Abstract
This study investigates the potential of cobalt ferrite-doped mango seed shell (CoFe2O4-MSS) as an innovative and eco-friendly approach for the treatment of crude oil-polluted water. CoFe2O4-MSS was synthesized by impregnating carbonized mango seed shell with cobalt ferrite nanoparticles through thermal precipitation. The study systematically evaluated the adsorption capacity, kinetics, and isotherm behavior of the developed material using standard equations. Experimental results demonstrate the effectiveness of the cobalt ferrite-doped mango seed shell in adsorbing crude oil components, with high removal efficiency of 98.3% at 80 °C after 50 min. The crystallite sizes of raw mango seed shell and CoFe2O4-MSS are 31.8 nm and 21.3 nm, respectively. The calculated adsorption capacity stood at 55.50 mg/g, the Brunauer–Emmett–Teller surface area of CoFe2O4-MSS was 1007.4m2/g with a porosity distribution of 1.685 \(\eta\) while the volume and pore diameter are 3.104m3/g and 7.212 nm, respectively. FTIR analysis revealed the presence of aliphatic, aromatic, and silicon compounds. The isotherm data matched well with Langmuir isotherm model while kinetic data fitted well with Bhattacharya–Venkobachar model. The unique properties of cobalt ferrite, a magnetic and iron-based material, combined with the abundant and biodegradable nature of mango seed shells, make this composite an attractive adsorbent for removing crude oil contaminants. This research contributes to the development of sustainable and cost-effective solutions for addressing environmental challenges posed by crude oil pollution. Also, this research has contributed immensely to the sustainable development goals of the united nation (UN-SDG) regarding environmental protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Generally, crude oil pollution is inevitable in most oil-producing countries of the world. The Niger Delta, a region in Nigeria, is known for its vast oil reserves and has been a significant contributor to the country's economy. However, the process of crude oil exploration has led to severe environmental pollution, causing detrimental effects on the region's ecosystem, inhabitants, and overall quality of life (Ekwueme et al. 2022; Uzoije et al. 2011; Reza et al. 2013). Crude oil exploration involves drilling, pipelines, and storage facilities, which often lead to oil spills and leaks. These incidents contaminate the soil and water sources, rendering them unfit for agricultural purposes and human consumption (Behnood et al. 2013; Angelova et al. 2011). The loss of arable land and clean water poses a significant threat to the livelihoods of the local communities, who heavily rely on fishing and farming for sustenance. Underground pipe breakage, social unrest, tanker accident, and careless handling by the operators in oil industries are also included in the identified causes of crude oil spillage (Uzoije et al. 2011; Kharoune et al. 2001; Gwendoline 2010). The burning of gas flares during the oil extraction process releases greenhouse gases and other toxic substances into the atmosphere. This air pollution not only exacerbates climate change but also poses severe health risks to the residents, including respiratory problems and increased vulnerability to diseases (Uzoije et al. 2011). The destruction of natural habitats due to oil exploration activities has led to the displacement of various plant and animal species. Some species may face extinction due to the loss of their ecosystems, while others may struggle to adapt to the altered environment. The contamination of soil and water sources has led to a decline in agricultural productivity, affecting the livelihoods of many farmers in the Niger Delta region (Ekwueme et al. 2022; Asadu et al. 2022a, 2022b). This loss of income can lead to increased poverty and food insecurity, and this is not good for the entire country, especially now that Nigeria wants to make agriculture her mainstay of the economy.
Crude oil pollution in water bodies poses a significant threat to aquatic life, ecosystems, and human health. Remediation of crude oil-contaminated water is essential to restore the health of these ecosystems and minimize the adverse effects on living organisms, and this has led researchers to find the best and effective ways to arrest the situation in order to sustain agricultural activities. Various techniques have been developed to address this issue, and some of the most effective ones are: mechanical skimming (Awual et al. 2019a), in situ burning (Ladhe et al., 2011; Awual et al. 2019b), use of dispersants ((Awual et al. 2019a), bioremediation (Kharoune et al. 2001), controlled drainage (Kumar, 2006), and adsorption method (Nwabanne et al. 2018; Thompson et al. 2020; Suidan et al. 2005; Ayotamuno et al. 2006; Yang et al. 2006). Comparative analysis of all these methods showed that they are not economic and environmentally friendly except adsorption process (Uzoije et al. 2011; Baars, 2002). Adsorption involves the use of materials, such as activated carbon, zeolites, or clay minerals, to bind and remove oil and other pollutants from contaminated water (Utsev et al. 2020; Iwar et al. 2021a, 2021b, 2022). This process can be applied on a large scale using floating barriers or in a laboratory setting for water treatment. Adsorption is an effective method for removing both dissolved and emulsified oil from water surface.
Modified biomass adsorbents are derived from natural, renewable, and organic sources, such as agricultural wastes. This makes them more sustainable and environmentally friendly compared to conventional adsorbents, which may be derived from non-renewable sources or involve synthetic processes, and they are mostly non-biodegradable (Awual et al. 2020; Naema et al. 2014; Olufemi et al. 2014). Modified biomass adsorbents are generally more cost-effective and abundant, as they can be obtained from waste materials or by-products of other industries. Conventional adsorbents, on the other hand, might be more expensive and less readily available due to their synthetic and non-renewable origins (Albert et al. 2016; Chinonye et al. 2018; Pelissari et al. 2014). Modified biomass adsorbents are considered due to their natural origin and potential for recycling or reuse. They also have a lower carbon footprint compared to some conventional adsorbents, and this informed the choice of mango seed shell as biomass for the synthesis of the adsorbent. To improve the adsorption ability of mango seed shells, they can be modified through processes like activation, surface functionalization, or by incorporating other adsorbent materials. These modifications can enhance the surface area, porosity, and chemical functionality of the mango seed shells, leading to better adsorption performance.
Recently, there has been a significant rise in research and development of advanced adsorbent materials for various environmental and industrial applications. Among these, the utilization of waste materials and biomass for the synthesis of eco-friendly adsorbents has gained substantial attention (Behnood et al. 2016; Thompson et al. 2020). One such promising adsorbent is the cobalt ferrite (CoFe2O4)-doped mango seed shell (MSS) nanoparticle. This research aims to produce a hybrid nanoparticle adsorbent by reacting cobalt ferrite with calcined mango seed shell to produce the cobalt ferrite-doped mange seed shell nanoparticle adsorbent (CoFe2O4-MSS) for efficient removal of crude oil from polluted water to meet the aspirational target of the UN-SDG regarding environmental management and protection.
The synthesis of cobalt ferrite-doped mango seed shell nanoparticles involves a series of steps. Firstly, the mango seed shells are collected, washed, and dried. Then, they are subjected to a calcination process at a specific temperature to remove the organic matter and obtain pure MSS. Subsequently, cobalt ferrite nanoparticles are synthesized using a co-precipitation method Gayathri and Raji 2019; Awual et al. 2019; Awual et al. 2020), followed by doping them onto the MSS surface. This process results in the formation of cobalt ferrite-doped mango seed shell nanoparticles. Metallic salt-doped biomass nanoparticles exhibit unique physicochemical properties due to the synergistic effects of both metallic salts and the biomass (Gayathri and Raji 2019; Awual et al. 2019). The doped CoFe2O4 nanoparticles contribute to the magnetic properties of the composite, enabling easy separation and removal of the crude oil layers by the adsorbent.
Materials and method
Among mango seed shell (MSS), stearic acid, crude oil, distilled water, NaOH, iron (III) chloride hexahydrate (FeCl3.6H2O, ≥ 98%), cobalt nitrate hexahydrate (Co (NO3)3.6H2O, 99%), H2SO4, HCl, and sieving net, MSS was sourced from local farmers, crude oil was gotten from Agip oil facility in Bayelsa State Nigeria while the other reagents were sampled from local market. MSS was cleaned by removing dirt, dust, or debris using a sieve and washing them gently under running water and thereafter spread on a clean surface and allowed to dry naturally under the sun for five days. The dried seed shell size was reduced into uniform size using a mechanical grinder and 50 µm mesh size.
Carbonization of the mango seed shell (MSS)
Process as given by Iwar et al. (2022) was adopted with little modification. Deionized water was used to remove leftover debris from the MSS and thereafter oven-dried (AGT-210I-JP (75L)) at 105 °C for 1. 5 h. The MSS was carbonized in the absence of oxygen in a muffle furnace (KWR 24/10 No: 22–21,345) at a temperature range 600 °C for 3 h. This temperature-controlled heating leads to the decomposition of organic matter, volatilization of impurities, and formation of carbonaceous material (biochar). The choice of temperature and heating rate plays a vital role in determining the adsorption properties of the resulting carbon (Utsev et al. 2020; Onwu et al. 2019a, b).
Preparation of cobalt ferrite-doped mango seed shell nanoparticle composite (CoFe2O4-MSS).
The procedures explained by Gayathri and Raji, (2019) was adopted with little modification. 6.1 g of iron (III) chloride hexahydrate (FeCl3.6H2O) and 3.6 g of cobalt nitrate hexahydrate (Co (NO3)3.6H2O) were added in a beaker containing 50 mL of deionized water. The solutions were then agitated for 20 min using magnetic stirrer. 20 g of carbonized mango seed shell was added to the metal nitrate solution and stirred for 50 min. In order to raise the pH of the solution up to 10, 30% NH4OH solution was added. After that, it was heated to 170 °C for 11 h in a hydrothermal vessel lined with Teflon. The solution was then filtered, and it was thoroughly dried. The composite powder was washed severally with deionized water. Lastly, the composite was sieved to obtain fine cobalt ferrite-doped mango seed shell nanoparticles (CoFe2O4-MSS).
Characterization of the raw MSS, carbonized MSS, and CoFe2O4-MSS
The synthesized CoFe2O4-MSS was characterized using various techniques such as X-ray diffraction (XRD), for phase identification, scanning electron microscopy (SEM) (model 302 Hitachi (CIQTEK model 3200, Japan), for morphological analysis, and Fourier transform infrared spectroscopy (FTIR) (BUCK model 500 M infrared spectrophotometer), for functional group identification. The American Society for Testing and Materials standard methods were used to analyze various properties of the adsorbents according to Didem (2012). Adsorption/desorption method was employed in determining the surface area, pore size distribution, and porosity of the composite material using the BET method as described by Onu et al., (2023) and Asadu et al., (2021). XRD, XRF, and BET analysis was done in the Faculty of Natural Sciences, Department of Chemistry, Walter Sisulu University, South Africa.
Demonstration of crude oil removal by raw MSS, carbonized MSS, and CoFe2O4-MSS
With minimal changes, the approach outlined by Ekwueme et al. (2022) and Onu et al. (2023) was used. In a 200-ml beaker, 100 mg/l of crude oil was created by combining water and crude oil for six minutes at room temperature. After standing for 25 min at 20 °C with a sieve net and keeping the pH at neutral, 0.4 g of CoFe2O4-MSS was added to the mixture, stirred, and separated. A vacuum drier was used to dry the adsorbent, and the weight was then recorded. The studies were conducted again using different oil–water concentrations of 200 mg/l, 300 mg/l, 400 mg/l, and 500 mg/l, as well as dosages of 0.8, 1.2, 1.6, and 2.0 g, and temperatures of 40, 60, 80, and 100 °C. Weight after adsorption and pre-weight of the adsorbent were used to calculate the concentration of oil absorbed by the adsorbent. Equation (1) is used to calculate the experiments adsorption capacity (qe), and Eq. (2) is used to calculate the amount of oil eliminated (Nwabanne et al. 2017; Ike et al. 2022; Chinonye et al. 2018).
Percentage removal was also calculated according to Eq. (2)
Isotherm modelling of oil removal by CoFe2O4-MSS
Isotherm studies play a crucial role in understanding the behavior of crude oil sorption onto various adsorbents. These studies help in determining the optimal adsorbent for specific applications, such as oil spill remediation or enhanced oil recovery (Asadu et al. 2022a, 2022b; Banerjee et al. 2006). The isotherm is a graphical representation of the equilibrium relationship between the amount of crude oil adsorbed onto an adsorbent and the concentration of crude oil in the surrounding environment at a constant temperature (Ekwueme et al. 2022). Several isotherm models have been developed to describe the adsorption process such as few under studies listed in Table 1. Each model has its own assumptions and limitations. By plotting the experimental data and fitting the results to the isotherm models in Table 1, model parameters was obtained, which provide insights into the adsorption behavior and performance of the CoFe2O4-MSS.
Model confirmation analysis.
Error analysis was employed to validate the performance of every model, as indicated in Table 1. The graphical plot was the standard by which earlier researchers judged which model best fit the data; however, this study employed additional equations, as indicated in Table 2, to bolster the model assessment where the adsorption capacities from laboratory tests and the adsorption capacities predicted by the isotherm models are denoted by the terms q(e,exp) and q(e,cal). P is the number of isotherm parameters or factors, and n is the number of experimental runs conducted.
Kinetic studies of oil removal by CoFe2O4-MSS
Kinetic studies of crude oil sorption onto adsorbents are essential in understanding the rate and mechanism of crude oil adsorption onto various adsorbent materials. These studies help in optimizing the design and selection of adsorbents for efficient crude oil recovery, environmental remediation, and pollution control (Banerjee et al. 2017; Ike et al. 2022). Six kinetic models have been selected to describe the adsorption process as shown in Table 3. Kinetic studies of crude oil sorption onto adsorbents aim to determine the rate at which the oil molecules are adsorbed onto the adsorbent surface.
Material and Method
Physicochemical properties of raw MSS and CoFe2O4-MSS
Table 4 shows the physical properties of the materials used. Energy of adsorption was found to have improved meaningfully after fusing together raw MSS and cobalt ferrite from 1.031 kj/mol to 5.321 l kj/mol, signifying more than a 300% increase. Energy of adsorption is a crucial concept in the field of surface chemistry and is closely related to the adsorption process that occurs when molecules, ions, or atoms from an adsorbate (crude oil) bind onto the surface of the adsorbent (Liu et al. 2019). According to Arica et al. (2019), the adsorption energy quantifies the strength of the interactions between the adsorbate and adsorbent and is a vital parameter in understanding and predicting the behavior of adsorption systems. Therefore, it can be deduced that the attraction forces that bind oil molecules onto the pores are higher in CoFe2O4-MSS than in raw MSS, indicating the superiority of CoFe2O4-MSS over raw MSS in removing crude oil which is in concurrence with the opinion of Arica et al. (2019).
The BET technique helps in determining the pore size distribution, surface area, and adsorption capacity of an adsorbent material. Table 4 and Fig. 1 show that the surface area of raw MSS increased from 298.4 m2/s to 1007.4m2/s after fusing with cobalt ferrite indicating a significant increase in the surface area. A higher surface area generally indicates a greater adsorption capacity for the adsorbents according to Onu et al., (2021); Chinonye et al. (2018) and Ezenwa et al., (2019).
Following the modification of raw MSS, there was a notable increase in both the pore radius and pore distribution. The pore diameter increased from 4.12 to 7.212 nm, the pore radius increased from 0.25 to 1.36 Å, and the pore volume increased from 0.012 to 3.104 m3/g. The diffusion of adsorbate (crude oil) onto the adsorbents is improved by increased pore size, volume, and distribution, according to Shokrollahi et al. (2011) and Onu et al. (2021). The microporous radius can impact the accessibility of the adsorption sites within the adsorbent structure. Smaller microporous radii may lead to more accessible adsorption sites, allowing for easier diffusion of adsorbate molecules into the pores as depicted by Iwar et al. (2022).
The disappearance of volatile organics during the heat treatment of the raw MSS and the fusing together of the carbonized MSS with cobalt ferrite salt with greater surface area is another factor contributing to the increase in the BET surface area. Additionally, at a pH of 6.88, a porosity distribution of 1.685 was observed on the CoFe2O4-MSS surface, suggesting a partial dominance of positively charged ions. The bulk density increased to 0.889 g/ml from 0.014 g. This is important because higher bulk densities can lead to higher adsorption capacities because of the increased surface area that is available for adsorption. The moisture content of the CoFe2O4-MSS dropped from 13.46% in raw MSS to 2.29% during activation, as was predicted. Furthermore, heat treatment caused a notable increase in the fixed carbon (33.86 to 82.88) % and a proportionate decrease in the volatile matter (31.13 to 11.45) %. As shown by Ekwueme et al., (2022), this may be explained by the inorganic contents in the biomass disappearing during the heat treatments. Pore diameter on the surface of an adsorbent is a crucial factor that significantly influences the adsorption process. The pore size distribution in these materials plays a vital role in determining their efficiency in various applications. These large pores have a relatively lower surface area, but are essential for the transport of adsorbate molecules within the adsorbent structure. They act as channels that allow the diffusion of molecules and particles throughout the adsorbent, ensuring efficient mass transfer. The pore diameter on the surface of an adsorbent can be controlled during synthesis by adjusting parameters like temperature, pressure, and precursor concentration.
SEM analysis of raw MSS and CoFe2O4-MSS
Scanning electron microscopy (SEM) is a powerful analytical technique used to visualize and analyze the surface morphology and topography of various materials, including adsorbents (Onwu et al. 2019a, 2019b). The SEM image of the raw MSS at 1500X amplifications is shown in Fig. 2a. The raw MSS adsorbent exhibits a rough and porous surface with irregularly shaped particles. The presence of numerous pores and crevices on the surface increases the available adsorption sites, which in turn enhances the adsorption capacity of the material. Moreover, the surface roughness and porosity facilitate the penetration and diffusion of crude oil into the adsorbent matrix, promoting efficient removal of crude oil from contaminated water.
Figure 2b shows the SEM image of CoFe2O4-MSS. SEM image analysis of cobalt ferrite-doped mango seed shell adsorbent reveals significant changes in the surface morphology compared to the undoped mango seed shell adsorbent in Fig. 2a. The incorporation of cobalt ferrite nanoparticles results in a more uniform and smoother surface with reduced porosity. This can be attributed to the interaction between the cobalt ferrite nanoparticles and the mango seed shell matrix, leading to the formation of a more compact structure. Despite the reduced porosity, the cobalt ferrite-doped mango seed shell adsorbent still retains a certain degree of surface roughness and irregularity, which can contribute to the adsorption process. The presence of cobalt ferrite nanoparticles also provided additional adsorption sites and enhanced the overall adsorption capacity of the composite.
Fourier transform infrared (FTIR) of raw MSS and CoFe2O4-MSS
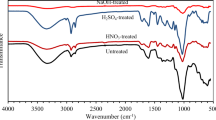
FTIR spectroscopy is an analytical technique used to study the molecular composition and functional groups present in various materials (Ike et al. 2022; Onu et al. 2023). Figure 3a and b depicts the analysis of the FTIR for raw MSS and CoFe2O4-MSS. O–H stretching was observed between 3183.1 and 3511.2 cm−1 while it was observed between 3133.5 and 3751.4 cm−1 in CoFe2O4-MSS (see Fig. 3a and b). This peak represents the presence of hydroxyl groups (O–H) in the MSS adsorbent. These hydroxyl groups play a significant role in adsorption processes due to their hydrogen bonding capabilities (Chinonye et al. 2018; Asadu et al. 2022a). C–H stretching was observed between 2851.4 and 2918.4 cm-1 in raw MSS while it was observed between 2751.4 and 2923.3 cm−1 in CoFe2O4-MSS. The presence of C–H stretching peaks indicates the presence of aliphatic and aromatic hydrocarbon groups in the adsorbents. These groups contribute to the overall structure and stability of the adsorbents (Ekwueme et al. 2022; Nwabanne et al. 2017). C = O stretching was observed between 1713 and 1990.4 cm−1 in raw MSS while it was observed between 1990.4 and 2113.4 cm−1 in CoFe2O4-MSS. The presence of carbonyl groups (C = O) in the adsorbents can be attributed to the presence of cellulose, hemicellulose, and lignin (Asadu et al. 2022b; Albert et al. 2016). These groups participate in various chemical reactions and contribute to the adsorption capacity of the raw MSS and CoFe2O4-MSS. C–O stretching was observed between 1375.4 and 1449.9 cm−1 in raw MSS while it was between 1364.7 and 1456.8 cm−1 in CoFe2O4-MSS. The presence of ester, ether, etc. was identified through these peaks. Nonetheless, silicon ozy compounds Si–O–Si and aromatic C–H were in plane bend and were located at 1375.4 cm−1 in raw MSS, but was found at 1364.7 cm−1 in CoFe2O4-MSS. However, the C–Cl were found to be between 700 and 900 cm−1 in raw MSS adsorbent.
X-ray fluorescence (XRF) of raw MSS and CoFe2O4-MSS
XRF is a nondestructive analytical technique used to determine the elemental composition of materials. According to Nick et al. (2021), this information may help determine the chemical composition of the shell, which may provide light on some of its possible uses or characteristics. Data presented in Table 5 showed that the main compounds found in raw MSS were alumina (20.96%), silica oxide (quartz) (40.19%), calcium oxide (8.79%), sodium oxide (8.03%), iron oxide (5.27%), and magnesium oxide (12.34%). Other minerals were present in trace amounts. Following the doping of MSS with cobalt ferrite, certain alterations in the mineral composition were noted. The percentage concentration of iron oxide increased to 10.10%. This could be as a due to addition of ferrite oxide. It was also observed that the percentage composition of silicon oxide remains relatively unchanged. While there is no presence of cobalt oxide in raw MSS, there appears 7.36% of cobalt oxide in CoFe2O4-MSS. This might be explained by the impact of cobalt ferrite doping of MSS. The high proportion of quartz in MSS and CoFe2O4-MSS, as shown in Table 4, could be the reason for the material has high chemically active surface area. This result validates the claims of Behnood (2013) and Bulut (2006) that quartz has a Mohs hardness of seven, which makes it incredibly resilient, inert, and heat resistant. It also preserves the micropores within the surface of the adsorbents and keeps its islands, fissures, cleats, and veins during heat treatments.
XRD analysis of CoFe2O4-MSS and raw MSS
XRD was used in this work to study the crystal structure and phase composition of materials in raw MSS and CoFe2O4-MSS as shown in Fig. 4a and b. Structural changes observed in the raw MSS spectrum could be due the effect cobalt ferrite incorporated into the mango seed shell, which changes its adsorption properties and pattern. In an X-ray diffractometer, the intensity of the scattered X-rays is measured as a function of the scattering angle (2θ). It is a common practice to use X-ray diffraction to reveal information about a substance's crystalline structure. According to Thompson et al. (2020), the XRD patterns show that the samples are crystalline and contain diffracting peaks to differing degrees and intensities. According to the raw MSS and CoFe2O4-MSS crystallographic parameters, it was confirmed at 2θ at alpha 90°, beta 90°, and gamma 120° that it was hexagonal. The spectrum shows that the XRF analysis in Sect. "X-ray fluorescence (XRF) of raw MSS and CoFe2O4-MSS" is consistent with the dominant structure of quartz. Prominent peaks with varying intensities that appear at nearly the same 2θ angle for both composite adsorbents are visible in the XRD spectra. This suggests that there are minor variations in the raw MSS and CoFe2O4-MSS levels of crystallinity. Das and Mondal (2011) noted similar findings regarding the crystalline nature of various coconut shells sourced from various sources. Equation (20) is used to calculate the standard crystallite size (D) of the adsorbents. The Scherrer’s equation according to Lin et al., (2006) was given as:
where the K is the Scherrer constant, λ is the wavelength, β is the XRD peak width at half peak height while θ is the diffraction angle as defined by Lin et al., (2006).
A decrease in the peak intensity was observed between the CoFe2O4-MSS and the raw MSS. This might be attributed to the integration of cobalt ferrite onto MSS in the composite CoFe2O4-MSS, which is explained. It can also be as a result weaker bond between the molecules in the composite and an increase in the adsorbent's surface area brought on by the cobalt ferrite nanosize. For the raw MSS and the CoFe2O4-MSS, the measured crystalline diffraction peak angles are 78.49° and 65.1°, respectively. Using OriginPro, the peak width at half peak height (β) was calculated. According to Scherer's equation, the crystallite sizes of raw MSS and CoFe2O4-MSS are 31.8 nm and 21.3 nm, respectively. The process of doping the MSS with cobalt ferrite salt may be the cause of the decrease in the crystallite size of the CoFe2O4-MSS when compared to the raw MSS, which is highly consistent with the postulate of Albert et al. (2016).
Effect of the process factors on crude oil removal by different adsorbents
Effect of adsorbent dosage
Effect of adsorbent dosage on the removal of oil layer from water surface using raw MSS, carbonized MSS and CoFe2O4-MSS was demonstrated as shown in Fig. 5. This was studied between adsorbent dosage of 0.4 g and 2.0 g at a constant temperature of 80 °C, time of 50 min, concentration of 100mgL, and pH of 4. Figure 5 shows that as the dosage increases, the amount oil layer removed (%) also increased. This is very much in agreement with the reports by Nwabanne et al., (2018); Onwu et al., (2019a), which states that at low dosages of the adsorbent, the oil layer may not be efficiently removed from the water because there is not enough adsorbent surface area available to hold and remove the oil particles, but at optimal dosage, as the dosage increases, the efficiency of oil removal from water also increases because more adsorption sites become available on the adsorbent surface, allowing for greater interaction with the oil particles. At this stage, the adsorbent is able to effectively capture and hold the oil, reducing the oil layer in the water. It was also observed that at saturation point, which is beyond a certain dosage, the efficiency of oil removal even decreases. This supports the claim by Reza et al., (2013) and Asadu et al., (2021) that this occurs when the adsorbent becomes saturated with oil particles, meaning there are no more available adsorption sites. At this point, adding more adsorbent will not significantly improve the oil removal process, indicating that excessively high dosages of the adsorbent may lead to issues such as increased sludge production, difficulty in separating the adsorbent from the treated water, and potential environmental concerns due to the excess adsorbent. Also, CoFe2O4-MSS was found to have performed better than raw MSS and carbonized MSS. This could be credited to the increase in the surface area of the adsorbent occasioned by doping of raw MSS with cobalt ferrite nanoparticles. The optimum percentage removal was found to be 96.3% with adsorbent dosage of 2 g.
Effect of temperature
The performance of the adsorbents under the ranges of temperatures between 20 and 100 °C (see Fig. 6). Keeping other factors constant, it was observed that optimum adsorption temperature was 80 °C with 98.3% of oil layer removal with CoFe2O4-MSS adsorbent. Figure 6 depicts as well that an increase in the temperature leads to an increase in the percentage of oil layer removal. The trend agrees with the works by Asadu et al., (2021); Ezenwa et al., (2019), and Onu et al., (2021), where they stated that as the temperature increases, the kinetic energy of the oil molecules also increases, which can lead to a higher rate of adsorption but also increasing the temperatures can also cause the oil molecules to desorb from the adsorbent surface, potentially reducing the overall efficiency of the process if properly not controlled. Therefore, an optimal temperature must be determined for the best oil–water separation performance. Figure 6 also reveals the superiority of CoFe2O4-MSS over carbonized MSS and raw MSS with 98.3% removal when carbonized MSS and raw MSS are 56.7% and 52.4% at the same process conditions. Improved performance of CoFe2O4-MSS over others can still be ascribed to the greater surface area occasioned by fusing of cobalt ferrite nanoparticles onto raw MSS.
Effect of crude oil concentration
Figure 7 depicts the effect of concentration on adsorption of crude oil onto the various adsorbent. This was studied using concentrations of crude oil in water ranging between 100 and 500 mg/L while keeping other factors constant. Figure 7 shows that as the concentration of crude oil in water increases at constant dosage of the adsorbent, the percentage removal decreases significantly. The maximum removal of crude oil occurred at 100 mg/L with 96.8% removal using CoFe2O4-MSS adsorbent. Carbonized MSS and raw MSS showed similar trends even though greater performance occurred with CoFe2O4-MSS. This result agrees with the reports by Ekwueme et al., (2022); Arivoli et al., (2019); Asadu et al., (2018), and Nwabanne et al., (2017) where they stated that if the concentration of crude oil becomes too high, it may cause the adsorbent to become saturated. When this happens, the adsorbent will reach its maximum adsorption capacity, and any additional crude oil molecules will not be adsorbed. However, Ekwueme et al., (2022) and Ike et al., (2022) further explained that, initially, as the concentration increases, the adsorption rate might increase due to the higher number of available hydrocarbon molecules. But at equilibrium point, the adsorption rate will decrease, as there will be no more available adsorption sites (Fig. 7).
Effect of pH
The effect of pH on crude oil removal was studies between the pH value of 2 and 10 at constant temperature, concentration, time and dosage as demonstrated in Figure 8. It can be seen that greater performance by the adsorbents occurred at pH 4 with highest percentage removal of 97.6%. The same pattern occurred with other adsorbents. At the initial stage of the experiments, there was a sharp rise in percentage removal between pH 2 and 4 with highest percentage removal by the three adsorbents being recorded at pH 4, but decreased continuously till pH 10 thereafter. This could be ascribed to the fact that, at low pH, the surface of the adsorbents become positively charged due to protonation of functional groups and this can lead to electrostatic attraction between the adsorbents and negatively charged crude oil components, thereby increasing the adsorption capacity and percentage removal, and this agrees with the reports by Ekwueme et al. (2022) and Ike et al. (2022)
Modelling of crude oil layers adsorption onto CoFe2O4-MSS
Adsorption isotherms are crucial in characterizing the adsorption capacity and selectivity of an adsorbent material. It is a fundamental prerequisite for the design of any adsorption system, as according Utsev et al. (2020), Chinonye et al. (2018), Iwar et al. (2021a), and Nwabanne et al., (2018). It is a relationship between the concentration of a substance at constant temperature and the amount of the material adsorbed from the liquid phase by unit mass of the adsorbents (Iwar et al. 2021a, 2021b; Albert et al. 2016; Utsev et al. 2020; Asadu et al. 2018). Experimental data generated in this work were fitted into the models demonstrated in Table 1 and analyzed with the corresponding isotherm data and R2 derived from the plots of the linear fittings (see Table 6).
R2 was the key for pointing out better performing models; however, the parameters in Table 2 for each model were used to confirm the conclusion regarding the model that efficiently described the process of adsorption. At the four targeted temperatures, the calculated quantity for the Langmuir isotherm, known as the separation factor RL, is < 1 but > 0 (0.03324, 0.05689, 0.0698, and 0.0890), signifying favorable adsorption. The uniform distribution of active sites on the CoFe2O4-MSS surface may be the cause of this observation which is in concurrence with the view of Onu et al., (2021) and Awual et al. (2020) that each adsorption site can accommodate only one adsorbate molecule, meaning that multiple layers of adsorbed molecules are not formed and there are no interactions between adsorbed molecules, and the energy of adsorption is constant for all sites for Langmuir isotherm.
According to Thompson et al. (2020) and Asadu et al. (2018), constant n indicates the intensity of adsorption, whereas constant Kf measures the adsorption capacity. The range of values for n for favorable adsorption is 1–10 according to the work done by Muhammed et al., (2014). Table 6 shows that at four distinct temperatures, the value of n remains between 1 and 10 (2.401, 5.1090, 5.8938, and 6.7860), signifying a beneficial adsorption for the sorption of crude oil onto CoFe2O4-MSS. Table 6 clearly shows that the Freundlich constant Kf rises with temperature, suggesting that high temperatures are favorable for oil sorption's adsorption capacity onto CoFe2O4-MSS. The Alexander Dubinin and Vladimir Radushkevich isotherm is based on the assumption that the adsorption process is a result of the statistical probability of adsorption sites interacting with adsorbate molecules. It considers the energy aspect of adsorption, where adsorption sites have a range of energies, and the adsorbate molecules interact with these sites depending on their energy compatibility (Awual et al. 2019a). According to Dubinin–Radushkevich, physisorption occurs when the energy of activation (E) is less than 8 kJ/mol; however, chemisorption occurs when the energy of activation falls between 8 kJ/mol and 16 kJ/mol. Additionally, according to Kudaybergenov et al. (2015), the mean free energy of adsorption per molecule (Bd) indicates that ion exchange is controlling adsorption, while E > 16 kJ/mol indicates that a particle diffusion mechanism is in charge of adsorption. Table 6 clearly shows that, at the four temperatures under investigation, the activation energy was less than 8 kj/mol, suggesting that CoFe2O4-MSS is adsorbing crude oil from the water surface via physisorption (a physical process). Moreover, the Bd values at all temperatures under study are < 16 kJ/mol, signifying that the particle diffusion mechanism may not have been the governing factor for the sorption of crude oil onto CoFe2O4-MSS. The Langmuir isotherm model appears to be the model of the best fit for the adsorption of crude oil onto CoFe2O4-MSS, considering that the R2 values at the four different temperatures were closer to unity.
Isotherm model performance validation
SD, HYBRID, ARE, MPSD, and RMSE were used to validate the proposal regarding the model that best described the process using the R2 criteria in Sect. "Modelling of crude oil layers adsorption onto CoFe2O4-MSS." The adsorption capacity predicted by the model (q_cal) and adsorption capacity from laboratory (q_exp) were used to validate the isotherm model of the best fit for the data generated in this work. Table 7 presents the findings of the analysis. According to Onu et al. (2021) and Asadu et al., (2022a), the more associated the data set and the better the goodness of fit, the smaller the values of HYBRID, SD, MPSD, ARE, and RMSE. Table 7 clearly shows that the adsorption of crude oil onto CoFe2O4-MSS matched the Langmuir model since it has the least values for all the statistical tools combined.
Kinetic modelling of crude adsorption by CoFe2O4-MSS
The kinetics of adsorption in the context of this work refers to the study of the rate at which crude oil layers are taken up by the pore on the surface of a solid material, such as adsorbents. This has been identified as one of the key elements in determining the adsorption efficiency (Nwabanne et al. 2017). Six common kinetic models were investigated in this study as listed in Table 3. Figure 9a–f shows the graph of the models at three selected temperatures, and Table 8 displays the R2 and kinetic constants that were determined.
a First-order kinetic model for crude oil removal by CoFe2O4-MSS. b Pseudo-first-order kinetic model for crude oil removal by CoFe2O4-MSS. c Pseudo-second-order kinetic model for crude oil removal by CoFe2O4-MSS. d Intraparticle diffusion model for crude oil removal by CoFe2O4-MSS. e Bhattacharya–Venkobachar kinetic model for crude oil removal by CoFe2O4-MSS. f Elovich kinetic model for crude oil removal by CoFe2O4-MSS
Plotting ln (Ct/Co) vs t and log (qe–qt) vs t was used to study the first-order and pseudo-first-order models, as illustrated in Fig. 9a and b. Low R2 values indicated that these kinetic models were unable to adequately describe the modelling of the oil layer removal. According to Onu et al., (2021) and Onwu et al., (2019a), the constant, k, is associated with the energy barrier that must be overcome for the reaction to proceed. A larger k value indicates that the reaction proceeds more quickly, as the energy barrier is lower. Table 8 shows that at every temperature, the rate constant (k) was very high, suggesting that the uptake of crude oil by CoFe2O4-MSS was a rapid procedure.
The pseudo-first order is a widely used concept in chemical kinetics and reaction engineering. It is a simplified representation of a more complex reaction mechanism, often employed to analyze and describe the behavior of reactions that involve intermediates. In such cases, the overall reaction may not strictly follow first-order kinetics, but it can be approximated as first order for practical purposes (Chinonye et al. 2018; Onwu et al. 2019b). Table 8 shows that the experimentally determined adsorption capacity of 55.50 mg/g was significantly larger than the adsorption capacity calculated in the pseudo-first-order model at three different temperatures with low correlation coefficients, signaling that the adsorption of crude oil by CoFe2O4-MSS may not have aligned well with pseudo-first order.
The pseudo-second order is particularly useful when studying systems where the rate law cannot be easily determined as a simple first-order or second-order reaction. In a pseudo-second-order reaction, the rate-determining step is assumed to involve an equilibrium between the reactant and a slow reacting species, rather than the reactant and a fast-reacting species (Nwabanne et al. 2017). The suitability of the model and intraparticle diffusion model was investigated using the plots of t/qe vs t and qt vs t0.5 shown in Fig. 9c and d, respectively. For pseudo-second order, the calculated adsorption capacity gradually dropped along with the rate constant (k) as temperature increases. This is because the adsorbate molecules' kinetic energy increases with temperature, resulting in a reduction in the amount of time they spend in contact with the adsorbent. This will therefore cause the amount adsorbed per gram of adsorbate to decrease. Also, the rate of uptake of the crude oil by CoFe2O4-MSS seems to occur at a speed that pseudo-second-order rate constant cannot clearly agree with, indicating that the uptake of crude oil by CoFe2O4-MSS may not have followed pseudo-second-order kinetic model.
Intraparticle focuses on the transport of reactants or solutes from the external surface of the porous material to the active sites located within the pores. The transport process can be governed by different mechanisms, such as diffusion. The intraparticle model aims to quantify these mechanisms and predict the overall reaction rate or mass transfer rate within the porous material (Nwabanne et al. 2018; Ike et al. 2022). As opined by the intraparticle diffusion, the adsorbent's adsorption capacity is linearly related to t0.5 instead of t. The experimental adsorption capacity and the adsorption capacities obtained from the intraparticle diffusion were comparable, signifying the model broad applicability. The possibility that the adsorbate molecules will diffuse from the adsorbent's surface into its pores is examined by the model (Girlie et al. 2020). Furthermore, both of these kinetic models had strong correlation coefficients (usually > 0.9), supporting their applicability in explaining the kinetics of uptake of crude oil from surface water by CoFe2O4-MSS.
Plotting ln (1–Ut) vs t at various temperatures was used to study the Bhattacharya–Venkobachar model. The model uses the concentration of a first-order reversible kinetic model to study the mechanism and properties of the sorption process. The effective diffusion coefficient Db, which varied from 1.67 × 10–10 to 2.21 × 10–10 m2/s, according to Muhammed et al., (2020), was calculated using the rate constant KB. Since the correlation coefficients were nearly equal to one, the Bhattacharya–Venkobachar model provided the best description of the removal of the crude oil spills. Additionally, the correlation coefficient increased as temperature increased, indicating that high temperatures were more ideal for the adsorption process.
The Elovich model was studied using the plot of qt vs t. The chemisorptive characteristics and extent of surface coverage were ascertained by means of the model (Onu et al. 2020). The slope and intercept were used to calculate the preliminary adsorption rate symbolized as α and the coverage extent symbolized as β. The very low R2 indicates that the removal of crude oil spill using this adsorbent cannot be described by the Elovich kinetic model. Since the Elovich model is only applicable to adsorption onto heterogeneous surfaces and is primarily applicable to chemisorption processes, Olufemi et al. (2014) state that the Elovich model is insufficient to fully describe the adsorption process. The possibility of the adsorbate diffusing into the adsorbent's internal pores and past its surface limits the application of the particle diffusion model to adsorption (Sivakumar and Palanisamy 2009). Only adsorption processes with low adsorbate concentrations are covered by the first-order model.
Mechanism of crude oil adsorption by CoFe2O4-MSS
Crude oil dispersion and adsorption in water by adsorbents is a process that plays a crucial role in environmental remediation, pollution control, and industrial applications. The mechanism involves the interaction between oil molecules, water molecules, and the adsorbent material in this case CoFe2O4-MSS as shown in Fig. 9a. Crude oil, being a hydrophobic substance, does not mix with water due to its nonpolar nature. When it is released into water, it forms droplets or a thin layer on the water surface, creating a separate phase, and this phenomenon is known as the oil–water emulsion or oil dispersion. The surface of the cobalt ferrite-doped mango seed shell adsorbent possesses various functional groups, such as hydroxyl (-OH) and carboxyl (-COOH) groups, which provide active sites for crude oil molecules to adhere to. This adsorbent has high affinity for oil molecules due to the positively charged surface. The adsorbent is introduced into the oil–water mixture to facilitate the separation process. As the CoFe2O4-MSS comes into contact with the oil droplets or the oil–water interface, the oil molecules are attracted to the adsorbent surface due to forces such as van der Waals forces, hydrophobic interactions, electrostatic interactions, and hydrogen bonding. These forces cause the oil molecules to adsorb or get trapped on the surface of the CoFe2O4-MSS, effectively removing them from the water phase. The cobalt ferrite-doped mango seed shell adsorbent exhibits magnetic properties due to the presence of cobalt ferrite nanoparticles. The oil molecules are physically trapped within the porous structure of the CoFe2O4-MSS enhancing the efficiency of the oil removal process. As the oil molecules are adsorbed onto the CoFe2O4-MSS surface, the water molecules are released from the system. This results in a cleaner water phase, with the adsorbent now carrying the adsorbed oil.
Conclusion
The treatment of crude oil-polluted water using cobalt ferrite-doped mango seed shell demonstrates a promising and eco-friendly approach to addressing water pollution. BET isotherm analysis revealed the surface area of cobalt ferrite-doped mango seed shell as 1007.4m2/s while that of raw mango seed shell is 298.4m2/s. Adsorption energy of raw mango seed shell increased from 1.031 kj/mol to 5.321kj/mol after doping with cobalt ferrite nanoparticles, signaling increase in the adsorption capacity. The dominant compounds in raw mango seed shell after XRF analysis are iron oxide, magnesium oxide, and silicon oxide. It was also observed that the percentage of iron oxide increased significantly from 5.16780 to 10.10328% after doping raw mango seed shell with cobalt ferrite nanoparticles. The crystallite sizes of raw MSS and CoFe2O4-MSS are 31.8 nm and 21.3 nm from XRD analysis. Batch adsorption studies shows that the optimum adsorption temperature was 80 °C with 98.3% of oil layer removal with CoFe2O4-MSS adsorbent. The experimentally determined adsorption capacity was 55.50 mg/g and adsorption data fitted well with Langmuir model. Bhattacharya–Venkobachar kinetic model provided the best description for the oil adsorption by CoFe2O4-MSS. The unique adsorption properties of CoFe2O4-MSS, combined with their low cost and abundant availability, make them an attractive solution for removing crude oil contaminants from water. This innovative method not only addresses the environmental concerns of crude oil pollution but also promotes the utilization of waste materials, such as mango seed shells, in a sustainable manner. As research in this field continues to advance, the potential applications of cobalt ferrite-doped mango seed shells in water treatment could significantly contribute to the preservation of our planet's water resources and the overall well-being of ecosystems worldwide.
References
Albert CA, Asadu CO, Onoh MI, Azubuike KA (2016) Kinetics and isotherm studies on divalent lead ions adsorption by zeolite solution. Int’l J Novel Res Eng Sci 3:49–61
Angelova D, Uzonov I, Uzonova S, Gigova A, Minchev L (2011) Kinetics of oil and oil product adsorption by carbonized rice husks. Chem Eng J 172(1):306–311
Arica TA, Kuman M, Gercel O, Ayas E (2019) Poly(dopamine) grafted bio-silica composite with tetraethylenepentamine ligands for enhanced adsorption of pollutants. Chem Eng Res Des 2019(141):317–327
Asadu CO, Egbuna SO, Ejikeme PCN (2018) Survey on kinetic decomposition of organic matter and bio-fertilizer synthesis by composting sawdust, vegetable waste and sewage sludge. J Chinese Adv Mater Soc. https://doi.org/10.1080/22243682.2018.1516161
Asadu CO, Ezema AC, Onu CE, Ike SI, Onoghwarite OE, Okwudili UE (2021) Development of an adsorbent for the remediation of crude oil polluted water using stearic acid grafted coconut husk (Cocos nucifera) composite. Appl Surf Sci Adv 6:100179
Asadu CO, Ezema CA, Onu CE, Ogbodo NO, Maxwell OI, Ugwele OF, Chukwuebuka AS, Onah TO, Godwin-Nwakwasi E, Ike SI, Ezeh EM (2022a) Equilibrium isotherm modelling and optimization of oil layer removal from surface water by organic acid grafted plantain pseudo stem fiber. Case Stud Chem Environ Eng 5:1–12. https://doi.org/10.1016/j.cscee.2022.100194
Asadu CO, Ekwueme BN, Onu CE, Onah TO, Ike SI, Ezema CA (2022b) Modelling and optimization of crude oil removal from surface water via organic acid functionalized biomass using machine learning approach. Arab J Chem 15:1–21. https://doi.org/10.1016/j.arabjc.2022.104025
Arivoli S, Hema M, Martin PD (2019) Adsorption of malachite green onto carbon prepared from borassus bark. The Arab J for Sci and Eng 34(2A):31–43
Awual MR, Hasan MM, Rahman MM, Asiri AM (2019a) Novel composite material for selective copper(II) detection and removal from aqueous media. J Mol Liq 283:772–780
Awual MR, Hasan MM, Asiri AM, Rahman MM (2019b) Cleaning the arsenic(V) contaminated water for safe-guarding the public health using novel composite material. Compos B Eng 171:294–301
Awual MR, Hasan MM, Asiri AM, Rahman MM (2020) Optimization of an innovative composited material for effective monitoring and removal of cobalt(II) from wastewater. J Mol Liq 298:112035
Ayotamuno MJ, Kogbara RB, Ogafi SOT, Probert SD (2006) Bioremediation of a crude oil polluted agricultural-soil at port-harcourt. Niger J Appl Energy 83:1249–1257
Baars BJ (2002) The wreckage of the oil tanker ‘Erika’-human health risk assessment of beach cleaning, sunbathing and swimming. Toxicol Lett 128 (1–3):55–68
Banerjee S, Chattopadhyaya MC (2017) Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab J Chem 10:S1629–S1638
Banerjee SS, Joshi MV, Jayaram RV (2006) Treatment of oil spill by sorption technique using fatty acid grafted sawdust. Chemosphere 64:1026–1031
Behnood R, Anvaripour B, Jaafarzadeh N, Farasati M (2013) Application of natural sorbents in crude oil adsorption. Oil Gas Sci Technol J 2(4):01–11
Behnood R, Anvaripour B, Jaafarzadeh N, Farasati M (2016) Oil spill sorption using raw and acetylated sugarcane bagasse. J Cent South Univ 23:1618–1625. https://doi.org/10.1007/s11771016-3216-8
Bhattacharya AK, Mandal SN, Das SK (2006) Adsorption of Zn (II) from aqueous solution by using different adsorbents. ChemEng J 123:43–51
Bulut Y, Aydin H (2006) A kinetic and thermodynamic study of methylene blue adsorptionon wheat shells. J Desalin 194:259–267
Chinonye OE, Oluchukwu AC, Elijah OC (2018) Statistical analysis for orange G adsorption using kola nut shell activated carbon. J Chinese Adv Mater Soc. https://doi.org/10.1080/22243682.2018.1534607
Das B, Mondal NK (2011) Calcareous soil as a new adsorbent to remove lead from aqueous solution: equilibrium, kinetics and thermodynamics study. Univers J Environ Res Technol 1(4):515–530
Didem O (2012) An Approach to the Characterization of Biochar and Bio-Oil. Bioengineering Department Yildiz Technical University, Turkey
Ekwueme BN, Ezema CA, Asadu CO, Onu CE, Onah TO, Ike IS, Orga AC (2022) Isotherm modelling and optimization of oil layer removal from surface water by organic acid activated plantain peels fiber. Arab J Chem 16:104443. https://doi.org/10.1016/j.arabjc.2022.104443
Ezenwa O, Asadu CO, Agaba A (2019) Optimization and kinetic modeling of the removal of lead from enugu coal by acid leaching. J Energy Res Rev 3(1):1–13
Gayathri Manju B, Raji P (2019) Green synthesis of nickel–copper mixed ferrite nanoparticles: structural, optical, magnetic, electrochemical and antibacterial studies. J Electron Mater 48:7710–7720
Gwendoline F (2010) The effects of oil spills of aquatic life and environments. J Sci Nat 2(4):23–30
Hikmat NA, Qassim BB, Khethi MT (2014) Thermodynamic and kinetic studies of lead adsorption from aquesous solution onto petiole and fiber of palm tree. American J Chem 4(4):116–124
Ike SI, Asadu CO, Ezema CA, Onah TO, Ogbodo NO, Godwin-Nwakwasi EU, Onu CE (2022) ANN-GA, ANFIS-GA and Thermodynamics base modeling of crude oil removal from surface water using organic acid grafted banana pseudo stem fiber. Appl Surf Sci Adv 9(2022):100259
Iwar RT, Iorhemen OT, Ogedengbe K, Katibi KK (2021a) Novel aluminium (hydr) oxide-functionalized activated carbon derived from Raffia palm (Raphia hookeri) shells: augmentation of its adsorptive properties for efficient fluoride uptake in aqueous media. Environ Chem Ecotoxicol 3:142–215
Iwar RT, Ogedengbe K, Katibi KK, Oshido LE (2021b) Meso-microporous activated carbon derived from Raffia palm shells: optimization of synthesis conditions using response surface methodology. Heliyon 7(6):e07301
Iwar RT, Ogedengbe K, Ugwudike BO (2022) Groundwater fluoride removal by novel activated carbon/aluminium oxide composite derived from raffia palm shells: optimization of batch operations and field-scale point of use system evaluation. Res Eng 14:100407
Kharoune M, Pauss A, Lebeault JM (2001) Aerobic biodegradation of an oxygenates mixture: ETBE, MTBE and TAME in an upflow fixed-bed reactor. Water Resour 35(7):1665–1674
Kudaybergenov KK, Ongarbayev EK, Mansurov ZA (2015) Oil sorption by heat- treated rice husks. J Pet Environ Biotechnol 6:5–13
Kumar KV (2006) “Comparative analysis of linear and non-linear method of estimating thesorption isotherm parameters for malachite green onto activated carbon.” J Hazard Mater 136(2):197–202
Ladhe UV, Wankhede SK, Patil VT, Patil PR (2011) Adsorption of eriochrome blackT from aqueous solutions on activated carbon prepared from mosambi peel. J Appl Sci Environ Sanitation 6(2):149–154
Lin H, Huang C, Li W, Ni C, Shah S, Tseng Y (2006) Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl Catal B: Environ 68(12):1–115
Liu D, Yuan J, Li J, Zhang G (2019) (2019) Preparation of Chitosan Poly(methacrylate) composites for adsorption of bromocresol green. American Chem Soc Omega 4:12680–12686. https://doi.org/10.1021/acsomega.9b01576
Lopez GEP, Madrid JF, Abad LV (2020) Chromium and cadmium adsorption on radiation-grafted polypropylene copolymers: regeneration, kinetics, and continuous fxed bed column studies. SN Appl Sci 2:400. https://doi.org/10.1007/s42452-020-2168-7
Nestor T, Natalia M, Fabiana M, Javier P, Carina P, Tomas C (2004) Phenol Adsorption onto powdered and granular activated carbon, prepared from eucalyptus wood. J Colloid Interface Sci 279:357–363
Nick OO, Christian OA, Ezema AC, Onoh MI, Onu CE, Ike SI, Ohimor EO (2021) Preparation and Characterization of activated carbon from agricultural waste (Musa-paradisiacapeels) for the remediation of crude oil contaminated water. J Hazard Mater Adv. https://doi.org/10.1016/j.hazadv.2021.100010
Nwabanne JT, Okpe EC, Asadu CO, Onu CE (2017) Application of response surface methodology in phenol red adsorption using kola nut (Colaacuminata) shell activated carbon. Int Res J Pure Appl Chem 15(4):1–14
Nwabanne JT, Okpe EC, Asadu CO, Onu CE (2018) Sorption studies of dyestuffs on low-cost adsorbent. Asian J Phys Chem Sci 5(3):1–19
Olufemi BA, Jimoda LA, Agbodike NF (2014) Adsorption of crude oil using meshed corncobs. Asian J Appl Sci Eng 3:63–75
Onu CE, Nwabanne JT, Ohale PE, Asadu CO (2021) Comparative analysis of RSM, ANN and ANFIS and the mechanistic modeling in eriochrome black-T dye adsorption using modified clay. South African J Chem Eng 36:24–42
Onu CE, Ekwueme BN, Ohale PE, Onu CP, Asadu CO, Obi CC, Dibia KT, Onu OO (2023) Decolourization of bromocresol green dye solution by acid functionalized rice husk: artificial intelligence modeling, GA optimization, and adsorption studies. J Hazard Mater Adv 9:100224
Onu CE, Igbokwe PK, Nwabanne JT, Nwanjinka OC, Ohale PE (2020) Evaluation of optimization techniques in predicting optimum moisture content reduction in drying potato slices. Artif Intell in gric 4:39–47
Onwu DO, Nick O, Cordelia ON, Asadu OO, CI MO (2019a) Optimization of process parameters for the treatment of crude oil spill polluting water surface by sorption technique using fatty acid grafted ogbono shell as a sorbent. J Mater Sci Res Rev 3(3):1–12
Onwu DO, Ogbodo ON, Ogbodo NC, Chime TO, Udeh BC, Egbuna SO, Onoh MI, Asadu CO (2019b) Application of esterified ogbono shell activated biomass as an effective adsorbent in the removal of crude oil layer from polluting water surface. J Appl Sci Environ Manage 23(9):1739–1746
Paulauskiene T, Jucike I, Juseenko N, Baziuke D (2014) The use of natural sorbents for spilled crude oil and diesel cleanup from the water surface. Water Air Soil Pollut 225:1959. https://doi.org/10.1007/s11270-014-1959-0
Pelissari FM, Amaral-Sobral PJ, Menegalli FC (2014) Isolation and characterization of cellulose nanofibers from banana peels. Cellulose 21:417–432. https://doi.org/10.1007/s10570-013-0138-6
Rabiul Awual Md, MunjurHasan Md (2014) A novel fine-tuning mesoporous adsorbent for simultaneous lead(II) detection and removal from wastewater. Sens Actuators B: Chemical 202:395–403
Reza B, Bagher A, Nematollah JHF, Masoumeh F (2013) Application of natural sorbents in crude oil absorption. Iran J Oil Gas Sci Technol 2:01–11
Sharma YC, Singh B, Uma (2009) Fast removal of malachite green by adsorption on rice husk activated carbon. Open Environ Pollut Toxic J 1:74–78
Shokrollahi A, Alizadeh A, Malekhosseini Z, Ranjbar M (2011) Removal of bromocresol green from aqueous solution via adsorption on Ziziphus nummularia as a new, natural, and low-cost adsorbent: kinetic and thermodynamic study of removal process. J Chem Eng Data 2011(56):3738–3746
Sivakumar P, Palanisamy PN (2009) Adsorption studies of basic Red 29 by a non-conventional activated carbon prepared from Euphorbia antiquorum L. Int J Chem Technol Res 1(3):502–510
Suidan MT, Esperanza M, Zein P, McCauley RC, Brenner AD (2005) Challenges in biodegradation of trace organic contaminants-gasoline oxygenates and sex hormones. Water Environ Res 77(1):4–11
Thompson CO, Ndukwe AO, Asadu CO (2020) Application of activated biomass waste as an adsorbent for the removal of lead (II) ion from wastewater. Emerg Contam 6:259–267
Utsev JT, Iwar RT, Ifyalem KJ (2020) Adsorption of methylene blue from aqueous solution onto delonix regia pod activated carbon: batch equilibrium isotherm, kinetic and thermodynamic studies. J. Mater. Environ. Sci. 11(7):1058–1078
Uzoije AP, Onunkwo A, Egwuonwu N (2011) Crude oil sorption onto groundnut shell activated carbon: kinetic and isotherm studies. Res J Environ Earth Sci 3(5):555–563
Yang G, Zhang L, Sun X, Jing W (2006) Photochemical Degradation of Crude Oil in Sea Water. Chinese J, Oceanol Limnol 24(3):264–269
Zawani Z, Luqman CA, Thomas SYC (2009) Equilibrium, kinetics and thermodynamic studies: adsorption of remazol black 5 on the palm kernel shell activated carbon. Eur j Sci Res 1(37):67–76
Acknowledgements
The authors wish to thank everyone that contributed to the success of this work in any way. Also, the authors wish to thank in a special way, David Umahi Federal University of Health Sciences, Uburu Ebonyi State Nigeria for making her lab available for this work.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors wish to declare that there no conflict of interest or competing interest associated with this work.
Ethical Approval
This Article: Treatment of crude oil-polluted water using CoFe2O4 -doped mango (Mangifera indica) seed shell composite (https://doi.org/10.1007/s13201-024-02212-4) does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asadu, C.O., Ujah, C.O., Ekwueme, B.N. et al. Treatment of crude oil-polluted water using CoFe2O4-doped mango (Mangifera indica) seed shell composite. Appl Water Sci 14, 161 (2024). https://doi.org/10.1007/s13201-024-02212-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02212-4