Abstract
The objective of the current study is to investigate the hydrogeochemistry of the Quaternary groundwater and evaluating its suitability for drinking and irrigation needs using statistical analysis, water quality indicators and physicochemical parameters, in west of the Nile Delta, Egypt. The study area has high population growth and agricultural activities, which require groundwater protection, and predicting probable environmental problems. For these reasons, 54 groundwater samples were collected during April 2021. Hierarchical cluster analysis, Pearson, and factor analysis used for statistical analysis. Biplots, Gibbs and Piper diagrams were used to infer the geochemical processes controlling groundwater chemistry. The groundwater is affected by silicate weathering, reveres ion exchange, dissolution of CO2, and recharge from the Nile. According to the WQICCME drinking value, the groundwater ranged between fair to marginal water quality. The distribution of integrated-weight water quality index of the samples, showed that it is excellent for irrigation (< 25). The nine physicochemical parameters sodium percent (Na%), permeability index, sodium adsorption ratio, Kelley Index, residual sodium carbonate, magnesium hazard, Potential Salinity, Corrosive Ratio and Chloro-alkaline Indices revealed that most of the groundwater are of good quality and can be safely used for agricultural activities, albeit few samples due the west needs some treatment. It is recommended to minimize the fertilizers and nutrients use to decrease the anthropogenic impact on the groundwater. The reuse of irrigation water without treatment should be limited. As well, the pumping rates should be controlled to avoid aquifer salinization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is one of the most important natural resources for all living organisms. It is considered as an important source for sustainable economic growth in society, especially in arid regions. Due to the high consumption of water by the increased population, urbanization and anthropogenic activities, water may be subjected to depletion. This requires appropriate management and assessment of this precious resource.
More than 85% of the total water budget of Egypt is consumed in agricultural uses (MWRI 2016). Lately, Egypt needs of water increased because of increasing population and introducing new strategic land reclamation projects and industrial development. These projects depend mainly on surface water for agricultural purposes; but recently, groundwater has been used to meet the agricultural requirements.
The chemistry of groundwater is affected by the hydrogeochemical reactions with the surrounding rocks (e.g. dissociation of minerals, cation exchange, precipitation of secondary minerals, evaporation, oxidation–reduction reactions, waters mixing, leaching of pesticides, fertilizers and manure, and contamination processes). Investigating the hydrogeochemical processes will help in elucidating the influencing factors on groundwater quality and the spatial variations of the groundwater chemistry (Matthess 1982, Kumar et al. 2006, Mohamed et al. 2015, Gad et al. 2021, Abu Salem et al. 2021 and 2022, Mohammed et al. 2022a, Nosair et al. 2022). As well, these processes provide important evidence for the nature of groundwater recharge and discharge, and interpreting the origin of groundwater components (Walton 1970).
In order to categorize the quality of water, water quality index (WQI) has been applied to groundwater results of the area of study. This methodology is useful to infer the quality of water to the people and policy makers in the concerned area. WQI is a mathematical relationship benefited to convert large batch of water data into one number (Stambuck-Giljanovic 1999; Stigter et al. 2006), which assesses the overall quality of water. This has the advantage of distinguishing between clean and contaminated waters with respect to location and time (Todd 1980; Tyagi et al. 2013). As well, the drinking water quality index (DWQI) is useful technique for evaluating water for drinking use (Bora and Goswami 2017).
In addition, the physicochemical parameters are used to assess the quality of water, and connecting information about water quality and water liability to contamination (Mondal et al. 2016). Many authors investigated the groundwater suitability to drinking, irrigation and industrial purposes through the hydrogeochemistry and water quality (Ekenta et al. 2015, Naaz and Anshumali 2015, Manoj et al. 2017, Sakram and Adimalla 2018, Egbueri 2019, Egbueri et al. 2019, Rajesh et al. 2019, Egbueri et al. 2020, Egbueri et al. 2021, Hussein et al. 2021 and Gad et al. 2023).
In the southwest of the Nile Delta water, which is available from the River Nile, irrigation canals and groundwater, is used to fulfill the needs of different uses. The aim of the current study is to investigate hydrogeochemistry of the Quaternary groundwater and evaluating its suitability for the drinking and irrigation purposes using water quality indicators and physicochemical parameters.
Study area
The area of the study is located to the west of the Rosetta Branch of the Nile River between latitudes 30° 31′ 26″–30° 56′ 06″ N and longitudes 30° 26′ 04″–30° 49′ 08″ E (Fig. 1). It is characterized by a desert arid climate (Nashwan et al. 2019). It has a warm winter season with rainfall falling in the coastal parts and a dry summer season. Day temperatures differ by season and alter with the dominant winds.
The area of investigation is dominated by agricultural activities, which is dependent on the groundwater tapping the Quaternary aquifer. The water-table ranges from 1 to 6 m. Pesticides and fertilizers are used extensively to increase the productivity of the agricultural crops. Hence, the pollution of groundwater is possible owing to geogenic activities and anthropogenic influences. The effect of applying compost and insecticides for agriculture have harmful bearings on the health of human. Furthermore, the anthropogenic aspects are exemplified by waste disposal in unsuitably designed septic cisterns. The present study investigates the hydrogeochemical status and the class of groundwater to evaluate it for drinking besides agricultural uses. Also, this work is of great importance for the strategic controlling of water resources and for protection of local inhabitants besides agricultural practices.
Hydrogeological setting
Geologically, sedimentary layers constitute the western Nile Delta succession, which belongs to Cenozoic Era (Fig. 2). The area of research is distinguished by a plain topography with little heights. The essential geomorphic units are the alluvial plains, which can be differentiated into young and old alluvial plains. Maryuit plain appears and attains about 110 m elevation to the west of old alluvial plains.
Stratigraphically, the sediments of Quaternary comprise the principal aquifer of the study area. The sedimentary column of the Delta attains ~ 4000 m thickness, where it unconformably overlay the basement.
The majority of the yearly recharge to the Quaternary aquifer is obtained from the direct infiltration from the surface canals and agricultural practices. The aquifer receives about 6.70 km3 of water yearly (Sherif et al. 2012; Salem et al. 2016). The surface canals run through highly porous Holocene sands and gravels. Consequently, the groundwater can be polluted from surface sources, which might contain excessive amounts of farming and anthropogenic wastes.
The Quaternary aquifer is of semi-confined nature (Mabrouk et al. 2013), and is occupying the whole Nile Delta, with thickness ranges between 200 m due south and 1000 m due north. The water table depth ranged from 1 to 2 m due north, 3–4 m due the middle, and ~ 5 m due the south (Morsy 2009; Mabrouk et al. 2013).
The aquifers of the Quaternary times could be distinguished into Mit-Ghamr and Bilqas Formations. Mit-Ghamr Formation forms the essential aquifer of the Delta (Rizzini et al. 1978; Mabrouk et al. 2013; Salem et al. 2016). It is composed of sand and gravel with existence of tiny clay interbeds. Mit-Ghamr Formation is capped by Holocene Bilqas formation, which is composed of fine detrital materials ranging between clay and silt with traces of sand.
Hydraulically, the Quaternary aquifer is unconnected with the formations found below which act as an aquiclude formation. The groundwater flows towards the northwest (Emara et al. 2007, Nasr et al. 2023).
Material and methods
Water sample collection and physicochemical parameters
In the current study, 54 samples were collected from different drilled groundwater wells distributed in the southwest area of Nile Delta in April 2021 (Fig. 1). The location of each water well was taken by Garmin GPS MAP 86sci. The collected groundwater samples were chemically examined for major and minor ions in agreement with the guidelines of US Environmental Protection Agency (USEPA 2018), and the American Public Health Association (APHA 1995). The physicochemical results of the analyzed samples of the study area are given in Table 1.
Before sampling, the used polyethylene containers were washed with 0.1% HNO3, and then rinsed with distilled water to avoid interferences with the parameters being sampled. From an operating well for at least 15 min, two samples from each wellhead were collected. The first one is taken at its normal pH after washing the container with the well water then well preserved (~ at 4 °C) for the analysis of anions. The second sample was acidified (after filtration through 0.45 by μm membrane filters) by adding 2 ml of HNO3 to pH < 2 for the analysis of cations and metals. Acidification was performed to minimize adsorption, hydrolysis, and precipitation effects. In situ, pH and electrical conductivity (EC) were measured by portable HI 991300 Hanna Instruments.
Analysis of major ions (Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, Cl− and NO3−) and minor ions (Fe, Mn, Pb, Cu, Cd, Cr, Ni, Zn, B, SiO2, NH4+, NO3−, and PO43−) were achieved on the water samples with the usual known techniques. All the analyses were done in the Central Lab of the Faculty of Science, University of Alexandria.
The ion balance errors calculation was used to ensure the precision of the analysis, using the following formula (1):
The results of chemical analysis were observed to be within the standard edge of ± 5% (Appelo and Postma 1999).
Titration methods were used to determine Ca2+ and Mg2+, HCO3− and Cl− concentrations. DR4000 HACH Spectrophotometer was used to determine SO42− concentration. Na+ and K+ were specified using PFP7 Flame Photometer. SiO2, NH4+, NO3−, and PO43− were measured using Hach DR 3900 Spectrophotometer.
Fe, Mn, Pb, Cu, Cd, Cr, Ni, Zn and B were measured using analytikjena contrAA 300 Atomic Absorption Spectrophotometer.
Statistical analysis
The statistical analysis of the analytical data (Descriptive statistics, Pearson correlation, Q-mode cluster analysis and factor analysis) was performed using SPSS© 17.0 software.
Groundwater categorization
The chemical character of the groundwater samples was studied using Piper diagram generated by AquaChem v 4.0.264 software. The hydrogeochemical processes, water types, concentrations of major ions and the relationship and ionic ratios were also investigated.
Water quality assessment
Drinking water quality index (DWQICCME)
This index is based on three factors that represent time, water type, and variables (EPA 2003):
F1 represents the scope that signifies the extent of water quality guideline non-compliance in the studied time interval (2).
F2 represents the frequency where the individual tests percentage do not fulfill objectives (tests that fail) (3).
F3 represents the amplitude denoting the amount where the failed tests do not fulfill their objectives. F3 is calculated through Eqs. 4, 5, 6, 7. Excursion represents how many times a particular concentration is less than (when the objective is a minimum) or greater than the objective (4).
As the test value should not fall below the objective (5):
Finally, the normalized sum of excursions (nse) represents the total amount by which individual tests are far of guidelines. It is determined by the sum of excursions of individual tests divided by the total number of tests (6) (Tests that meet and do not meet the objectives).
F3 is computed via a function (asymptotic) that scales the nse from objectives to produce a range from 0 to 100 (7).
The WQI is finally estimated as (8):
Integrated-weight water quality index (IWOI)
The quality index of water represents one of the good indicators that is useful in groundwater quality management (Pei-Yue et al. 2010, Hameed et al. 2015). The weighted arithmetic water quality index is calculated for eleven parameters. IWQI is calculated using the following Eqs. (9–11) (Călmuc et al. 2018; Gao et al. 2020 and Zhang et al. 2021):
where Wi represents the unit weight that reflects the significance of a parameter in the WQI calculation, Qi is the quality relative value of ith parameters. Qi and Wi are calculated using the following equations:
where Vi is the measured concentration of the parameter, Vo is the ideal value of the parameter. All parameters have an ideal value of 0 except pH has a value of 7. Si is the agriculture water quality guideline standard (Ayers and Westcot 1994) and K is a proportionality constant that is calculated according to the formula:
Water quality can be categorized into different grades depending on IWQI (Bora and Goswami 2017) as indicated in Table 2.
Assessment of water for irrigation
Nine physicochemical parameters are used to assess groundwater quality for irrigation, they are Na%, PI, KI, SAR, MH, RSC, PS, CR and CAI (Table 3).
Results and discussion
Statistical analysis
Descriptive statistics of the physicochemical parameters
The minima, maxima, and standard deviations of the groundwater from the Quaternary aquifer were calculated and compared to some standard guidelines (World Health Organization (WHO 2011), Environmental Protection Agency (EPA 2014), and the Egyptian Health Authority (EHA 2007)) (Table 1).
The groundwater salinity of the collected water samples varies from 353 (fresh) to 1026 (slightly saline) (Mayer et al. 2005). The values of pH of the Quaternary groundwater vary from 7.1 to 8.1, showing slight alkaline water, which shows normal ranges for drinking water according to the guidelines (Table 1). The EC values vary from 551 to 1602 µmhos/cm, which indicate slight to moderate water salinity. High EC reflects slow soil–water ion exchange, high solute dissolution and soluble minerals. The maximum concentrations of Na+, SO42−, B, Cr, Mn, Fe, NO3−, Cd, Ni, and Pb increase by ~ 1.3, ~ 1.5, ~ 1.4, 1.6, 2.4, 5.4, 7.8, 13.3, 16, and 94 folds the EHA (2007) guidelines for drinking water (Table 1).
Conversely, Cu, Cl−, and Zn maximum concentrations are less than the EHA (2007). Regarding anions concentrations, alkalinity values vary from ~ 219 to 621 mg/L revealing the dominance of this anion followed by SO42− that changed from 11 to 386 mg/L that is in the normal limit for irrigation depending on (FAO 1985).
Pearson correlation
The application of Pearson bivariate correlation to the studied variables shows strong positive correlation of TDS and HCO3− (0.791), Na+ (0.757), SO42− (0.7), and K+ (0.634) indicating that the salinity is attributed to these ions (Table 4). In addition, strong positive correlation exists between Na+ and SO42− (0.760) (Table 4).
Moderate positive correlations found between Mg2+ and HCO3− (0.627), Na+ and K+ (0.665), B and NO3− (0.534), K+ and SO42− (0.526), K+ and NO3− (0.502), and B and NO3− (0.560) (Table 4).
These correlations could be attributed to the use of secondary nutrients (Mg2+ and S) and micronutrients (B, Na+, and Mn) in cultivation processes. In addition, anthropogenic impact is evidenced by NO3−. Weak positive correlations found between TDS and Mg2+ (0.473), NO3− (0.418), and B (0.401) (Table 4).
Factor analysis (FA)
According to Kaiser (1958), statistical factor analysis is a familiar method to reduce large variables into significant factors using a satisfactory rotation of loadings. Factor analysis was applied to the Quaternary groundwater samples where the Kaiser–Meyer–Olkin (KMO) and Bartlett’s sphericity tests were first used to check the reliability of applying the factor analysis (Field 2009; Abu Salem et al. 2017). After that, a varimax rotation with Principal Component Analysis (PCA) extraction was made (Table 5). The best adequacy results were obtained using 13 variables (Na+, B, SO42−, NO3−, Cd, Mn, Mg2+, PO43−, Cu, Cr, Fe, Ca2+, and Pb). The KMO equals 0.615 implying the feasibility of factor analysis using these variables. Moreover, Bartlett’s sphericity results (Chi-Square ~ 196, degree of freedom = 78, and p = zero) prove that variance is shared among the examined variables. To extract factors, eigenvalue more than one was used giving five factors with total cumulative variance of ~ 69% (Table 5).
The first factor represents ~ 23% of the variance where a strong positive loadings exist on Na+ (0.908), B (0.822), SO42− (0.806), and NO3− (0.715). The second factor represents ~ 15% of the variance and provides strong positive loading on Cd (0.794), Mn (0.792), and Mg2+ (0.671). The first and second factors possibly could be related to anthropogenic impacts. The third factor represents ~ 11% of the variance and shows strong positive loading on PO43− (0.81), and moderate positive loading on Cu (0.552). This factor could be named the fertilizers factor. The fourth factor represents ~ 11% of the variance and shows strong positive loading on Cr (0.74), moderate positive loading on Ca2+ (0.565), and strong negative loading on Fe (− 0.645). This factor could be named the geogenic factor. The fifth factor represents ~ 9% of the variance and shows strong positive loading on Pb (0.903). This factor could be termed the lead factor.
Hierarchical cluster analysis (Q-mode) (HCA)
One of the well-known methods for identifying various classes and groupings within the data under investigation is the HCA which can display the findings with a dendrogram representation (Davis and Sampson 1986; Jia et al. 2020; Gao et al. 2022,).
To eliminate mistakes caused by the orders of magnitude and the variance of the variable, the dataset was normalized before performing HCA to the Z scores. HCA was applied to the Quaternary groundwater samples through the variables (pH, TDS, Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, Cl−, Fe, Mn, Pb, Cu, Cd, Cr, Ni, Zn, B, SiO2, NH4+, NO3−, and PO43−) (Fig. 3a). The HCA was represented by a dendrogram where the pheon line was chosen at distance 10 (Fig. 3a) where five main clusters were distinguished (Fig. 3b). The minima, maxima and means of the different clusters and their representation were given in Table 6 and Fig. 4. Samples of cluster 1 (C1, 33 samples) have the lowest salinity while those of cluster 5 (C5, 2 samples) have the highest salinity as well as SO42−, Na+, Cu and NO3−. This reflects the proximity of C1 samples to the sources of recharge (seepage from irrigation canals and the River Nile). Since the groundwater flow is due northwest, C5 samples lie far west of the delta reflecting the decrease of freshwater recharge from the River Nile and seepage from irrigation canals as well as the effect of lithologic variation (Table 6 and Fig. 3b). Cluster 2 (C2, 10 samples) show the highest Fe and NH4+ contents (Table 6) explaining the effect of lithology as a source of iron (Abdelhameed et al. 2019) and the anthropogenic outputs from cultivation that increase NH4+ (Nasr and Abdel-Motelib 2023). Additionally, cluster 3 (C3, 2 samples) show the highest Cl−, Pb, Cr and B (Table 6), while cluster 4 (C4, 7 samples) show the highest HCO3−, Mn, Ni, and PO43− (Table 6) explain the proximity to the River Nile and irrigation canals and their role in the bicarbonate facies of the groundwater. The higher Mn, Ni, and PO43− in C4 samples could be attributed to local anthropogenic sources in these localities.
Hydrogeochemical facies
Ionic dominance and water types
The ionic concentrations of the groundwater samples have the sequence Na+ > Ca2+ > Mg2+ and alkalinity > SO42− > Cl−. High concentration of Na+ ions is possibly because of the ion exchange with Ca2+ in the aquifer sediments and possibly due to the leaching of clay minerals and silicate weathering (El Osta et al. 2020). Elevated values of alkalinity might be related to carbonate dissolution and silicate weathering (Obeidatt and Alawneh 2019). Regarding the concentration of NO3−, its concentration ranges from 1 to 351 mg/L, indicating a very high concentration with a possible effect of contamination due to the use of fertilizers.
The occurrence of trace elements is in the following sequence: Fe > Mn > Pb > B > Ni > Cu = Zn > Cr > Cd. No obvious effects on the suitability of agricultural water due to the low values of trace constituents (Gad et al. 2020).
Geochemical controlling mechanism
The diagram of Gibbs (Gibbs 1970) is a relation between TDS and the ratios (Na+ + K+)/(Na+ + K+ + Ca2+) and Cl−/(Cl− + alkalinity). The plot of groundwater samples shows the essential processes that affect the groundwater chemistry are that the rock dominance and weathering (Fig. 5) where all the investigated samples lie between the range of TDS from 100 to ~ 1000 mg/L.
Piper diagram
The diagram of Piper was used to represent the compositional change of the groundwater samples (Piper 1944). Most of the groundwater samples (~ 76%) plotted on the secondary alkalinity zone that is dominated by weak acidic anions and alkaline earths (Fig. 6) reflecting the recharge water of the River Nile (most samples of clusters 1 and 4 as well as some samples of cluster 2). The remaining samples plotted in the mixed zone (~ 22%, the rest of samples of cluster 2 and the samples of cluster 3), while only one sample (~ 2%) plotted in the primary salinity zone that is dominated by alkali metals and strong acidic anions (Fig. 6, cluster 5). The mixed zone samples reflect the effect of interference of different waters and anthropogenic impacts.
The diagram of Piper of the studied samples, A1, A2, S1 and S2, stands for primary alkalinity, secondary alkalinity, primary salinity, and secondary salinity; respectively. Samples of clusters 1, 2, 3, 4, and 5 are represented by hollow squares, blue triangles, yellow squares, green diamonds, and red triangles
Ionic relationship of groundwater samples
The groundwater composition and the origin of solutes can be indicated by the ionic relations (Mohamed et al. 2015; Hussein et al. 2017; Abu Salem et al. 2021, Mohammed et al. 2022a). The relation between Na+ and Cl− shows that most of the studied water from C1, C4, and C3 and all samples from C2 and C5 fall above the halite dissolution line reflecting the silicate weathering process (Fig. 7a, reactions 13 and 14), while the rest of the studied samples of C1, C4, and C3 could be controlled by the reuse of irrigation water and/or reverse ion exchange due to the proximity of these samples to the irrigation canals. The (Ca2+ + Mg2+)–(SO42− − HCO3−) against Na+–Cl− relationship was defined by (Fisher and Mullican 1997) and represented in (Fig. 7b). If the dominant process is ion exchange, this relation must be linear with a slope of -1. The plot of the studied samples shows that the process of ion exchange is not an effective one in the controlling processes on water chemistry. The relationship between SO42− + HCO3− against Ca2+ + Mg2+ indicates that the majority of water samples of C1, C4, and C3 and all samples from C2 and C5 plot above the equimolar line suggesting silicate weathering (Fig. 7c). The Na+ against Ca2+ + Mg2+ relationship indicates that majority of the samples of C1, C3, and C4 and some samples of C2 plot below the equimolar line reflecting the reverse ion exchange process that could have a role in the water chemistry of the groundwater (Fig. 7d, reactions 15 to 18). The rest of C2 samples and all the C5 samples plot above the equimolar line indicating a slight increase in the sodium content in these samples. Additionally, the plot of the samples on the Ca2+ against HCO3− and Ca2+ against SO42− precluded gypsum-carbonate dissolution (Fig. 7e and f) and suggested the dissolution of CO2 as a possible process that leads to increase in bicarbonate content (Fig. 7f, reaction 19).
Silicate weathering
Albite Kaolinite silicic acid
Anorthite Kaolinite
Reverse ion exchange
Dissolution of CO2 gas
Water quality assessment
Drinking water quality index (DWQI)
Water quality index (WQI) is used for water quality assessment through the determination of physicochemical parameters of water. It can act as indicator of water contamination due the effect of natural and anthropogenic sources (WHO 1997, Mohammed et al. 2022b, Abu Salem et al. 2023), so it represents an efficient tool to assess water quality for the policy makers and environmentalists (USEPA 2008). Therefore, this index has a number that expresses the overall water quality of a certain location and time (EPA 2014).
There are several indices with many variations and limitations based on several water quality variables being used. WQICCME is selected to assess water quality in relation to contamination characterization and water classification according to the guidelines of the (EHA 2007). This index varies from 0 (poor quality) to 100 (excellent quality).
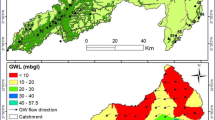
The studied groundwater samples range in quality from fair to marginal. The water seems to be threatened due the east of the area of study, because of the extensive agricultural activities and the vast distribution of the canals and drains (Fig. 8).
Integrated-weight water quality index (IWQI)
Calculation of IWQI comprises many known steps; the first step is calculation of the unit weight to each parameter. The greatest unit weight is 0.29 for K+ and PO43− followed by 0.11 for Mg2+ and 0.08 for pH reflecting the importance of these parameters in calculating IWQI. The standard values of different parameters that are taken in calculating the IWQI and the unit weight are illustrated in Table 7.
IWQI values for groundwater samples ranged from 0.709 to 4.65 (Table 2) revealing that the IWQI for the studied samples is considered excellent (< 25) (Table 8) (Fig. 9). This may be attributed to the continuous recharge from irrigation water and infiltrated water from irrigation canals.
Suitability of water for irrigation purposes
Nine hydrochemical parameters have been used to evaluate the validity of water for use in irrigation, they are: Na%, PI, SAR, KI, RSC, MH, PS, CR and CAI.
Sodium percentage (Na%)
The Na% is usually benefited for identifying the validity of groundwater for agricultural needs. High Na+ concentration comparing to Ca2+ and Mg2+ concentrations decrease the permeability of soil causing soil structure deterioration leading to the development of stunning plant (Doneen 1964, Todd 1980, Saleh et al. 1999, Sundaray et al. 2009, Purushothman et al. 2012).
In the groundwater samples, Na% ranges from 20.15 to 76.43 with an average value of 36.26 and standard deviation of 11.28 (Table 9). Most of the groundwater samples are of good class for irrigation (76%), about (19%) of the samples are of permissible water class and the remaining (5%) fall in the doubtful water class (Table 9). The distribution contour map of Na% reflects an increase in value from eastern to western parts of the study area (Fig. 10a).
Results of chemical analysis of the samples of the area of study were plotted on Wilcox diagram (Wilcox 1948) (Fig. 11), reveal that about (61%) of the samples fell in the good water to permissible water class, (37%) of the samples fell in the doubtful to unsuitable water class, and (2%) fell in the permissible to doubtful water class. According to these results, the investigated samples are suitable for irrigation.
Permeability index (PI)
Suitability of irrigation water is assessed using the PI, where it is affected by the long period of contact with elevated concentration of Ca2+, Mg2+, Na+ and alkalinity ions (Ravikumar et al. 2011).
In the groundwater samples, PI ranges from 41.96 to 94.70 with an average value of 62.13 and standard deviation of 10.54 (Table 9). The investigated samples were grouped into: (1) suitable class (I) which represents 13% of the total samples, and (2) good class (II) which represents 87% of the total samples (Table 9). The distribution contour map of PI reflects an increase in value from eastern to western parts of the study area following the same pattern as Na% (Fig. 10b). According to these results, the investigated samples are suitable for agricultural uses.
Sodium adsorption ratio (SAR)
The sodium hazard in groundwater (Subramani et al. 2005; Srinivasamoorthy et al. 2014) can be assessed using the SAR. SAR has a strong relation to the soil’s exchangeable sodium percentages (Suarez et al. 2006). Values of SAR of the studied samples varied from 0.88 to 8.26 with an average value of 2.02 and standard deviation of 1.32 (Table 9). All the groundwater samples have SAR less than 10, indicating excellent water for irrigation depending on the SAR classification (Table 9). The distribution contour map of SAR reflects an increase in value from eastern to western parts of the study area (Fig. 10c).
Elevated concentration of Ca2+ and Mg2+ in water used in irrigation decreases the permeability of soil (Kelley 1951; Tiwari and Manzoor 1988; Kumar et al. 2007; Arumugam and Elangovan 2009). The EC values are plotted against the SAR to rate irrigation water (USSL 1954). Most groundwater samples (74%) fell into the C3–S1 group while the rest (26%) fell into the C2–S1 group indicating high salinity and low sodium content of the samples (Fig. 12). Depending on the USSL Classification, the studied samples are plotted in the low salinity field (< 2250 mmhos/cm), so, the groundwater samples are suitable for agricultural practices.
Kelley index (KI)
KI is another parameter for assessing the water suitability for agricultural practices reflecting the sodium excess in water (Sudhakar and Narsimha 2013). Water with a KI > 1 is considered unsuitable for irrigation, whereas that of KI < 1 is suitable for irrigation. Values of KI in the area of research vary from 0.24 to 3.17 with an average value of 0.63 and standard deviation of 0.49 (Table 9). According to the results of KI, it is found that 89% of the studied samples have (KI < 1) indicating good water class for irrigation, whereas 11% of the samples are unsuitable (Table 9). The distribution contour map of KI reflects an increase in value from eastern to western parts of the study area (Fig. 10d).
Residual sodium carbonate (RSC)
RSC reflects the excess in alkaline earth metals (Ca2+, Mg2+) in water (Kelley 1951; Hem 1995; Subramani et al. 2005; Suarez et al. 2006; Kumar et al. 2007; Arumugam and Elangovan 2009; Sundaray et al. 2009; Ravikumar et al. 2011; Sudhakar and Narsimha 2013; Srinivasamoorthy et al. 2014). The applicability of groundwater for agricultural practices is affected mainly by the alkalinity content in the studied groundwater samples. In the area of study, the RSC values vary between − 3.76 and 3.52 meq/l with an average value of − 0.41 meq/l and standard deviation of 1.29 (Table 9). The RSC results revealed that 94% of the samples are less than 1.25 meq/l and located in the good class and desirable for agricultural practices, while 2% of the water samples located in the marginal class and 4% of the water samples located in the unsuitable class for irrigation (Table 9). The distribution contour map of RSC reflects an increase in value from eastern to western parts of the study area reflecting the effect of the reverse ion exchange processes in the groundwater quality (Fig. 10e).
Magnesium hazard (MH)
When water with high Mg2+ is applied for agricultural needs, it can cause damage to soil structure and high salinity. High magnesium concentration in groundwater will exchange with Na+ causing the soil to become alkaline and decreasing the yield of crops (Anim-Gyampo et al. 2019, Zhao et al. 2021). MH values in the water samples varied between 21.37 and 77.62 with an average value of 40.21 and standard deviation of 8.43 (Table 9). About 91% of the water samples have MH values < 50%, which reflect a valid water for agricultural practices. The rest 9% of the water samples reflect unsuitable water for irrigation (Table 9). The spatial distribution of MH reflects no specific trend indicating that the increase in magnesium content could be due to the use of Mg rich fertilizers (Todd et al. 1976, Fig. 10f).
Potential salinity (PS)
PS is another water quality parameter-based index for classifying irrigation water (Doneen 1964). It has been reported that the less soluble salts precipitate and accumulate in the soil, whereas highly soluble salts increase the salinity of the soil (Gholami and Srikantaswamy 2009). The PS ranged from − 0.838 to 4.46 meq/l with an average value of 1.61 meq/l and standard deviation of 1.33 (Table 9). According to Delgado et al. (2010), all the water samples are of good class for agricultural practices (Table 9). The spatial distribution of PS shows several trends suggesting the effect of localized anthropogenic impact promoted by the increase in chloride and sulfate contents (Fig. 10g).
Corrosivity ratio (CR)
CR value should be < 1 for the transportation of groundwater through pipes (Ryner 1944). CR of the studied water samples range from 0.25 to 1.96 with an average value of 0.59 and standard deviation of 0.29 (Table 9). It was found that 94% of the studied samples are less than 1 and are non-corrosive while 6% of the samples are corrosive (Table 9). The distribution contour map of CR shows no specific trend of increase and decrease (Fig. 10h).
Chloro alkaline indices (CAI)
The CAI are usually applied to determine the reactions of ion exchange between water and the bearing rock (Schöeller 1965). The values CAI in groundwater of the area of study range from − 5.19 to 0.32 with an average value of − 0.77 and standard deviation of 1.16 (Table 9). These negative values (about 84%) of the samples reflect the chloro-alkaline disequilibrium or indirect base-exchange reaction, which means that the host rocks are not considered to be the main source of dissolved solids in the water (Table 9). The distribution contour map reflects CAI increase from southern parts to northern fringes with some limited abnormalities in some parts of the area of study (Fig. 10i).
Conclusions
The groundwater salinity varies from fresh to slightly brackish and the pH values show normal ranges for drinking water. Among the studied parameters, Na+, SO42−, B, Cr, Mn, NO3−, Fe, Cd, and Ni maximum concentrations are higher than the standard limits for drinking. Additionally, Pb2+ maximum concentration far exceeds the guidelines by 94 folds.
HCA distinguishes five main clusters showing variable salinities and element concentrations. The spatial distribution of clusters shows good coordination with the hydrogeologic setting and the proximity of the lower salinity samples to the River Nile. The Pearson bivariate correlation revealed the effect of using secondary nutrients (Mg2+ and S) as well as micronutrients (B, Na+, and Mn2+) in cultivation process, in addition to anthropogenic impacts as evidenced by higher NO3−. Additionally, factor analysis gave five factors with total cumulative variance of ~ 69% showing the anthropogenic, geogenic, and fertilizer effects on the groundwater.
The use of biplots of ionic ratios indicates that the possible reactions affecting the groundwater chemistry are silicate weathering, reverse ion exchange, dissolution of CO2 gas and the reuse of irrigation water. ~ 76% of the water samples located in the secondary alkalinity zone on Piper diagram reflecting the recharge water of the River Nile. The other water samples were located in the mixed zone (~ 22%), while only one sample (~ 2%) plotted in the primary salinity zone. The mixed zone samples reflect the effect of interference of different waters and anthropogenic impacts.
The WQICCME values for the groundwater samples range from fair to marginal water quality. The water seems to be threatened due to extensive agricultural activities to the east of the study area. IWQI values for samples show excellent water for irrigation that could be used without any cautions on soil or crops. This may be attributed to the continuous recharge of River Nile water and the infiltrated water from irrigation canals.
The suitability of water for irrigation was assessed using several parameters e.g., Na%, PI, SAR, KI, RSC, and MH where the majority of groundwater samples are suitable for irrigation.
Based on the results of this study, it can be recommended to use the minimum limit of fertilizers and nutrients to decrease the anthropogenic impact on the groundwater. The reuse of irrigation water without treatment imposes impacts on the water quality so, it is recommended to minimize this usage. The groundwater in the west of the study area shows higher salinities than in the east, so, the pumping rates should be controlled to avoid aquifer salinization.
References
Abdelhameed AT, Salem, ZE, Osman OM (2019) Sedimentological characteristics of the quaternary groundwater aquifer, northwestern Nile Delta, Egypt. Groundw Nile Delta, pp 161–186
Abu Salem H, Gemail KS, Nosair AM (2021) A multidisciplinary approach for delineating wastewater flow paths in shallow groundwater aquifers: a case study in the southeastern part of the Nile Delta. Egypt J Contam Hydrology 236:103701
Abu Salem H, Abu Khatita A et al (2017) Geo-environmental evaluation of Wadi El Raiyan Lakes, Egypt, using remote sensing and trace element techniques. Arab J Geosci 10:224. https://doi.org/10.1007/s12517-017-2991-3
Abu Salem HS, Gemail KS et al (2022) An Integrated approach for deciphering hydrogeochemical processes during seawater intrusion in coastal aquifers. Water 14(7):1165
Abu Salem HS, Albadr M et al (2023) Unraveling the hydrogeochemical evolution and pollution sources of shallow aquifer using multivariate statistical analysis and hydrogeochemical techniques: a case study of the Quaternary aquifer in Beni Suef area. Egypt Environ Monit Assess 195(6):670
Anim-Gyampo M, Anornu GK et al (2019) Quality and health risk assessment of shallow groundwater aquifers within the Atankwidi basin of Ghana. Groundw Sustain Devel 9:100217. https://doi.org/10.1016/j.gsd.2019.100217
APHA (American Public Health Association/American Water Works Association/Water Environment Federation; Washington DC, USA) (1995) WPCF, Standard Methods for the Examination of Water and Wastewater
Appelo CAJ, Postma D (1999) Chemical analysis of groundwater, geochemistry, groundw and pollution. Balkema, Rotterdam
Arumugam K, Elangovan K (2009) Hydrochemical characteristics and groundwater quality assessment in Tirupur Region, Coimbatore District, Tamil Nadu, India. Environ Geol 58:1509–1520
Ayers RS, Westcot DW (1994) Water quality for agriculture, FAO: Rome, Italy, 29:77042–77044
Bora M, Goswami DC (2017) Water quality assessment in terms of water quality index (DWQI): case study of the Kolong River, Assam. India Appl Water Sci 7(6):3125–3135
Călmuc VA, Călmuc M et al (2018) Various methods for calculating the water quality index. Ann Dunarea Jos Univ Galati Fascicle II Math Phys Theor Mech 41:171–178
CONOCO (1987) Geological map of Egypt, 1: 500,000. In: Klitzsch E, List FK, Pöhlmann G (eds) Map sheets NH36NW and NH35NE. CONOCO with cooperation of The Egyptian General Petroleum Corporation, Cairo
Davis JC, Sampson RJ (1986) Statistics and data analysis in geology. Wiley, New York
Delgado C, Pacheco J et al (2010) Quality of groundwater for irrigation in tropical karst environment: the case of Yucatan. Mexico Agric Water Manag 97(10):1423–1433. https://doi.org/10.1016/j.agwat.2010.04.006
Doneen LD (1964) Notes on water quality in agriculture. Department of Water Science and Engineering, University of California, California
Eaton SN (1950) Significance of carbonate in irrigation water and soil. Science 39:123–133
Egbueri JC (2019) Evaluation and characterization of the groundwater quality and hydrogeochemistry of Ogbaru farming district in southeastern Nigeria. SN Appl Sci. https://doi.org/10.1007/s42452-019-0853-1
Egbueri JC, Mgbenu CN, Chukwu CN (2019) Investigating hydrochemical processes and quality of water resources in Ojota and environs using integrated classical methods. Model Earth Syst Environ. https://doi.org/10.1007/s4080-019-00613-y
Egbueri JC, Ezugwu CK, Unigwe CO, Onwuka OS, Oyemesili OC, Mgbenu CN (2020) Multidimensional analysis of the contamination status, corrosivity and hydrogeochemistry of groundwater from parts of the Anambra Basin Nigeria. Anal Lett. https://doi.org/10.1080/00032719.2020.1843049
Egbueri JC, Mgbenu CN, Digwo DC, Nyigide CS (2021) A multicriteria water quality evaluation for human consumption, irrigation and industrial purposes Umunya area southeastern Nigeria. Int J Environ Anal Chem. https://doi.org/10.1080/03067-319-2021.1907360
EHA (Egyptian Health Authority) (2007) Guideline for drinking water and household. http://extwprlegs1.fao.org/docs/pdf/egy83626.pdf 458
Ekenta OE, Okoro BU, Ezeabasili ACC (2015) Hydrogeological characteristics and groundwater quality analysis for selected boreholes in Oghara Local government Area, Anambra state Nigeria. Am Sci Res J Eng Technol Sci 14(2):198–210
El Osta M, Masoud M, Ezzeldin H (2020) Assessment of the geochemical evolution of groundwater quality near the El Kharga Oasis, Egypt using NETPATH and water quality indices. Environ Earth Sci 79:56. https://doi.org/10.1007/s12665-019-8793-z
Emara MM, El Sabagh I et al (2007) Evaluation of drinking groundwater for the rural areas adjacent to the nearby desert of Giza governorate of Greater Cairo, Egypt. Environmental security in harbors and coastal areas: management using comparative risk assessment and multi-criteria decision analysis. Springer, Netherlands, pp 379–394
EPA (2003) Long term 2 enhanced surface water treatment rule, toolbox guidance manual. In: United States environmental protection agency Washington
EPA (2014) Advice Note.15: Optimization of Chemical Coagulant Dosing at Water Treatment Works. Version 1
FAO (1985) Water quality guidelines for agriculture, surface irrigation and drainage. FAO: rome Italy
Field A (2009) Discovering statistics using SPSS: book plus code for E version of text (896). SAGE Publications Limited
Fisher RS, Mullican WF (1997) Hydrochemical evolution of sodium sulfate and sodium-chloride groundwater beneath the northern Chihuahuan Desert, Trans-Pecos, Texas, USA. Hydrogeol J 5(2):4–16. https://doi.org/10.1007/s100400050102
Gad M, Elsayed S et al (2020) Combining water quality indices and multivariate modeling to assess surface water quality in the northern Nile Delta. Egypt Water 12:2142
Gad M, Abou El-Safa MM et al (2021) Integration of water quality indices and multivariate modeling for assessing surface water quality in Qaroun Lake. Egypt Water 13:2258
Gad M, Saleh AH, Hussein H, Elsayed S, Farouk M (2023) water quality evaluation and prediction using irrigation indices, artificial neural networks, and partial least square regression models for the Nile river. Egypt Water 15(12):2244. https://doi.org/10.3390/w15122244
Gao Y, Chen J et al (2022) Hydrogeochemical characteristics and processes of groundwater in an over 2260 year irrigation district: a comparison between irrigated and nonirrigated areas. J Hydrol 606:127437
Gao Y, Qian H et al (2020) Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J Clean Prod 260:121006. https://doi.org/10.1016/j.jclepro.2020.121006
Geologic Survey and Mining Authority of Egypt (1981) Geologic map of Egypt, scale 1:2000000. Ministry of Industry and Mineral Resources, Cairo
Gholami S, Srikantaswamy S (2009) Analysis of agricultural impact on the Cauvery river water around KRS dam. World Appl Sci J 6:1157–1169
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Hameed A, Jawad M et al (2015) Evaluating water quality of Mahrut River, Diyala, Iraq for irrigation. Eng Tech J 33:830–837
Hem JD (1995) Study and interpretation of the chemical characteristics of natural water. USGS Water Supply Paper 264:117–120
Hussein H, Abd El-Raouf A, Almadani S, Abdelrahman K, Ibrahim E, Osman OM (2021) Application of geochemical modeling using NETPATH and water quality index for assessing the groundwater geochemistry in the south Wadi El-Farigh area. Egypt J King Saud Univ Sci. https://doi.org/10.1016/j.jksus.2020.101284
Hussein H, Ricka A et al (2017) Groundwater hydrochemistry and origin in the south-eastern part of Wadi El Natrun. Egypt Arab J Geosci 10:170. https://doi.org/10.1007/s12517-017-2960-x
Jia H, Qian H et al (2020) Alterations to groundwater chemistry due to modern water transfer for irrigation over decades. Sci Total Environ 717:137170
Kaiser HF (1958) The varimax criterion for analytic rotation in factor analysis. Psychometrika 23:187–200
Kelley WP (1940) Permissible composition and concentration of irrigated waters. In: Proceedings of the A.S.C.F 607
Kelley WP (1951) Alkali soils: their formation, properties and reclamation. Reinhold, New York
Kumar M, Ramanathan AL et al (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. J Environ Geol 50:1025–1039
Kumar M, Kumari K et al (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab. India Environ Geol 53(3):553–557
Mabrouk MB, Jonoski A et al (2013) A review of seawater intrusion in the Nile Delta groundwater system—the basis for assessing impacts due to climate changes and water resources development. Hydrol Earth Syst Sci Discuss 10:10873–10911
Manoj S, Thirumurugan M, Elango L (2017) An integrated approach for assessment of groundwater in and around uranium mineralized zone, Ggi region, Karnakata, India. Arabian J Geosci https://doi.org/10.1007/s12517-017-3321-5
Matthess G (1982) The properties of groundwater. Wiley, New York, p 498
Mayer XM, Ruprecht JK, Bari MA (2005) Stream salinity status and trends in south-west Western Australia, Department of Environment, Salinity and land use impacts series, Report No. SLUI 38
Mohamed EA, El-Kammar AM et al (2015) Hydrogeochemical evolution of inland lakes’ water: a study of major element geochemistry in the Wadi El Raiyan depression. Egypt J Adv Res 6:1031–1044
Mohammed MS, Mohamed EA et al (2022a) Geochemical evolution of groundwater in the quaternary aquifer in Beni Suef area. Egypt Egypt J Chem 66(8):319–338
Mohammed MS, Elbeih SF et al (2022b) Spectral indices based study to evaluate and model surface water quality of Beni Suef Governorate. Egypt Egypt J Chem 65:631–645
Mondal NC, Tiwari KK et al (2016) Diagnosis of groundwater quality from a semiarid region in Rajasthan, India. Arab J Geosci 9:1–22
Morsy S (2009) Environmental management to groundwater resources for Nile Delta region. PhD thesis Fac Eng Cairo Univ Egypt
MWRI (Ministry of Water Resources and Irrigation, Egypt) (2016) Water scarcity in Egypt: the urgent need for regional cooperation among the Nile Basin Countries, p 5. http://www.mfa.gov.eg/SiteCollectionDocuments/Egypt%20Water%20 Resources%20 Paper_2014.pdf
Naaz A, Anshumali L (2015) Hydrochemistry of fluoride–rich groundwater in semiarid region central India. Arabian J Geosci. https://doi.org/10.1007/s12517-015-1936-y
Nashwan MS, Shahid S, Wang X (2019) Assessment of satellite-based precipitation measurement products over the hot desert climate of Egypt. Rem Sens 11(5):555. https://doi.org/10.3390/rs11050555
Nasr SA, Abdel-Motelib A et al (2023) An integrated assessment of the groundwater chemistry of the aquifers at North Giza Governorate, using multivariate statistical analysis, ionic ratios, and geochemical modeling. Eg J Geol 67(1):147
Nosair AM, Shams MY et al (2022) Predictive model for progressive salinization in a coastal aquifer using artificial intelligence and hydrogeochemical techniques: a case study of the Nile Delta aquifer. Egypt Environ Sci and Poll Res 29:9318–9340
Obeidatt A, Alawneh M (2019) Hydrochemistry and groundwater quality assessment in Mafraq Province, Jordan. Open Access Libr J 6:1–10
Pei-Yue L, Hui Q, Jian-Hua W (2010) Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, Northwest China. E-J Chem 7:S209–S216
Piper AM (1944) A graphic procedure in the geochemical interpretation of water- analyses. EOS Trans Am Geophys Union 25:914–928
Purushothman P, Rao MS et al (2012) Drinking and irrigation water quality in Jalandhar and Kapurthala Districts, Punjab, India: Using hydrochemistry. Int J Earth Sci Eng 5:1599–1608
Rajesh R, Elango L, Brindha K (2019) Methods for assessing groundwater quality book chapter GIS and geostatistical techniques for groundwater sciences, 10/1016/B978-0-12-815413-00006-7
Ravikumar P, Somashekar RK, Angami M (2011) Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin Belgaum District, Karnataka State, India. Environ Monit Assess. https://doi.org/10.1007/s10661-010-1399-2
Richards LA (1954) Diagnosis and improvement of Saline and Alkali Soils, U.S. Department of Agriculture: Washington DC USA
Rizzini A, Vezzani F et al (1978) Stratigraphy and sedimentation of a Neogene-Quaternary section in the Nile Delta area (A.R.E.). Marine Geol 27:327–348
Ryner JW (1944) A new index for determining amount of calcium carbonate scale formed by water. J Am Water Assoc 36:472–486
Sakram G, Adimalla N (2018) Hydrogeochemical characterization and assessment of water suitability for drinking and irrigation in crystalline rocks of Mothkur region, Telanagana state, south India. Appl Water Sci. https://doi.org/10.1007/s1320-018-0787-6
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Salem ZE, Al Temamy AM et al (2016) Origin and characteristics of brackish groundwater in Abu Madi coastal area, Northern Nile Delta. Egypt Estuar Coast Shelf Sci 178:21–35
Schöeller H (1965) Geochemistry of groundwater. Groundwater studies–an international guide for research and practice. UNESCO, Chapter 15, Paris, pp 1–18
Sherif MM, Sefelnasr A, Javadi A (2012) Incorporating the concept of equivalent freshwater head in successive horizontal simulations of seawater intrusion in the Nile Delta aquifer. Egypt J Hydrol 464:465
Srinivasamoorthy K, Gopinath M et al (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu, India. J King Saud Uni Sci 26:37–52
Stambuck-Giljanovic N (1999) Water quality evaluation by index in Dalmatia. Water Res 33(16):3426–3440
Stigter TY, Ribeiro L, Carvalho Dill AM (2006) Application of a groundwater quality index as an assessment and communication tool in agro-environmental policies—two Portuguese case studies. J Hydrol 327:578–591
Suarez DL, Wood JD, Lesch S (2006) Effect of SAR on water infiltration under a sequential rain-irrigation management system. Agric Water Manag 86:150–164
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Sudhakar A, Narsimha A (2013) Suitability and assessment of groundwater for irrigation purpose: a case study of Kushaiguda area, Ranga Reddy district, Andhra Pradesh, India. Adv Appl Sci Res 4:75–81
Sundaray SK, Nayak BB, Bhatta D (2009) Environmental studies on river water quality with reference to suitability for agricultural purposes: Mahanadi river estuarine system, India—A case study. Environ Monit Assess 155:227–243
Szabolcs I, Darab C (1964) The infuence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th international congress of ISSS, transmission, 2:803–812
Tiwari TN, Manzoor A (1988) River pollution in Kathmandu valley (Nepal) suitability of river water for irrigation. Indian J Environ Prot 8:269–274
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, NewYork, p 535
Todd DK, Tinlin RM, et al. (1976) Monitoring ground-water quality: monitoring methodology. U S National Technical Information Service Springfield, Va, Report PB 256 068; U S Environmental Protection Agency, Report No EPA-600/4-76-026:154
Tyagi S, Sharma B et al (2013) Water quality assessment in terms of water quality index. Am J Water Resour 1(3):34–38
USEPA (2008) Stage 2, disinfectant and disinfection by-products, rule consecutive systems guidance
USEPA (2018) Drinking water standards and health advisories EPA 822-F-18–001. Office of water, US environmental protection agency Washington (DC)
USSL Staff (1954) Diagnosis and improvement of saline and alkali soils, agricultural handbook No. 60. USDA. Agricultural research service, soil and water conservation research branch, Washington, DC, USA
Walton WC (1970) Groundwater resources evaluation. McGraw-Hill, New York
WHO (World Health Organization) (1997) Guidelines for drinking-water quality. 2nd ed 3 Surveillance and control of community supplies. Geneva: WHO
WHO (World Health Organization) (2011) Guidelines for drinking-water quality. 4th edn. 104–108
Wilcox L (1948) The quality of water for irrigation use. US Department of Agricultural Technical Bulletin 1962. US Department of Agriculture, Washington, DC
Wilcox LV (1955) Classification and use of irrigation waters. United States Department of Agriculture, Washington
Zhang Q, Qian H et al (2021) Groundwater quality assessment using a new integrated-weight water quality index (IWQI) and driver analysis in the Jiaokou Irrigation District China. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2021.111992
Zhao X, Guo H et al (2021) Groundwater hydrogeochemical characteristics and quality suitability assessment for irrigation and drinking purposes in an agricultural region of the North China plain. Environ Earth Sci 80:162
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by all authors commenting on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article.
Human and animal participants
There is no Human Participants and/or Animals in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussein, H., El Maghraby, M.M.M.S. & Abu Salem, H.S. Application of water quality index and statistical-hydrochemical techniques in groundwater assessment of the Quaternary aquifer, southwest Nile Delta of Egypt. Appl Water Sci 14, 143 (2024). https://doi.org/10.1007/s13201-024-02189-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02189-0