude oil has the highest weight percent concentration of asphaltenes (7.31 %) and Bonny Export, the least (1.34 %). Bodo crude oil also has a higher percentage of resins (9.66) than Bassrah(3.77). 0API gravity, wt % of n-alkanes, asphaltenes, resins and the ratio of asphaltenes to resins were compared in two of the crudes: Bodo and Bassrah. The asphaltenes to resins ratio is one of the key parameters that control the stability of asphaltenemisceles in crude oils. This work demonstrated that Bassrah crude has a higher asphaltenes to resins ratio than Bodo crude. NAASAR method employed in this work is cost effective because it does not require sophisticated equipment as in SARA group type of analysis (saturates, aromatics, resins and asphaltenes) according to Fan and Buckley (2002). It requires small samples of crudes and solvents and also it is a reliable means of crude oil analysis when compared with other existing methods which require the use of fairly large samples and solvents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The problems encountered in oil production have been associated with heavy organics such as paraffin/wax, resins, asphaltenes, diamondoid, mercaptans and organometallic compounds in crude oil in various quantities and forms. Such compounds could precipitate out of the crude oil solution due to various forces causing blockage in the oil reservoir, in the well, in the pipelines and in the oil production and processing facilities (Mansoori 1995). Solid particles suspended in the crude oil may stick to the walls of the conduits and reservoirs. The toughness of the precipitates creates a lot of damage in the production and processing facilities whether there is asphaltene present in the crude oil even in minute quantities. Asphaltenes, which are highly polar compounds, could act as glue and mortar in hardening the deposits and, as a result, causing barrier to the flow of oil. Hence, this research is directed towards the determination of n-alkanes, resins and asphaltenes in various crudes as the economic implications of these problems are tremendous, considering the fact that a problem well workover for cleaning out asphaltene restrictions and restoration of well productivity could get as high as a quarter of a million dollar (Leontaritis and Mansoori 1988). The tackling of this problem is beset with a lot of shortfalls such as the unavailability of an adequate precipitation theory, lack of sufficient understanding of the process to adopt a rational approach to develop more effective dispersants and inhibitors. Unstable asphaltenes can form a separate phase that might plug the oil-bearing rock formation near a well. They can also aggregate at oil/water interfaces where they stabilize water-in-oil emulsions or at oil/solid interfaces where they can alter surface wetting properties or accumulate and plug well bores and flow lines. The first step towards predicting and avoiding any of these problems is knowing how to evaluate asphaltene stability.
The stability of asphaltenes depends on:
-
1.
the quantity of n-alkanes in the crude oil.
-
2.
the ratio of asphaltenes to resins in the crude oil, and
-
3.
the properties of asphaltene fractions.
Thus, in this research, there is reasonably good knowledge of quantitative composition of asphaltenes, resin and n-alkanes using NAASAR (n-alkanes, asphaltenes, aromatics and resins) method of crude oil analysis instead of using SARA method of analysis (saturates, aromatics, resins and asphaltenes) which may lead to loss of volatile material that contain saturates.
Urea adduction has been severally reported (Nwadinigwe and Eze 1990; Nwadinigwe and Nwobodo 1994; Mullinc 2007) as inexpensive and forms well-defined filterable channel complexes with n-alkanes. n-Heptane precipitation method has been extensively used to separate asphaltenes from crude oils (Buckley et al. 1998; Long 1981). The central part of the asphaltenemiscelle consists of high-molecular weight compounds surrounded and peptized by neutral resins of aromatic hydrocarbons (Hak-Hee kim et al. 1996). Asphaltenes separated with n-heptane are amorphous, shiny black solids. Column chromatography was used to separate the resins from deasphalted crude oil.
In this present study, n-alkanes, asphaltenes and resins have been successfully extracted from five different crudes as in Fig. 1 and the ratios of asphaltenes to resins compared. For example, it has been established through this work that the ratio of asphaltenes to resins is higher in Bassrah crude (1.254) than in Bodo crude (0.756), Table 3. This finding obviously lends support that the asphaltenes/resins ratio is not the only factor that affects the asphaltene or heavy organics deposition potential of a crude (Leontaritis and Mansoori 1988). Interplay of other parameters such as the ratio of aromatics to saturates, electrokinetics and polydispersivities might also be important.
Experimental
Sample collection
The five crude oil samples used for this study viz: Escravos, sourced from Western Niger Delta, Bodo, Bonny-Export, Penningston sourced from Eastern Niger Delta and Bassrah crudes (imported crude from Iraq) were obtained from the Research and Development Division of the Nigerian National Petroleum Corporation (NNPC), Port-Harcourt and Kaduna, Nigeria.
Some physical properties of the crude oils
The crude oil densities were determined with specific gravity bottles; viscosities were measured using the Ferranti portable viscometer. The viscosity units in poises were converted to mm2/s. In the urea-n-alkane adduction procedure, the weighed crude (Mettler H315) was poured into a reaction vessel and diluted with a calculated volume of dichloromethane (CH2Cl2) to obtain a viscosity of 1.5 mm2/s. The diluted samples were mixed at room temperature (30 °C) with an equal weight of urea activated with 4 % aqueous methanol (CH3OH). The mixture was stirred for 60 min at 1,400 rpm using a mechanical laboratory stirrer (Heldolph RZ-RI n-280-2200/35-250). The content was filtered with the suction pump. The residue, solid urea-n-alkane adduct was washed with benzene (100 ml) and then decomposed with warm water at 60 °C in a separatory funnel to give semi-solid/solid n-alkanes (upper layer) and an aqueous (lower) layer which was carefully drained off. The n-alkanes were melted, kept at 75 °C and mixed with 1 g of anhydrous granular calcium chloride (CaCl2) to remove traces of water. The hot mixture was then centrifuged at 2,000 rpm for 3 min to separate the n-alkanes from the CaCl2 XH2O precipitate. The decanted n-alkanes were cooled and weighed.
Analysis of the extracted n-alkanes by gas chromatography
Gas chromatography (GC) was performed using GC Model Agilent 6980 and HP-5 columns. The method of analysis was United States Environmental Protection Agency (USEPA) 8270. Injection volume was 3 μl with helium as the mobile phase and flame ionization detector (FID), injection and detection temperatures were 250 and 340 °C respectively.
Precipitation of asphaltenes
250 cm3 of the filtrate obtained after extraction of n-alkanes using urea adduction method was distilled at the temperature of 230 °C. 40 cm3 of heavy organic-rich residue was obtained. N-heptane was added in the ratio of 30 ml of n-heptane to lg of the crude oil residue. It was thoroughly agitated on a hot plate with suitable stirrer until solubilized. The flask was properly covered and kept in the refrigerator at a temperature of 4–6 °C for 48 h (normally this is sufficient time for flocculation). The centrifuge tubes were labeled and weighed. The sample was transferred into the centrifuge tubes and the flasks rinsed 2–3 times with 10 ml of n-heptane. The samples were centrifuged for 30 min to obtain n-C7asphaltene (n-heptane insoluble fraction) which was shiny black amorphous solid. The solvents were decanted. The resulting asphaltenes were dried in an oven at 105–110 °C for 30 min. The weights of different fractions were determined.
The Separation of resins from crude oil by column chromatography
The deasphalted crude oil was concentrated by heating at 40 °C to 25 ml. The weights of the two crudes, Bassrah (Iraq crude) and Bodo (Nigerian crude) were determined. Silica gel was activated at 240 °C for 4 h. The activated silica gel was packed into a glass column (150 × 1.5 cm, id). Dichloromethane/methanol (1:1) was used as a mobile phase to elute the resins at an elution rate of 1.5 cm3/s. The solvents were evaporated on a water bath at 40 °C after which the samples were centrifuged at 1,500 rpm for 30 min followed by decantation to obtain the resins.
Results and discussion
The results of some physical properties (viscosities, densities, 0API gravity and kinematic viscosities) for the five crudes are shown in Table 1. Crude oils with 0API of 40–450 are classified as very light crudes, 34–390 as light crudes, 22–330 as medium crudes and less than 220 as heavy crudes (Tissot and Welte 1984). Bassrah crude proved to be the heaviest. The calculated 0API gravity shows that Escravos, Penningston, Bonny Export are light crudes while Bodo and Bassrah crudes are medium crude oils.
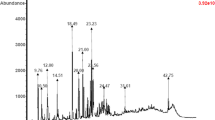
Gas chromatographic analysis data of the n-alkanes extracted by urea adduction are presented in Table 2 and Figs. 2, 3, 4, 5, 6 and also represented as a bar chart in Fig. 7. The results of the total concentration of n-alkanes in Fig. 7 show that Bassrah crude has the highest concentration of n-alkanes in mg/l while Bodo crude oil has the least concentration of n-alkanes. The values of pristane/phytane (Pr/Ph) ratios: 1.01, 2.41, 1.08, 1.48 and 1.51 for crudes from Escravos, Penningston, Bonny Export, Bodo and Bassrah, respectively, obtained from the chromatograms (Figs. 2, 3, 4, 5, 6) as represented in Fig. 8 were indicative of source rock and depositional environment. Penningston crude with the highest pr/ph ratio (2.41) is consistent with source rock deposited in a sub-oxle to oxle environment according to Shangunam (Shangunam1985).
Table 3 displays oAPI, wt % of n-alkanes, asphaltenes, resins and asph/resin ratios. The results show that Bodo crude has the highest wt % of n-alkanes which characterize it as a light crude. The highest weight percent of n-alkanes in Bodo crude makes it to precipitate more asphaltenes according to Nellensteyn (Nellensteyn 1924) than in Bassrah crude. Petroleum resins provide a transition between the polar (asph) and the relatively non-polar (oil) fractions in petroleum, thus preventing the assembly of polar aggregates that would be non-dispersible in the oil (Dickie and Yen 1967). The result of 0API gravity, lower n-alkanes weight percent, higher ratio of asph\resin in Bassrah crude are indicative that Bassrah crude is more viscous than Bodo crude oil. The increase in viscosity of Bassrah crude as a result of higher asph/resin ratio shows that the asphaltene is not fully dispersed and forms large-sized aggregates. The ratios of asphaltenes to resins in Bodo crude oil and Bassrah crude oil were compared and it was found that the Bodo crude oil has the lower asphaltenes to resin ratio than Bassrah crude. Therefore, Bassrah crude oil with higher asph/resin ratios is more prone to heavy organic deposition. Crude oils with higher asphaltene to resin ratios are usually more prone to heavy organics deposition but this is not the only factor to be considered in evaluating the deposition potential of a crude (Leontaritis and Mansoori 1988) as shown by the result of this work.
Conclusion
The quantitative assessment of N-alkanes, asphaltenes and resins from crude oils and also the gas chromatographic analysis of the n-alkanes were successfully effected from crude oils using the NAASAR method at lower cost and short-cut than those routinely employed in SARA analysis. The major but quite important advantage of NAASAR method of crude oil analysis over SARA (Ali and Notal 1994) is that, in NAASAR method, n-alkanes are obtained directly from diluted crude by urea adduction procedure but in SARA analysis one has to get the saturates first before n-alkanes extraction and there may be loss of volatile material that contains saturates. From the results obtained, the quantity of n-alkanes in the crude oil and the ratio of asphaltenes to resins were determined which are the factors on which the stability of asphaltenes depends apart from the properties of the asphaltene fractions. Therefore, NAASAR method of crude oil analysis is very important to evaluate this heavy organic deposition problem on oil production and processing by determining the factors above, in which the asphaltenes stability depends.
References
Ali MA, Notal WA (1994) Fuel Sci Tech Int 12:21–33
Buckley JS, Hirasaki GJ, Liu Y, Von Drasek S, Wang JX, Gill BS (1998) Asphaltene precipitation and solvent properties of crude oils. Pet Sci Technol 16(3–4):251–285
Dickie JR, Yen TF (1967) Macro structures of the asphaltic fractions by various instrumental methods. Anal Chem 39:1847
Fan T, Buckley JS (2002) Rapid and accurate SARA analysis of medium gravity crude oils. Energ Fuels 16:1571–1575
Kim Hak- Hee, Chung Kwang- Bo, Kim Myung-Nyn (1996) Measurement of the asphaltene and resin content of crude oil. J Ind Eng Chem 2(1):72–88
Leontaritis KJ, Mansoori GA (1988) Asphaltene deposition: a survey of field experiences and research approaches. J Pet Sci Eng 1:229
Long RB (1981) The concept of asphaltenes. In: Bunger JW, Li NC (eds) Chemistry of asphaltenes. American Chemical Society, Washington, DC, pp 17–27
Mansoori GA (1995) Arterial blockage in the petroleum and natural gas industries-heavy organics (asphaltene/bitumen, resin, organometallics, paraffin/wax, diamondoids, etc) deposition from petroleum fluids. http://www.uic.edu/~mansoori/HOD_html
Mullinc OC (2007) Asphaltenes, heavy oils and petroleomics. Springer, New york
Nellensteyn FJ (1924) The constitution of Asphalt. J Inst Petrol Technol 10:311
Nwadinigwe CA, Eze SO (1990) Deparaffination of light crudes through urea n-alkane channel complexes. Fuel 69:126–127
Nwadinigwe CA, Nwobodo IO (1994) Analysis of n-paraffins in light crudes: molecular sieve and urea adduction techniques revisited. Fuel 73(5):779–782
Shangunam G (1985) Significance of coniferous rain forests and related oils, Australia, American Association of Petroleum Geologists. Bulletin 69:241–1254
Tissot BP, Welte DH (1984) Petroleum formation and occurrence, 2nd edn. Springer, Berlin, Heidelberg, New York, Tokyo, pp 370–478
Acknowledgments
The authors gratefully acknowledge Nigerian National Petroleum Corporation (NNPC) Kaduna and Port Harcourt for the supply of crude oil samples and FUGRO Consultants Nig. Ltd, Port-Harcourt, for the gas Chromatographic (g.c) analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nwadinigwe, C.A., Alumona, T.N. NAASAR procedure for quantitative assessment of n-alkanes, asphaltenes and resins in crudes. J Petrol Explor Prod Technol 5, 383–390 (2015). https://doi.org/10.1007/s13202-014-0145-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-014-0145-7