Abstract
Climate change has clearly affected the desert city of Bechar, located in southern Algeria, and this miserable situation for the supply of drinking water prompted the authorities to provide capabilities and funds to bring groundwater located 250 km away and transfer it to the city of Bechar. The characterization of these underground waters presents a bicarbonate-magnesian facies according to the diagram of Schöeller and Berkaloff; the representation of the data on the triangular Piper diagram shows that Boussir ground water has the magnesium bicarbonate facies. The calculation of the quality index (GWQI) shows that all samples taken from the boreholes belong to the good quality category. The long distance of diversion of this underground water and the quality of the materials used in the project under a dry desert climate made us carry out the process of monitoring and tracking the quality of the water from the well until it reaches the consumer. The results revealed that all the levels of the physic-chemical parameters do not exceed the WHO portability standards, except that a variation of certain values was observed at the level of the storage tank, this variation due to the mode of filling and the mixing of water in tubular form, without eliminating the effect of water stagnation. If we technically know how to produce high-quality drinking water, we cannot always ensure a safe and sustainable water supply of the same quality in distribution networks and reservoirs; it is from this principle that our article is based in order to reinforce the monitoring role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sustainable access to water resources is a major concern for all countries in the Mediterranean Basin. Climate change, urban and demographic growth as well as the expansion of agricultural activities, expected in the region, is likely to exacerbate the water stress situation already affecting most of the southern and eastern Mediterranean countries (Kahlerras et al 2018; Mebarki et al. 2021).
Water intended for human consumption must not pose direct or indirect risks to health; for the water producer and the distributor, this requires obligations of results in terms of water quality parameters, but also obligations of means whether for raw water (where standards are dictated), the establishment of a protective perimeter around the water intake, an approval of the treatment sector and the reagents used, as well as materials, finally an obligation to maintain hydraulic structures (Bendida 2019).
The complexity of the chemical or biological reactions that occur on the network requires acting on the quality of the water produced but also in the operation of the distribution network (Bendida et al. 2021). Experiments show that good water quality can only be maintained if a certain number of instructions for operating the networks and their accessories are followed (Bendida 2019; Kendouci et al. 2016).
Water supply monitoring generates data on the safety and adequacy of the drinking water supply to help protect human health. Most current models for monitoring urban water supplies come from developed countries and have significant shortcomings if applied directly elsewhere. There are differences not only in socioeconomic conditions, but also in the nature of water supply services, which often include a complex mix of formal and informal services (Benalia 2012; Kendouci et al. 2013a).
The management and design of the drinking water supply network stimulate several factors that can lead to deterioration of water quality. In a distribution system, the reservoirs perform three main functions: providing a buffer reserve to compensate for the differences between production and consumption, maintaining the pressure in the network, constituting a reserve in the event of incidents and, in some cases, ensuring fire defense. The design of the reservoirs must make it possible to meet these essential functions, in particular, in the determination of the altimetry heights and the volume (Kendouci et al. 2019). However, in order to avoid the significant risks of degradation of the quality of the water in these structures, certain design rules must be taken into account from the outset of the project.
As in the other parts of the network, the renewal of water in the reservoirs is a necessary condition for the preservation of water quality. The residence time directly depends on the storage volumes. These volumes make it possible to ensure sufficient security of supply without exaggerating the residence time of the water in the structure. More generally, it is considered that the storage volume must be renewed within an interval of 1 to 3 days (Kendouci et al. 2019).
The shape of the tanks as well as the filling and emptying devices must allow sufficient circulation of the water to avoid the formation of areas of stagnant water and therefore prevent the proliferation of germs. The design of these devices often requires the realization of a study on a physical model to optimize the mixing conditions.
The conservation of water quality is facilitated by a reduction in the residence time in the network. In urban networks, there is often a high mesh, which ensures quantitative security, imperatives of fire safety, makes it possible to deal with peak hours and facilitates water stoppages if necessary. However, it increases the residence time of the water in the network (Kadhim et al.2021).
In reservoirs, the residence time of water can increase depending on operating constraints, or even result from design errors for the design of singular points on the network.
The water residence time is not a single value for the network, but is represented by a statistical distribution. The average residence time of water in the network can be of the order of a few days, but certain volumes of water can stagnate for more than ten days in areas of the network where the flow is weak or the demand for almost no water. Studies have shown that stagnation problems favoring corrosion and deposits appear as soon as the water velocity is less than 0.01 m/s (Kendouci 2013b).
The interactions between the water and the materials of the distribution network, that is to say the container, can be the cause of degradation of the quality of the water distributed. Direct contact between water and metal (cast iron) must be avoided in order to prevent any corrosion phenomenon.
Algeria, like countries affected by water stress (Kendouci et al. 2023), is in the category of poorest countries in terms of water potential, below the theoretical scarcity threshold set by the World Bank at 1,000 cubic meters per capita and year (Mebarki et al. 2021).
To meet the satisfaction of drinking water needs, for the city of Bechar, located in southwest Algeria, technicians and decision makers have sought other additional water resources, namely in the Jurassic aquifer of Mougheul 45 km north of the city, the Turonian aquifer of Ouakda 20 km northeast of the city and the great groundwater resource of the Boussir well field which is 250 km north of the town of Bechar which exploits the waters of the Albian aquifer.
The objective of this work is to characterize and monitor the physic-chemical quality of water from the well field to the consumer. To do this, a sampling campaign was undertaken during the year 2023, in four locations on the course of the water flow, starting with the drilling then the pumping station, the buffer tank then the reservoir storage and ultimately to the consumer.
The physical and chemical parameters measured at the site and in the laboratory made it possible to characterize these waters. The results made it possible to identify and evaluate the quality of water for human consumption (WHO standards).
Material and methods
Description of case study area
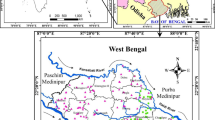
Bechar is located in southwest Algeria about 58 km south of the Moroccan border and 950 km southwest of the capital of Algiers with a surface area of 5050 km2. It is located at an altitude of 747 m, on the banks of the Oued Bechar which crosses the city from the northeast to the southwest (Fig. 1). The municipality of Bechar presents almost 63.01% of the total population of the province. In 2023, it has approximately 228,553 inhabitants.
Beni-Ounif, commune of the province of Bechar, is located northeast of the capital Bechar (Fig. 1). It extends over 16,600 km2, this commune is cut (crossed) by Oueds of Zousfana, Nemous, Melias and that of Sidi Abdelkader. The Boussir region well field (commune of Beni-Ounif) is located in an Albian water well field which extends over an area of 2,773.67 km2, north of the province.
The climate of Bechar city is monitored by a weather station located in longitude 31° 37′ 00″ N and in latitude 02° 14′ 00″ W and with an altitude of 772 m, indexed by 13 01 32. The station is managed by the National Agency for Hydraulic Resources (Bekhira et al. 2018) (Kendouci et al. 2013a). As for the climate, the city of Bechar is located in the arid zone with a cold winter and hot summers. According to rainfall and temperature in Bechar city during the period 1985–2015, the maximum average annual rainfall appears in October with 21 mm; the minimum of 2.3 mm is in July; the average annual precipitation becomes 72.97 mm. The rainy season extends from September to March, with a maximum in October. Minimum temperatures range from 3.4 to 26.6 °C, and maximum temperatures from 16.1 to 39.6 °C (ADE, 2023).
From a geological point of view, the study region is part of the transition zone between the Saharan atlas and the Saharan platform (Menchikoff 1936). It includes training:
-
1.
The Jurassic: well developed in the Jebel but of limestone and dolomite nature. On the other hand, at the level of Djebel Bou Yala and Fendi the Jurassic is perhaps developed represented by evaporitic clays and dolomites.
-
2.
The Cretaceous: poorly exposed, it is essentially the Lower Cretaceous (Albian) of sandstone nature and it is an excellent water reservoir.
-
3.
The tertiary sector: can also develop in the Béni Ounif region. Forms a thin calcareous shell.
-
4.
The Quaternary: represented by the alluvium of the wadis, the regs and the sebkha.
In the study area, only one aquifer is exploited: the Lower Cretaceous sandstone aquifer (known as Albian) whose thickness is greater than 100 m, it is made up of more or less permeable hard sandstones, permeable sandy sandstones, semipermeable sandy sandstones and impermeable red clays, covering almost the entire syncline either in outcrop or at depth.
Water sources available
Dependence on groundwater remains to provide drinking water to the region still exists. Groundwater resources in the Bechar region are:
-
1.
The Ouakda aquifer consisting of two aquifer levels (Turonian and Quaternary). The water resources of this aquifer estimated by the ANRH at 6700m3/d and are in a state of decline, because it is a field which has been exploited in the agricultural field in a random and unorganized way, in addition to the unauthorized drilling, which requires putting it to rest and reducing production by him to restore his energy, thinking another resource plus a meme of meet the needs of the city (ADE 2023).
-
2.
The drinking water supply of Bechar has been strengthened by the recent commissioning in the region of Mouhguel in the north of Bechar near the Algerian–Moroccan border. The Mougheul aquifer is a semi-captive aquifer with an average thickness of 64 m contained in the very cracked dolomitic limestones of the Lower Jurassic of the Koudiat El Haïdoura formation. It is the most important permeable base of the entire system in this region and even in the northern part of Bechar (Mebarki et al. 2021). Mougheul aquifer at a flow rate of 2600 m3/d in 2023.
-
3.
The large-scale transfer project over 250 km of the Albian water pipeline network from the well field of the Boussir region (municipality of Beni-Ounif—north of province of Bechar) to the municipality of Bechar, partially commissioned since July 2020. The project currently allows the delivery of a quantity of 11,000 m3 for the needs of the drinking water supply of the population, with a global offer and daily capacity of 30,000 m3/d. The project considers as insufficient and unsecured, and these are the type of pumping channels which have proved to be little practical, especially in the event of leaks which produce there, which increases the time of repair and direct pumping for more than 48 h at least, which leads to a fluctuation in distribution given the flagrant lack of storage capacity in city (ADE, 2023).
Drinking water supply in the city of Bechar
The drinking water supply network of Bechar city extends over 620 km. For the gravity and pressure adduction, works are used as: 30 drillings, 5 pumping station of 36,190 m3 /d and 11 storage and distribution tanks with a total capacity of 34,250 m3. The network of Bechar city covers more than 620 km of which: (i) 60% of pipes with a diameter of less than 200 mm (minimum diameter 15 mm), (ii) 35% of pipes with a diameter greater than or equal to 200 mm. The largest diameter is the DN 800-mm ductile iron (ADE, 2023).
Sampling and analytical methods
The sampling of Bechar waters has been carried out in the month of March 2023, was conducted in laboratory; we have conducted a total of (20) levies for the physicochemical analysis divided as follows: ten (10) drilling of Boussir, 04 drilling capturing the Mougheul and (01) for the Ouakda (W20), and 4 samples to follow the change in physicochemical parameters are: at the drilling level, in the pumping station, in the buffer tank, in the reservoir storage and finally at the consumer. The geographic coordinates (X, Y, Z) of each water point were measured using a GPS (Global Positioning System) (Table 1).
The methods of analyze described by Rodier (1996) were followed during field and laboratory work. The pH was measured by a pH meter, conductivity is given directly in µS/cm by conductivity meter and the turbidity was measured by turbidimeter. The other chemical parameters by different methods:
-
The calcium (Ca+2) and magnesium (Mg+2), chloride (Cl−) by titrimetry.
-
The total hardness or the hydrotimetric title of the samples is determined by complexometry by titration with acid ethylene-diamine-tetracetique (EDTA).
-
Sodium Na+ and potassium K+ are dosed by photometry of the flame.
-
Ions sulfate (SO4−2) rushed to the state of barium sulfate and assayed by gravimetry.
-
Posphate, nitrite, manganese and iron are dosed by spectrophotometer.
-
Nitrate is dosed by colorimetry.
The calculation of the ion equilibrium error is evaluated by taking the relationship between the total cations and the total anions for each sample of water. It is recognized that the anion-cation balance is less than ± 5% (Domenico and Schwartz 1997).
Results and discussion
Chemical facies of groundwater
Hydrochemical processes occurring in aquifers may affect groundwater chemistry.
The distribution of groundwater facies is primarily attributed to local geology and lithology, particularly in arid to semiarid regions (Al-Mashreki et al. 2023).
The purpose of the interpretation of the results of the analyses is to determine the chemical facies; for this, we used the graphic methods of Schöeller and Berkaloff. According to the diagram of Schöeller and Berkaloff in the year 2023 (Fig. 2), we have found that drillings waters Mougheul and Boussie have the bicarbonate-magnesian facies, and the water point of W 20 has the chloride sodium facies.
The Piper’s trilinear diagram (Piper 1944) includes three triangles. (Back and Hanshaw 1965) have defined the subdivisions of the diamond field that have represented water type or categories that form the basis for one common classification scheme for natural waters. Lithology, solution kinetics and flow patterns of the aquifer control hydrochemistry of any facies (Nemčić-Juree et al. 2017).
The representation of the data on the triangular Piper diagram of the companions 2023 (Fig. 3) shows that water points of Mougheul and Boussir have the magnesium bicarbonate facies, and the water of drilling W20 has the facies mixed type.
To classify the groundwater and to identify the hydrochemical processes, a Chadha diagram (Chadha, 1999) is used (Fig. 4). This diagram is a modified version of Piper diagram (Piper, 1994) and the expanded Durov diagram (Durov, 1948) (Fig. 5) (Madhuri, 2013).
Applying Chadha diagram to groundwater allows us to see which samples from Mougheul and Boussir are found in group 1, drilling W20 in group 2 (Fig. 4). Group 1 contains water samples located near the limit of limestones; these are therefore the recharge waters. This is water with a low dry residue content and a calcium bicarbonate type. Those in group 2 are waters characterized by an excess of Ca and Mg to the detriment of Na + K. These are waters of medium salinity where the presence of Ca and Mg is due to the alteration of Ca2+rich minerals other than carbonates, probably gypsum and/or anhydrite.
Durov diagram (Fig. 5) can explain group (a) corresponds to the dissolution of minerals rich in calcium (calcite and gypsum). In group (b), we find the box where the composition of the water is the result of the reactions of mixed waters of different origins. Group (c) indicates water mixing and ion exchange.
Irrigation water quality
Irrigation has made a significant contribution to supporting the population’s expanding food demands, as well as promoting economic growth in irrigated regions (Eid et al. 2023).
Groundwater has become the primary source of water use in the agricultural sector of many countries where river systems, drainage and wastewater recycling are not sufficient. Therefore, poor groundwater quality for irrigation purpose is a matter of worry in recent years (Kishan et al. 2018).
Exchangeable sodium percentage (ESP)
The percentage of Na has been calculated using Eq. (1).
where all the concentration units are in meq/l. The sodium reacts with soil to reduce its permeability; higher amount of sodium in water has reduced crop yield. (Wilcox 1955) has used ESP and EC to classify groundwater and divided into five categories. It is recommended that the ESP should not exceed 60% in water which is used for irrigation purposes (Nemčić-Juree et al. 2017). All the Mougheul and Boussir water samples have low concentration of Na% and fall in excellent category; the water of the well W20 lies in the category of good (Fig. 6). Hence, generally water is suitable for agriculture application.
Water quality indices for human consumption
To guarantee the protection of the environment and human health, it is important to examine water quality. The primary step involves assessing the overall water quality and then detecting the source of pollutants to mitigate pollution levels.
The groundwater quality index (GWQI) method reflects the composite influence of different equation in accordance with WHO standards:
with Ci, concentration of each parameter; If, limit value of each parameter set by WHO; wi, the weight of each parameter according to its relative importance in the quality of drinking water; qi, quality rating for each parameter; Wi, the relative weight; and SIi, the subindex of parameter i.
The calculation of the quality index (GWQI) shows that all samples taken from the boreholes belong to the good quality category.
Physicochemical parameters
Hydrogen potential (pH)
pH is important in almost all phases of water treatment. The ideal pH level of drinking water is between 6 and 8.5. The pH value of water is used to determine whether water is hard or soft. Pure water has a pH of 7, and water lower than 7 pH is considered acidic. Acidic water sometimes contains metal ions such as manganese, copper, lead and iron. Acidic water causes damage to metal piping and has a sour taste. A neutralizer is typically used to treat a low-pH water system. Water with a pH above 8.5 is considered hard water and typically has a bitter taste. Hard water typically does not pose any health risks.
According to Fig. 7, the pH has an increase from the drilling 7.71 toward the buffer tank 7.88, and a decrease until the consumer 7.83, the rate of increase of Ph at the consumer compared to the drilling is reached 2%.
Electric conductivity (EC)
The conductivity is determined by measuring the electrical resistance of the solution. From the conductivity, we can assess the degree of salinity of the water; it is also a function of the temperature; it increases with the concentration of ions in solution and the temperature.
Figure 8 shows that the conductivity in the water may increase from the drilling to the buffer tank (760 μS/cm, 765 μS/cm), then a large increase in the reservoir storage (up to 871 μS/cm), which is justified by the addition of chlorine in the tank, the mode of filling and the mixing of water in tubular form, the mode of filling, the mixing, the stagnation and the residence time of the waters in the reservoirs, finally the value decreased to 738 in the case of distribution to consumer the rate of decrease of conductivity at the consumer compared to the drilling is reached 3%. In addition to the WHO standard, the results obtained are lower than the standards (2700 μs/cm) for the conductivity of drinking water.
Salinity
Salinity is measured by reading the electrical conductivity of water. The relationship between them is direct, the higher the salinity, the greater the conductivity. Figure 9 shows that the salinity content underwent a stabilization between the drilling to the buffer tank of around 0.3 mg/l; then an increase between the buffer tank and the refill tank (0.4 mg/l), finally a decrease until distribution to the consumer. The peak increase the salinity in the reservoir is justified by the same reasons previously mentioned in the conductivity.
Turbidity
Turbidity is the measure of relative clarity of a liquid. It is an optical characteristic of water and is a measurement of the amount of light that is scattered by material in the water when a light is shined through the water sample. At high flow, water velocities are faster and water volumes are higher, which can more easily stir and suspend and cause higher turbidity. Many consumers equate turbidity with safety and consider turbid water as being unsafe to drink. This response is exacerbated when consumers have been accustomed to receiving high-quality water. As a guide, “crystal clear” water has turbidity below 1 NTU, and water becomes visibly cloudy at 4 NTU and above (WHO 2017).
Figure 10 shows that the turbidity is stable 0.5NTU between the drilling and the pumping station, then decreased in the buffer tank, then increased to 0.9 NTU in the reservoir storage (the turbulent), finally decreased to 0.4 NTU at the consumer (the stagnation), and the rate of decrease of conductivity at the consumer compared to the drilling is reached 20%.
Turbidity should ideally be kept below 1 NTU because of the recorded impacts on disinfection. This is achievable in large well-run municipal supplies, which should be able to achieve less than 0.5 NTU before disinfection at all times and an average of 0.2 NTU or less, irrespective of source water type and quality (WHO 2017).
The complete alkalimetric title (CAT)
The alkalimetric total title corresponds to water content in free alkaline, carbonates and hydrogen carbonates which originally comes from the limestone bedrock of the watershed that gives water its bicarbonate nature (Jamali and Amir 2017).
Figure 11 shows that the complete alkalimetric title underwent an increase between the drilling and the buffer tank 25 mg/l; then a decrease to the consumer 10 mg/l, the rate of decrease of CAT at the consumer compared to the drilling is reached 15%.
The highest desirable limit prescribed by WHO is 250 mg/l for drinking purposes. So, the alkalinity is in many samples above the prescribed limit. However, the alkalinity characteristic has been demonstrated contribute in the stability of water by control its aggressiveness to the pipes and appliance (WHO 2006). In fact, for some metals, alkalinity (carbonate and bicarbonate) and calcium (hardness) also affect corrosion rates.
Hydrotimetric title (total hardness)
Total hardness of water is not pollution parameter but indicates water quality mainly in terms of Ca2+ and Mg2+ expressed as CaCO3. The calcium is generally the dominant element of the drinking waters, and its content varies essentially following the nature of the lands crossed (chalky or gypsies land) (Rodier 2009). High hardness promotes scaling; low hardness promotes corrosion.
Figure 12 shows that the total hardness underwent an increase between the drilling and the buffer tank 29 mg/l; then a decrease to the consumer 20 mg/l, the rate of decrease in total hardness at the consumer compared with drilling is 26%. (The use of soda therefore makes it possible to lower the hardness of water.) The results obtained show that all the waters analyzed (100%) are fresh, according to the drinking water standards established by the WHO.
Oxidizable materials
Oxidability tests are used instead of total organic carbon (TOC) tests to determine the concentration of organic matter present in a water supply. The limit for oxidizability is 5 mg/l of oxygen.
According to Fig. 13, the oxidizable matter (OM) has undergone an increase between the drilling and the pumping station 1.2 mg/l; and a decrease measured at the consumer 0.6 mg/l, the rate of decrease in oxidizability at the consumer compared with drilling is 40%.
Dry residue
The dry residue corresponds to the weight of all dissolved solids per liter of water; the water is obtained by evaporation after elimination of suspended solids. The purity of water is measured by the amount and size of dry residue it contains. Water with a low residues level is healthier and more pleasant to drink.
Results of the determination of the total amount of minerals (calculated as dry residue at 180 °C) are shown in Fig. 14; the dry residue content has undergone an increase since drilling up to the consumer 419 mg/l, justifying this progressive increase by justifying this gradual increase by the increase in water discharge and then the sediment deposits in the pipes. According to the total mineral quantity (in mg/L), the waters of the samples belong to the category of low mineral waters (< 500). The rate of increase of dry residue at the consumer compared to the drilling is reached 40%.
Calcium (Ca+2) and Magnesium (Mg+2)
Calcium is generally the dominant element in drinking water, and its content varies mainly depending on the nature of the terrain crossed (limestone or gypsum soil) (BRGM 2006). The magnesium is an element that often accompanies calcium, and comes from the dissolution of dolomites, dolomitic limestones and ferromagnesian minerals (BRGM 2006).
Maximum allowable limit of calcium and magnesium in the drink water is 200 mg/L and 150 mg/L as suggested by the WHO limit. In the samples, the calcium and magnesium content in the water varied from 32 to 48 mg/L and 33–40.8 mg/L, respectively (Fig. 15).
From the figure below, the contents of calcium and magnesium underwent a small increase between the drilling and the buffer tank; and a decrease between the buffer tank and the reservoir storage is a final stabilization up to the consumer. (The use of soda therefore makes it possible to lower the hardness of water.) The rate of decline of the calcium of the consumer in relation to the drilling is 27%, and the rate of decrease of the magnesium of the consumer compared to the drilling is 13%.
Sodium (Na+) and Potassium (K+)
Sodium is mostly found in natural waters, whereas the potassium content of natural water is usually less than that of sodium.
According to Fig. 16, we note that the sodium content has undergone a small increase between the drilling and the pumping station of 31 mg/l; and a final decrease to the consumer 13 mg/l, in well water the sodium concentration is higher than the piped. The potassium content underwent a small increase between the drilling and the pumping station, then a decrease in the buffer tank, then an increase in the reservoir storage, finally a decrease at the consumer. The rate of decrease of the sodium of the consumer in relation to the drilling is 57%, and the rate of increase of the potassium of the consumer compared to same source is 133%.
The results indicate that the sodium and potassium contents in all the water samples are well within the permissible limit as per guideline laid down by the WHO standard (250 mg/l and 12 mg/l, respectively).
Chlorides (Cl−) and Sulfates SO4 −2
Both chlorides and sulfates contribute to the total mineral content of water. High concentrations of either sulfate or chloride ions add to the electrical conductivity of water. Chlorides can have several origins and are mainly linked to the dissolution of salt fields. The dissolution of these salts is very easy; hence, their presence in high concentrations in the waters having crossed the clay-sandy or clayey formations. Thus, they can also come from human action from highway relief, or by contamination by wastewater (BRGM 2006). Chlorides give an unpleasant taste and pose the problem of corrosion in pipes and tanks from 500 mg/l (WHO standard) (Mebarki et al. 2021).
Sulfates are present in natural waters at very variable levels, and they can come from the dissolution of gypsum. They also depend on industrial waste. Sulfates are very soluble and also very stable elements (BRGM 2006).
Figure 17 shows that the chloride content underwent a small increase in the pumping station of 0.6 mg/l; then and a remarkable increase up to the subscriber 180 mg/l. The sulfate content underwent a small increase between the drilling and the pumping station of 0.5 mg/l; and a successive decrease until the consumer 73 mg/l. The rate of increase of the chlorides of the consumer in relation to the drilling is doubled due to the addition of chlorides in water treatment technologies, and the rate of decrease of the sulfates of the consumer compared to same source is 14%.
Iron (Fe2+) and Manganese (Mn2+)
Iron and manganese are an abundant metal found in the Earth’s crust. It is naturally present in water but can also be present in drinking water from the use of iron coagulants or the corrosion of steel and cast iron pipes during water distribution (Elahcene et al. 2019).
Iron is an essential element in human nutrition. (WHO 2006) states that, values of up to 0.3 mg/L. The studied sample ranged from 0.01 to 0.04 mg/L, so it does not present a hazard to health. High levels of manganese also cause objectionable tastes in the water, but there are no particular toxicological connotations. The WHO recommends a guideline value of 0.1 mg/L. The average manganese of water in samples varied between below the limit of detection to 0.03 mg/L.
Figure 18 shows that the iron content underwent stabilization between the drilling and the buffer tank of around 0.04 mg/l; and a decrease to the consumer 0.01 mg/l. The content of manganese underwent stabilization between the drilling and the pumping station 0.01 mg/l; then an increase between the pumping station and the buffer tank, then stabilization toward the reservoir storage, finally an increase dear to the consumer of 0.03 mg/l. The rate of decrease of the iron of the consumer in relation to the drilling is 7%, and the rate of increase of the manganese of the consumer compared to same source is doubling twice.
Nitrates (NO3 −) and Nitrites (NO2 −)
It is a very soluble form, and its presence in water is linked to fertilizers, nitrate ion (NO3−) is the most oxidized form of nitrogen. Natural sources of nitrate are mainly rain and interactions with soil and vegetation. Anthropogenic sources of nitrate are numerous and are mainly linked to the leaching of fertilizers and to domestic and industrial discharges (BRGM 2006). The nitrate contents are between 11 at consumer and 67 mg/l at drillings level which exceeds the limits recommended by the WHO (50 mg/l).
While the increase in the nitrite because either ammonium oxidation or reduction of nitrate by denitrification (Mebarkia et al. 2017). All the water samples had traces of nitrite 0.01–0.02 mg/L (Fig. 19). The World Health Organization required that nitrites be minimal because they constitute substances that cause cancer and anemia (WHO 1999).
According to Fig. 19, we note the content of nitrate in the drilling 67 mg/l underwent a decrease until the subscriber 11 mg/l, and the rate of increase in nitrate at the consumer compared to the drilling reached 16%. The content of nitrite underwent a stabilization of 0.01 mg/l between the drilling and the buffer tank then and an increase until the reservoir storage finally a decrease until the consumer 0.01 mg /l due to water treatment techniques.
Phosphate (PO4 3−)
The major sources of nitrate and phosphate come from human activities, primarily through the addition of fertilizers to crops, lawns and gardens, from municipal and home sewage systems and animal feed. The World Health Organization (WHO) has set safety limits for the allowable concentration of phosphate for no more than 1 mgL − 1, respectively, in drinking waters (Yang et al. 2010). Therefore, effective removal of excess nitrate and phosphate from water is critical to prevent eutrophication and restore water quality.
Figure 20 shows that the phosphate content increased between the drilling and the buffer tank of 0.08 mg/l then decreased to the consumer of 0.05 mg/l and the rate of increase phosphate at the consumer compared to the drilling reached 150%.
Conclusion
This study was conducted to give the monitoring the spatial evolution of groundwater quality during its diversion in the drinking water supply network Bechar city, in order to assess its quality for drinking water supply and irrigation. The need for this research is due to the knowledge of the effect of the length of supply pipes and distribution in the drinking water supply network, the mixing of water in the reservoirs and their filling mode, and the residence time of the stagnation of water in reservoirs and in pipes on the spatial evolution of groundwater quality. The physicochemical study of the groundwater of Bechar city shows that according to the diagram of Schöeller and Berkaloff, we have observed that the groundwaters of Mougheul and Boussir have the bicarbonate-magnesian facies, and the water point of W 20 has the chloride sodium facies. The representation of the data on the triangular Piper diagram shows that water points of Mougheul and Boussir have the magnesium bicarbonate facies, the water of drilling W20 has the facies mixed type. Application of the Chadha diagram to groundwater allows us to see that the waters of Mougheul and Boussir are found in the recharge waters, and the W20 drilling waters are waters characterized by an excess of Ca and Mg to the detriment of Na + K. These are waters of medium salinity where the presence of Ca and Mg is due to the alteration of minerals rich in Ca2 + other than carbonates, probably gypsum and/or anhydrite. The Durov diagram shows that drilling W20 corresponds to the dissolution of minerals rich in calcium (calcite and gypsum). The waters of Mougheul are found in the box where the composition of the water is the result of the reactions of mixed waters of different origins. The waters of Boussir indicate the mixing of water and the exchange of ions.
The classification according to the Wilcox diagram shows that all the Mougheul, Well W20 and Boussir water samples are good quality. The calculation of the quality index (GWQI) shows that all samples taken from the boreholes belong to the good quality category.
The monitoring the spatial evolution of groundwater quality during its diversion in the drinking water supply network Bechar city shows that the total hardness of all the waters analyzed (100%) is fresh. The measure of turbidity is evidence that the water is pure. The dry residue shows that the waters of the samples belong to the category of low mineral waters. The water samples remain drinkable, according to the drinking water standards of the WHO, except that a variation of certain values was observed at the level of the storage tank, this variation due to the mode of filling and the mixing of water in tubular form, without eliminating the effect of water stagnation.
We can justify the changes that occurred in the path of diverting water to the consumer as follows:
The variation in water quality at the level of boreholes is generally due to:
Infiltration of runoff water, penetration of microfauna and introduction of contaminated materials due to design flaws due to the direct access route above the body of water or poorly protected, ventilation holes above from the body of water or poorly protected, poorly protected overflow drainage pipe and the catchment exposed to daylight.
At reservoir level, the main causes of changes in water quality are:
-
Water stagnation due to over sizing for fire defense and when the reservoir acts as an end and balance reservoir.
-
Infiltration of runoff water, penetration of microfauna and introduction of contaminated materials due to ventilation openings above the water body or poorly protected.
-
Heating of the water due to insufficient thermal insulation, especially since the region of Bechar is an arid Saharan zone where the temperatures are very high.
-
Poorly protected overflow water drainage pipe and defective external sealing which allow the penetration and infiltration of runoff water.
At the network and consumer level, quality has suffered a decrease due to:
-
Stagnation of water in the pipes (main and secondary) and formation of deposits due to:
-
High mesh of secondary pipes (creation of balancing zones).
-
Over sizing of secondary antennas for fire protection or for other non-permanent reasons.
-
Isolated neighborhoods with low consumer density.
-
-
Leaks which would allow an intrusion of polluted water in the event of depression.
-
High flow velocity (excessive consumption, bleeding and rupture of a pipe).
-
Flooding of a suction cup manhole.
-
Depression in the public distribution network (water cutoff, excessive consumption and rupture of a pipe) or pressurization of a private distribution installation of drinking water, raw or rainwater
-
Warming of the water due to the strong summer heat with warming of the ground.
-
Permeable nature of conveying material and unsuitable material for potable water supply.
References
ADE (2023) Algerian for Waters, technical sheet of the municipality of Bechar (Situation of AEP stopped on April 30, 2023), pp 1 and 2.
Al-Mashreki MH, Eid MH, Saeed O, Székács A, Szűcs P, Gad M, Abukhadra MR, AlHammadi AA, Alrakhami MS, Alshabibi MA, Elsayed S, Khadr M, Farouk M, Ramadan HS (2023) Integration of geochemical modeling, multivariate analysis, and irrigation indices for assessing groundwater quality in the Al-Jawf Basin, Yemen. Water 15:1496. https://doi.org/10.3390/w15081496
Back W, Hanshaw B (1965) Chemical geohydrology. In: Chow VT (ed) Advances in hydroscience, vol 2. Academic Press Inc, New York, pp 49–109
Bekhira A, Habi M, Morsli B (2018) hydrological modeling of floods in the Wadi Bechar watershed and evaluation of the climate impact in arid zones (southwest of Algeria). Appl Water Sci 8:185
Benalia O (2012) Modélisation de la demande en eau dans une région aride. Cas de la Province de Djelfa. Revue Nature Technologie 6:93–105
Bendida A, Kendouci M A, Tidjani AE-B (2021) Characterization of Algerian Sahara groundwater for irrigation and water supply: Adrar region study case. J Water Land Dev 49(IV–VI):235–243
Bendida A (2019) Epuration des eaux usées par un système de marais artificiels : Approches et modélisation [Wastewatertreatment by an artificial marsh system: Approaches and modeling]. Ph.D. Thesis. Oran. USTO-MB, Algérie, p 168
BRGM (2006) Guides techniques, qualité des eaux souterraines. Méthodes de caractérisation des états de références des aquifères français, France
Domenico PA, Schwartz S (1997) Physical and chemical hydrogeology. Wiley, New York, p 528
Eid MH, Elbagory M, Tamma AA, Gad M, Elsayed S, Hussein H, Moghanm FS, Omara AE-D, Kovács A, Péter S (2023) Evaluation of groundwater quality for irrigation in deep aquifers using multiple graphical and indexing approaches supported with machine learning models and GIS techniques, Souf Valley, Algeria. Water 15:182. https://doi.org/10.3390/w15010182
Elahcene O, Abd El-Azim H, Aziouz A (2019) Physico-chemical and bacteriological analysis of water quality in different types of water from the Ain Zada Dam of Bordj Bou Arreridj (Algeria). Egypt J Aquat Biol Fish Zool 23(3):423–439
Jamali Y, Amir S (2017) Study and evaluation of the physico chemical groundwater quality of an agricultural region around Beni Mellal City. Int J Dev Res 7(1):11055–11064
Kadhim NR, Khalid A, Athraa Hashim M (2021) The management of water distribution network using GIS application case study: AL-Karada area. J Phys Conf Ser 1895:012038. https://doi.org/10.1088/1742-6596/1895/1/012038
Kahlerras M, Meddi M, Benabdelmalek M et al (2018) Modeling water supply and demand for effective water management allocation in Mazafran basin (north of Algeria). Arab J Geosci 11:547
Kendouci MA, Bendida A, Khelfaoui R, Kharroubi B (2013a) The impact of traditional irrigation (Foggara) and modern (drip, pivot) on the resource non-renewable groundwater in the Algerian Sahara. Energy Procedia 36:154–162
Kendouci MA, Kharroubi B, Maazouzi A, Bendida A (2013b) Study of Physic-Chemical Quality of Wastewater Discharged into the Natural Environment the Case of Bechar River Algeria Energy Procedia 36287–292. https://doi.org/10.1016/j.egypro.2013.07.033
Kendouci MA, Kharroubi B, Mebarki S, Bendida A (2016) Physicochemical quality of groundwater and pollution risk in arid areas: the case of Algerian Sahara. Arab J Geosci 9:146
Kendouci MA, Bendida A, Mebarki S, Kharroubi B (2019) study of the management efficiency of the drinking water supply in arid areas: case of Bechar city (southwest of Al- geria). Appl Water Sci 9:192. https://doi.org/10.1007/s13201-019-1081-y
Kendouci MA, Mebarki S, Kharroubi B (2023) Investigation of overexploitation groundwater in arid areas: case of the lower Jurassic aquifer, Bechar province Southwest of Algeria. Appl Water Sci 13:102
Kishan SR, Singh S, Gautam SK (2018) Assessment of groundwater quality for irrigation use: a peninsular case study. Appl Water Sci 8:233
Mebarki S, Benali K, Kendouci MA (2021) Physicochemical evolution and evaluation of groundwater quality in Mougheul area (Southwest of Algeria). Appl Water Sci 11:40. https://doi.org/10.1007/s13201-021-01368-7
Mebarkia A, Haouchine A, Boudoukha A, Nedjai R (2017) Assessment of nutrient contamination in surface water, case study of Ain Zada Dam (North North-East of Algeria). J Fundam Appl Sci 9(3):1358–1377
Menchikoff N (1936) Etude géologique sur les confins algéro-marocains du Sud. C. R. Som. S. C. France, pp 131–148
Nemčić-Juree J, Singh SK, Jazbee A (2017) Hydrochemical investigations of groundwater quality for drinking and irrigational purposes: two case studies of Koprivnica-Križevci County (Croatia) and district Allahabad (India). Sustain Water Resour Manag 1:1. https://doi.org/10.1007/s40899-017-0200-x
Rodier J (1996) The analysis of the water: natural water, waste water, sea water, 8th edn. Dunod, Paris
Rodier J (2009) L’analyse de l’eau, Dunod, Parie, Edition 9, 2009, P1438.
WHO (2006) Guidelines for drinking-water quality Recommendations, vol 1, 3rd edn. Word Health Organization, Geneva
WHO (2017) Guidelines for drinking-water quality, fourth edition incorporating the first addendum. World Health Organization, Geneva
Wilcox LV (1955) Classification and use of irrigation waters, vol 969. U.S. Department of Agriculture Circular, Washington, DC, p 19
Yang S, Yang F, Zhimin F, Wang T, Lei R (2010) Simultaneous nitrogen and phosphorus removal by a novel sequencing batch moving bed membrane bioreactor for wastewater treatment. J Hazard Mater 175(1–3):551–557
Acknowledgements
The authors would like to express their gratitude to the MESRS of Algeria, for the support. We also acknowledge the Algerian for waters (ADE) and the ANBT.
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript. This study was funded by author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mebarki, S., Kendouci, M.A. & Bendida, A. Monitoring the spatial evolution of groundwater quality during its diversion in the drinking water supply network in arid areas, case of Bechar city (Algeria Sahara). Appl Water Sci 14, 118 (2024). https://doi.org/10.1007/s13201-024-02157-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02157-8