Abstract
The aim of this study is to apply a water treatment technique (phyto-purification) in the Algerian Sahara, an arid region, to resolve the scarcity of irrigation, recycled wastewater and preserve Saharian ecosystems composed of sand and two species of aquatic plants: Typha latifolia and Imperata cylindrica. The choice of these plants was determined based on the natural vegetation, soil and climatic characteristics of the study area. To this end, we developed an experimental pilot composed of three tanks measuring 30 cm × 35 cm × 45 cm, arranged with a filter bed of sand and gravel. Two of these tanks are sown with the above-mentioned plants, while the third tank, serving as a control, is not. The values of the main wastewater pollution parameters, namely total nitrogen compounds, ammonium (NH4+), chemical oxygen demand (COD) and suspended solids measured at the outlet of the device, substantiate the performance of this treatment system. Indeed, the nitrogen reduction rate increased from 63% in the unplanted control filters to around 80% in the planted filters, and from 81 to 88% for NO3−. Regarding chemical oxygen demand (COD), the reduction rate exceeds 88.37% for the three tanks. The study of the analytical approach to modeling the purification kinetics reveals that the kinetics of COD and NH4+ are well correlated with the first-order model, with an explained variance varying between 68.1 and 81.6% for COD and 83.5 and 92.3% for ammonium. The Riverside diagram highlights that all treated water samples fall into the low sodium risk and high salinity risk class. Build on the Na% values; all purified waters have characteristics suitable for water use for irrigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, safeguarding our water resources and our environment depends on our ability to purify wastewater before release them into the natural environment (Bendida 2012). Wastewater is liquid water which, after industrial, agricultural or domestic use, is considered essential to preserve the immediate human environment as well as to preserve watercourses and groundwater. The term “water” describes the relationship between the demand for liquid and the variability of its availability (Saini et al. 2021). Insufficient water resources are quickly becoming a major challenge for many developing countries, which implies a lack of sufficient water (quantity) and no access to safe drinking water (quality) (Kendouci et al. 2019). A growing population imposes countless demands on natural water resources (Bendida et al. 2021). Most developing Nations face significant environmental challenges, particularly regarding wastewater treatment. (Jean-Marie et al. 2008).
In semi-arid and arid regions where the imperatives of population growth and improvement in living standards are met, the volume of wastewater produced is increasing significantly and will continue to increase steadily (Kendouci et al. 2016). Its consideration is therefore of the utmost importance and its valorization must therefore be integrated into the Sustainable Development Goals, provided that they are purified. However, wastewater treatment is virtually absent in arid areas due to the high cost of investment and maintenance. As a result, urban waste water generated in these areas is released to receiving environments without treatment. It is therefore necessary to explore new technologies for purification. That is adapted and reliable to the realities in these regions. Scientists are always looking for the best way to purify waste water with the least expense and maximum efficiency (Mimeche 2014).
The tension on water resources has never been so strong, and is increasing: we must find a way out in the face of droughts, and stop wasting. The recycling of water and its reuse is now encouraged. Knowing what is meant by wastewater requires having several key elements: what is the initial use of the water, what is its nature, its degree of contamination, and what technique is used to “clean up” it.
Recycling wastewater consists of recovering wastewater after treatment, to reuse it. This recycling, therefore, fulfills a double objective of saving resources: It allows both to save resources upstream by reusing them and also to reduce the volume of polluted waste. Its interest is, however, limited when there is no quantitative pressure on the water resource in the sector concerned. The availability and supply of drinking water are major challenges for the coming decades. It is even to be feared that, occasionally or more chronically, no country will be spared from this problem. Although the industry has been at the forefront of recycling, wastewater recycling has been gaining acceptance in recent years, particularly for irrigation and green space maintenance. It may soon be considered as a solution, including for domestic use. The reuse of wastewater is primarily for agricultural purposes. Indeed, agriculture is the activity that consumes the most water in terms of net consumption.
In Algeria, only 0.2% of treated wastewater is reused. Some countries, such as Italy and Spain, manage to reuse 8 and 14% of their treated wastewater.
Agriculture (irrigation) and green spaces (watering gardens, golf courses, stadiums, etc.) already use Reut (Reusing treated wastewater). Water from industries (effluents) and treatment plants can be used in a closed circuit, for cleaning, energy production (biomethanation) and heating. Another use is drinking water. The unpolluted water can even, in some cases, recharge the water table. Agricultural irrigation water, watering green spaces, cleaning vehicles or structures: There are many uses! As a reminder, in Algeria only 0.2% of treated wastewater is reused, so together let’s fight to preserve the environment while benefiting from business opportunities such as the resale of water to be reused or a significant reduction in your water consumption.
The advantages of using treated wastewater are of different kinds. In the context of environmental degradation, the societal expectation to avoid wasting water is growing. Companies can also see an economic interest in using recycled wastewater. We can observe three types of advantages when we talk about Water Reuse:
-
Environmental saving the water so far taken; fewer inputs injected into the natural environment because the wastewater, treated and reused, contains organic matter; limitation of polluting discharges at the source, in industry.
-
Societal favored circular economy; fight against waste and use a new resource.
-
Economy in the internal circuit, the industry can reduce its bill by reducing its water consumption by 50–80%.
Phyto-purification is gaining popularity in the physical and biological purification of wastewater treatment. Biological filtration technology offers the advantage of reduced operating costs, limited spatial footprints, high pollutant removal rates, as well as flexible collection. The flow type plays a crucial role in antibiotic removal, as it determines the redox conditions of the system and, therefore, influences microbial degradation.
To take advantage of the advantages of one species to compensate for the disadvantages of another, a balanced approach must be adopted. The vertical flow provides favorable conditions for nitrification (aerobic) can be compensated by a horizontal flow that is more effective in denitrification (hypoxia) to improve treatment effectiveness (Bendida et al. 2013).
Planted with macrophytes has shown its effectiveness in treating industrial and domestic wastewater. Phyto-purification represents an alternative method of treating domestic wastewater, thus meeting present environmental needs (Cristina et al. 2008). Several projects have proved their ability to effectively clean up urban wastewater worldwide, and artificial marshes have been used for the purification of municipal wastewater and industrial effluents. In addition, using the local plants of economic interest on these systems as macrophytes makes them more attractive because they can be self-financing (Bendida 2012). Wastewater treatment in filter marshes is carried out using a combination of both physical, chemical and biological methods. Plants play an essential role in the purification process, specifically by promoting the development of micro-organisms and oxygenating the environment (Mimeche et al. 2010). The artificial filter marshes have the advantage of having reduced installation and operating costs, requiring no chemicals and little or no energy, compared to conventional methods wastewater treatment. In addition, they provide a natural habitat and benefit from strong social acceptance. Inspired by natural wetlands, they are man-made ecosystems that are capable of treating all types of wastewater, including industrial, agricultural and municipal, effluents. They purify wastewater by filtration, using specific plants and selected filter media (El Hashemi et al. 2012; Mimeche et al. 2010).
This work consists of two parts. In the first part, we focus on wastewater purification in a desert city with a domestic drainage system, treated using planted filters. We were second, characterizing the wastewater treated with the cultivated filter for use in irrigating the nearby arid lands.
Material and methods
The physio-chemical parameters were analyzed by the experimental protocols described by the standard methods.
pH, conductivity and salinity
Measuring the pH of wastewater indicates the alkalinity or acidity of this water, it varies depending on the nature of the basic or acidic effluents. The latter influences most of the chemical and biological mechanisms in the aquatic environment. However, it is important for the viability and growth of micro-organisms. The level of electrical conductivity is probably one of the simplest and most crucial parameters for controlling wastewater quality. It is the paramount importance when considering the reuse of wastewater in agriculture. The salinity of water corresponds to its concentration of dissolved salts as a whole (Derradji2015). These parameters were measured with the WTW 197i multiparameter probe.
Suspended solids (SS) and chemical oxygen demand (COD)
Chemical oxygen demand is expressed in mg of oxygen per liter. It represents the total content of water in oxidizable materials (Rodier 2009). The principle of measuring Chemical Oxygen Demand (COD) involves treating a sample for two hours in an acidic medium, with the addition of potassium dichromate (an oxidizing agent), silver sulfate (catalyst of oxidation), and mercury II sulfate (chloride ion complexes). Then the excess potassium dichromate is measured. Suspended matter includes all mineral or organic matter that does not dissolve in water. Knowledge of the concentration of these particles in wastewater is necessary in assessing the impact of pollution on the aquatic environment. The SS is filtered paper, the water is filtered and the weight of material retained by the filter is determined by differential weighing (Rodier 2009).
Nitrogen forms
Kjeldahl nitrogen in turn is made up of organic nitrogen and the ammonium ion. The release of nitrogen into the receiving environment, such as water, causes oxygen consumption, leads to eutrophication, creates toxicity for fish, and constitutes an obstacle to the production of drinking water (Bendida 2012). The measurement was carried out using an udo 129 distiller kjeldahl. Ammoniacal nitrogen is mainly found in the toxic form of NH4+. It serves as a good indicator of the pollution of the waterways by discharges of urban effluents. Its presence in water usually reflects an incomplete process of organic matter degradation. For ammonium ions (NH4+), the determination is based on the reaction of ammonium ions with chlorine in an alkaline medium to form monochloramine. Together with thymol, it forms a blue inphenol. Nitrates are the salts of nitric acid. The presence of nitrates in water is an indicator of pollution of urban, agricultural or industrial origin. Nitrate compounds were measured using an AL800 spectrometer. The nitrate ions (NO3−) were determined by the 8507 method based on the chromotropic acid. The principle of this essay consists in reducing the nitrate ions to nitrite ions in an acid medium. These form an azo-yellow-orange coloring (Kendouci 2012). The measurement was carried out using an AL800 Spectrometer.
Sulfate and chlorides
Sulfate is of multiple origins: natural (gypsum, pyrite, and volcanoes), industrial (tanneries, paper mills, and textile industry) and agricultural treatment products (pesticides, fertilizers, etc.). The sulfate dosage was carried out by precipitation of the latter in a hydrochloric medium in the form of Barium sulfate. The precipitate thus obtained is stabilized using a two-solution (Maazouzi 2010). The homogeneous suspensions are measured with a spectrophotometer at 650 nm. Chlorides are elements of natural origin (sea: 27 g/l NaCl, and salty land), humans (wastewater; 10–15g NaCl in urine/day) or industrial (agrifood, electroplating, etc.). At the level of the estuary, the chloride contribution is mainly marine (Kendouci 2018). Chlorides were evaluated by Mohr volume try. By a solution of silver nitrate, in the presence of potassium chromate as a colored indicator, the AgCl precipitates as long as there is Cl−, the end of the essay is detected by the appearance of a red color.
Sodium and potassium
Inside a biological entity, sodium plays a vital role by participating in a large number of biological phenomena. IT can take the place of potassium ions when entering the body and can be essential in the functioning of enzymes. Potassium is involved in the development of a large number of biological phenomena. All living things need potassium (Kendouci 2018). The principle of potassium measurement is based on the excitation of these atoms using specific rays for each ion at the flame photometer. The sodium concentration is obtained after the calibration of the device with a standard NaCl solution (JENWA clinco LPFP7 Flame Spectrophotometer).
Calcium and magnesium
Calcium ranks as the fifth most abundant natural element, while magnesium ranks eighth. These two elements are found in all natural waters. Calcium quantification was performed using the EDTA titrimetric method. As this essay is done at a high pH (12–13), the calcium is precipitated in the form of hydroxide and does not intervene, so the chosen indicator Murixide only combines with the calcium to form a red complex (Maazouzi 2010). The magnesium assay is based on the EDTA titrimetric method to which Black Eriochrome is associated as a specific color indicator that the magnesium complex is purple (Derradji 2015).
Experimental pilot
The experimental pilot is composed of a plastic bin with a height of 30 cm, width of 35 cm and 45 cm length. The bin is filled from the bottom upwards with gravel of the following diameters (gravel 8/15 (5 cm), gravel 3/8(5 cm)) and sand (10 cm) (Fig. 1). Each bin is inclined with a 5% slope toward the downstream side.
Schema of the experimental pilot (Bendida 2012)
The sand
The great Erg Occidentale is located in the northwest of the Sahara, 600km as the crow flies south of the Mediterranean, 500km long from WSW to ENE, 150 to 200km wide and covers an area of nearly 100,000 km2 (Kendouci 2012). To determine the sandy site’s economic interest, we conduct mineralogical and particle size studies of these fine sediments. These vast expanses of sand, commonly called Ergs, create an iconic landscape of the Sahara, giving it a distinctive singularity. The media coverage of these sites contributes greatly to the region’s economy, as highlighted by Kendouci et al. (2013).
The plant
Collection of experimental plant
The two plants, Typha Latifolia and Imperata cylindrica are harvested from the Oued Bechar discards; the species are transplanted into polyethene tanks and transported to the laboratory. The roots are rinsed several times with twice distilled water and then implanted in the bin (Fig. 2).
Digital photographs of the experimental pilot (Bendida 2012)
Typha latifolia
It exists in public gardens and waterways. Typha, also called cattail, is a tall perennial water plant that evokes rushes or reeds. It is characterized by creeping roots and long flattened leaves. The best-known species remains the broad-leaved cattail which reaches between 1.50 and 3 m in height with flowers ending in dense cylindrical spikes and a downy ovoid fruit of brown color (Fig. 3). The cattail grows in freshwater marshes, near quiet streams in tropical and temperate regions; it is sometimes planted at the edge of ponds and in aquatic gardens (Bendida 2012). The root system of Typha latifolia is specially made up of submerged rhizomes which form creeping roots and which allow this plant to quickly colonize large areas. Typha latifolia grows in stagnant or slow-flowing water, preferably at a depth between 0 and 50 cm. They are present up to an altitude of 1800m.
Imperata cylindrical
Imperata cylindrica is a Mediterranean and tropical species present throughout the Sahara in hot and damp places. Imperata cylindrica is a perennial plant whose height varies between 30 and 150 cm (Fig. 4). The aerial stems are short, and erect and arise from rhizomes. The roots are fibrous and develop from the base of the culm and rhizome nodes. (Bendida 2012).
Results and discussion
The sand
The sand examined is classified in the category of fine sand, according to Kendouci (2012). This classification is corroborated by its low porosity rate, which amounts to 42%; close to those found in the literature, the usual values of porosity for soils range between 30 and 60%, which more or less fine sand can also be quantified by the effective diameterd10estimated from particle size analysis which is of the order of 0.17mm, the fineness modulus (FM) 2.16 for sand; and the very low permeability of the sandwiches, measured at approximately 7.26 × 10−4 m/s, classifies them as having good permeability. A uniformity coefficient of less than 2 indicates a uniform grain size (Kendouci 2012) (Table 1).
The physical features of water
Electrochemical results
The pH at the outlet of the three tanks shows a slight variation concerning the rejection value (Fig. 5). The value of the latter is 8.14. The pH reduction rates for the three tanks are 5.89% for Typha, 5.89% for Imperata and 3.43% for the control tank. The decrease in pH reflects acidification of the environment, which can result from the oxidation of ammonium and organic matter (Bendida et al. 2013). The oxidation of organic matter generates carbon dioxide (CO2) which acidifies the environment. The nitrification (oxidation of ammonium) leads to acidification of the water in the tanks. The optimum progression pH of Typha latifolia is between 3 and 8.5 (Cristina et al. 2008). In our work, pH values remain in the range 6.5–8.5.
The electric conductivity of the treated wastewater for both planted tanks is higher than that of the unplanted tank and raw wastewater (Fig. 6). The conductivity at the inlet (before filtration) is 1965 µS/cm, the conductivity increase rate for the Typha tank is 21.77%, that of the Imperata tank is 24.55%, and that of the control tank is 13.92%. The increase in electrical conductance in ponds is clarified by the increasing accumulation of salts in the filtration beds, resulting from vaporization processes. This difference in results would be related to the type of plant used. Regarding the effect of conductivity on the species used, Typha latifolia is characterized by a low and high tolerance to salinity between 4 mS/cm and 9 mS/cm (Cristina et al. 2008). According to Shu et al. (2001), most species survive in environments with conductivity varying from 0 to 2 mS/cm. Sensitive plants are impacted by conductivity of 4–8 mS/cm; while, tolerant plants can thrive in environments where conductivity exceeds 8 mS/cm.
The salinity of the three tanks during the experiment varies from 1.98–2.95 g/l (Fig. 7); there is an increase in the salinity value for the three tanks as a purpose of the residence time. The salinity increase rates of Typha, Imperata and Witness tanks were 23.23%, 48.98% and 12.62%, respectively. The salinity of the discharge before filtration is 1.97 g/l. During the treatment period, the conductivity of our medium remained between 3 and 5 mS/cm. These waters are moderately saline and should be used with moderate restrictions for irrigation (Abissy and Mandi 1999).
Chemical oxygen demand (COD) and suspended solids (SS)
The COD and SS values for release before filtration are 150 mg/land and 215 mg/l, respectively. Based on the results shown in the figures below, a reduction is observed for the two parameters (chemical oxygen demand and suspended solids) in the three tanks, the residence time plays a very important role to reach the reduction rate. In the case of SS, this effect was expected because of the filter function itself; for COD, physicochemical reactions are at the origin of this decrease. It is then observed that the colloidal and particulate elements are entrained during the decantation. At the filtration inlet, the COD will therefore be mainly in soluble form. The COD removal rate is 98% for the Typha tank, 95.35% for the Imperata tank and 88.37% for the control tank, almost total COD mitigation is observed for the Typha tank (Fig. 8). This is apparently due to the physical accumulation and the oxidation of organic matter in the tanks. These results are close to the removal rate of 91.99% obtained by Zhang et al. (2007) probably using Pseudacorus I as the plant species. These results are probably linked to the presence of plants which create physicochemical conditions favorable to the organic matter oxidation by the microbial flora. These provide oxygen to the filter bed via their rhizomes and roots. (Brix 1994; Kroer et al. 1998); where the major reduction in COD is achieved by microbial decomposition (Greenway et al. 1999; Bindu et al. 2018).
The elimination of suspended solids (SS) is significant. From the first filtration stage, between 76 and 81% of the SS are retained (Fig. 9). This rate reaches 88% at the end of the process. These high performances allow the system to result quality water for suspended solids. The water collected at the end of purification complies with the standard for wastewater discharge into the natural environment, which is 50 mg/l (Bendida 2012). The reduction in the SS concentration in the different tanks is due to physical filtration, which retains the coarse particles on the surface and the finer ones, whether by obstructing between the pores, by interception and fixation on the grains, or by chemical reactions. (Bendida et al. 2013.) The removal of 85.5% of the SS from the Typha planting tank and 87.7% from the imperative tank is similar to that documented by Jean et al. (2008). (85.5%). These important results are explained by the fact that the mass implanted by a macrophyte allows good removal of suspended solids and organic matter degraded by bacterial activity at the roots.
Azote forms
The principal indication of nitrogen in wastewater and ammonium-based detergents is urine, and ammonification reactions can turn organic nitrogen into ammonium. As ammonium has a high oxygen requirement, odorous molecules (nitrogen compounds) are what produce offensively since. Kjeldahl nitrogen presents worrying concentrations, reaching a maximum of 62.18 mg/l in discharges; while, the value of ammonium is 26.60 mg/l. The maximum nitrate concentration is 7.94 mg/l.
According to Fig. 10, there is a decrease in the concentration of kjeldahl nitrogen. The elimination rate is 79.27% for the Typha tank, 81.98% for the Imperata tank and 63.96% for the control tank, the latter being compared with the 65.60% elimination rate obtained by Tama et al. (2009). This result is due to the biological oxidation of nitric acid by bacteria and plants. Degradation of organic matter and denitrification of nitrogen in the root region of plants is carried out by micro-organisms. This zone will allow the proliferation of micro-organisms which are the main decomposers of organic matter in the root zone.
The ammonium contents of the waters studied show a variation as a function of the filtration time for the three tanks (Fig. 11). A decrease in the ammonium content is observed, the removal rates are 91.11% for the Typha tank, 90% for the Imperata tank and 66.66% for the sandbank. A removal rate of 80.75% ammonium is obtained after 5 days as retention time by Tama et al. (2009) in the presence of (Typha. latifolia) as a species. The production rates obtained here of 91.11% for Typha and 90% for Imperata compared with the control tank which is 66.66% shows that the ammonium ion is well assimilated by the two species (Typha and Imperata) which use it as nitrogen sources. Two other processes can reduce ammonium in the environment: ammonia volatilization and nitrification. The volatilization is favored at high pH (pH = 9) and will be responsible for the 21% reduction in nitrogen (Zimmo et al. 2003). In our work, the pH remains below 9, which means that the ammonia volatilization rate remains low.
It can be seen from Fig. 12 that the nitrate contents of the waters for the three tanks are lower than the release before filtration, the reduction rates of Typha, Imperata and the control tank are respectively 87.63%, 86.84% and 80.57%. According to Chan et al (2008), nitrification is easy with dissolved oxygen contents 2–3 mg/l, the phenomenon is not then negligible under aerobic conditions leading to the development of nitrates. The removal of nitrates from the two planted tanks is likely due to good nitrification. The ideal proportions of nitrate to ammonium vary depending on the plant, stage of development, the nitrogen concentration supplied and environmental conditions (Zou et al. 2000, Tylova et al. 2005; Chang et al. 2010). Nitrates can be effectively removed by microbial denitrification in the presence of bacteria.
Sulfate and chlorides
Sulfate and chlorides have very high concentrations, this augmentation are probably due to the nature of urban discharges containing sulfatic-based detergents (metastable state) which transform (oxidation) into sulfate. The sulfate concentration is 795.76 mg/l; while, the value of chloride is 923 mg/l. According to the curves representative of the sulfate evolution (Fig. 13) for the three tanks, a decrease in the sulfate content in the three tanks compared to the water before treatment. For the Typha tank, the sulfate elimination rate is 47.49%, for the Imperata tank 51.09% and 61.9% for the control tank. According to Fig. 14, we first observe a decrease in the concentration of chlorides, however, after the 4th sampling, we notice an augmentation compared to the water before treatment. For the Typha tank, the chloride increase rate is 24%, 28% for the Imperata tank and 8% for the control tank. Concerning the chlorides, their concentration in the three tanks is higher than that of the water before treatment, this is explained by an increase in evaporation as well as by the increase in electrical conductivity measured in the treated wastewater during the culture time. (Abissy and Mandi 1999). Sulfate and chlorides are proportional to the salinity of the environment and can influence photosynthesis. They act on the stability of calcium and magnesium in the tissues by decreasing and inducing an increase in the cushions of K and Na in the tissues, which influences photosynthesis.
Potassium and sodium
The evolution of the sodium and potassium contents shows that there is an increase for the two parameters in the three tanks compared to the rejection (Figs. 15, 16). The potassium and sodium values for release before filtration are 33.19 and mg/l 27.12 mg/l, respectively. The rate of increase in sodium varies from 89% for the Typha tank to 100.5% for that of Imperata and 107.94% for the control tank; for potassium, it is 26.80% for Typha, 28.14% for Imperata and 13.40% for the unplanted tank (Sand). These increases are probably due to the phenomenon of water evaporation during this period.
Calcium and magnesium
The calcium and magnesium contents of the rejection before filtration are respectively 134.26 mg/l and 68.53 mg/l. The evolution of the calcium and magnesium contents shows that there is a decrease for the two parameters (Figs. 17, 18) in the three tanks compared to the rejection. The calcium elimination rate is 7% for the Typha tank, 8.5% for the Imperata tank and 4.23% for the unplanted tank. For magnesium, the reduction rates for Typha, Imperata and the control tank are 7%, 9.3% and 5.89%, respectively.
The Concentrations of the wastewater pollution indicators (Kjeldahl nitrogen, ammonium and suspended solids), measured at the outlet of the device, confirm the effectiveness of the planted filters. The reduction rates in the unplanted control sand filter are approximately 88.37% for COD, 85.50% for SS, 63.96% for NKT and 66.66% for NH4+ (Fig. 19). The reduction rates in planted filters are around 98% of COD for Typha latifolia and 93.53% for Imperata cylindrica, 85.5% of MES for Typha Latifolia and 87.7% of MES for the Imperata cylindrica (Figs. 20, 21). All these results confirm the effectiveness of the use of plants to purify wastewater. The elimination of pollutants makes it possible to obtain good quality water that can be reused in the sector of industrial and irrigation and can solve one of the water shortage problems in our country.
The kinetics adopted for the degradation of carbon and nitrogen pollution
The kinetics of degradation is described by a variety of mathematical expressions increasing complexity as attempts are made to integrate the many variables impacting the disappearance of organic matter.
In our study, we propose a “1” order model that describes well the decreasing part of the carbonaceous and nitrogenous material:
With:
\(\frac{{\text{d}}X}{{\text{d}}t}\): Pollution degradation kinetics in (mg/l/d).
X: Pollution concentration in (mg/l).
t: residence time in (d).
a and b are the kinetic constants of the model, expressed in (d−1) and (mg/l/d), respectively.
In this study, we focused on carbon pollution (COD) removal characteristics and nitrogen pollution (Kjeldahl nitrogen, ammonium and nitrate), depending on the variation in residence time. 14 day expertise; the sampling step shall be distributed as follows: 17 h, 41 h, 89 h, 161 h, 233 h and 353 h.
In Fig. 22 we have shown the results according to the kinetic model of the first order; the variance applied for COD removal is between (0.68 and 0.82), meaning that this model is representative of our case.
Figures 23, 24, and 25 show the removal kinetics of nitrogen pollution as a role of time, the initial concentrations of Kjeldahl nitrogen, Ammonium and Nitrate are 62.18 mg/l, 26.60 mg/l and 7.94 mg/l, respectively. According to Fig. 23, which shows the kinetics of ammonium elimination, the variance applied is between (0.83 and 0.92), so there is a strong correlation between the removal of the ammonium concentration and the residence time. The strong correlation is noted in the Typha tank, which means that this model is representative of our work.
Regarding the kinetics of Kjeldahl nitrogen and Nitrate elimination, the variance applied is comprised (0.20 and 0.50) so there is a weak correlation between the concentration of Kjeldahl nitrogen, Nitrate and the residence time. That means this model is not representative of the degradation of Kjeldahl nitrogen and Nitrate (Figs. 24, 25).
Evaluation of treated water for irrigation
The chemical study of irrigation water appeared necessary to highlight any danger that certain chemical elements may present.
pH
In irrigation water, the pH should be between in the range 5.5 and 8. The pH values obtained for the three samples are in the range of 7.66–7.86; this pH is allowed for crop irrigation (Bendida et al. 2021).
Alkalinity hazard
Sodium is an element of alkaline and alkaline earth bases, playing an important role in maintaining the permeability of the solution for irrigation. To determine this risk, consider creating a classification that considers the ratio of sodium absorbed to total mineralization (SAR), in large quantities in irrigation water. The calculation of the SAR value is carried out using the following formula.
The SAR values of treated water are between 0.92 and 1.09 (Table 2). These values did not exceed the recommended threshold values (SAR ≤ 10, therefore a minimal risk of sodium accumulation). All samples belonging to class (S1) correspond to low sodium content. Extreme SAR (too much Na+ relative to Ca2+ and Mg2+) in irrigation water significantly affects crop yield (reduction in quality and quantity) and alters soil physical condition (Bendida et al. 2021).
Application of Richard’s method to treated water shows that it belongs to class (C4-S1) (Fig. 26). This class is described as poor quality, highly encrusted water which may be appropriate for irrigation of salt-tolerant plants, particularly on well leached and drained soils.
Na%
The percentage of sodium is frequently used to assess the appropriateness of water for irrigation according to the method described by Kendouci et al. (2023). The sodium percentage value allows water to be classified into five classes excellent, good, permissible, doubtful and unsuitable according to Mebarki et al. (2021). The calculation of the sodium percentage is carried out as follows:
The Na% values of treated water are between 14.94 and 18.93% (Table 2). Wastewater treated for the three tanks falls in the excellent category. In the case of high sodium concentration in irrigation water, sodium ions tend to be adsorbed by clay particles, thereby causing the displacement of Mg and Ca ions. This sodium exchange process in water against Ca and Mg present in the soil reduces permeability and ultimately leads to soil with poor internal drainage capacity (Bendida et al. 2021). Therefore, this limits the circulation of air and water during periods of wet weather, and these floors tend to become hard when dry.
The Wilcox diagram (1948) is a graphical representation of the (% Na+) as a role of the electrical conductivity, making it possible allows the risk of water salinity to be assessed. This classification identifies five categories of water: excellent, good, acceptable, poor and poor. The increase in Na + concentration hurts plant development. (Bendida et al. 2021). The Wilcox diagram shows that the treated waters are classified to the mediocre class (Fig. 27).
Kelly index
Kelly integrates a parameter (Bendida et al. 2021) depend on the concentrations of Na+ , Ca2+ and Mg2+ to assess the classification and quality of irrigation water. The KI value was calculated according to the following equation.
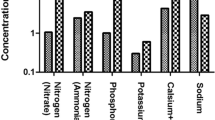
Water with a KI value less than one is suitable for irrigation, higher values is considered unsuitable (Bendida et al. 2021). KI values ranged from 0.19 to 0.24 meq/l (< 1) (Table 2). All samples were suitable for irrigation.
Chloride–sulfate ratio
The ratio between chloride/sulfate concentrations is used to assess the appropriateness of irrigation water (Bendida et al. 2021). Chloride–sulfate ratio was determined by following equation:
If the ratio (\({{\text{Cl}}}^{-}\)/\({{\text{SO}}}_{4}^{2-}\)) is less than one, water is considered suitable for irrigation. On the other hand, a higher value is considered unsuitable and saline (Bendida et al. 2021). The chloride–sulfate ratio of the three samples is 3.57, 3.95 and 4.28 meq/l, respectively (Table 2). All samples indicated a chloride–sulfate ratio greater than one.
Conclusion
The performance of artificial marshes in purifying wastewater depends on the role of all component beds. Each of these takes on a specific role depending on the environment in which it is located and the quality of the water to be clarified. This explains the importance of all purification processes such as nitrification, denitrification and oxidation decrease.
The objectives of this experimental study are:
-
Demonstrate the role of the substrate (sand) in the purification (biofiltration). We achieved remarkable results for the different parameters with abatement rates of 88.37% for COD, 85.50% for MES, 63.96% for NKT and 66.66% for NH4+.
-
Highlight the performance of planted filters. We recorded remarkable results for the different parameters with abatement rates around 98% of COD for Typha latifolia and 93.53% for Imperata Cylindrica, 85.5% of SS for Typha Latifolia and 87.7% of SS for Imperata Cylindrica. Plants can contribute to purification processes in a number of ways, such as settling suspended matter, or by providing surface micro-organisms.
-
According to the Riverside diagram, all treated water samples belong to the C4S1 class, which is characterized by high salinity risk and low sodium risk. All samples had Na% below 20%, indicating that the water is suited for agriculture.
In agriculture, the treated wastewater is valued by reason of its composition rich in mineral and organic matter. Irrigation with this water reduces the need to use fertilizers and this water can also be used to irrigate golf courses, forests and market gardening, because their treatment is suitable for this use.
This work is of major importance in the management and purification of wastewater from small towns in an arid or semi-arid climate. The results obtained confirm the effectiveness of the artificial marshland technique in the purification of domestic wastewater in the Bechar region where the natural elements used, sand and plants, are generally available.
In order to generalize the use of this purification system across the Algerian Sahara, we plan to test certain conditions in the future:
-
Analyze the effect of the hydroclimatic conditions of the region on the purifying performance of this system;
-
Tester of other macrophyte plants to compare their powers purifiers;
-
Use hybrid systems;
-
Study the effect of stress generated by accidental industrial releases.
References
Abbissy M, Mandi L (1999) Utilisation des plantes aquatiques enracinées pour le traitement des eaux usées urbaines : cas du roseau [Use of rooted aquatic plants for the treatment of urban wastewater : case of the reed]. Rev Sci Eau 12:285–315. https://doi.org/10.7202/705353
Bendida A, Tidjani AE-B, Kendouci MA, Nabou M (2013) Treatment of domestic wastewater from the town of Bechar by a sand filter (sand of Beni Abbes Bechar Algeria). Energy Procedia 36:825–833. https://doi.org/10.1016/j.egypro.2013.07.095
Bendida A, Kendouci MA, Tidjani AE-B (2021) Characterization of Algerian Sahara groundwater for irrigation and water supply: Adrar region studies case. J Water Land Dev 49:235–243. https://doi.org/10.24425/jwld.2021.137117
Bendida A (2012) Contribution à étude de Phyto traitement des eaux domestiques de la ville de Béchar pour leur réutilisation en irrigation. [Contribution to the study of Phyto treatment of domestic water from the city of Bechar for its reuse in irrigation]. Masters Thesis. University of Bechar
Bindu T, Sylas VP, Mahesh M, Rakesh PS, Ramasamy EV (2008) Pollutant removal from domestic wastewater with Taro (Colocasia esculenta) planted in a subsurface flow system. Ecol Eng 33(1):68–82. https://doi.org/10.1016/j.ecoleng.2008.02.007
Brix H (1994) The function of macrophytes in constructed wetlands. Wat Sci Tech 29:71–78. https://doi.org/10.2166/wst.1994.0160
Chan S, Tsang Y, Chua H, Sin S, Cui L (2008) Performance study of vegetated sequencing batch coal slag bed treating domestic wastewater in suburban area. Bioresour Technol 2008(99):3774–3781. https://doi.org/10.1016/j.biortech.2007.07.018
Chang J, Liu D, Cao H, Chang S, Wang X, Huang C, Ge Y (2010) NO3−/NH4+ratios affect the growth and N removal ability of the Acoruscalamus and Iris pseudacorus in a hydroponic system. Aquat Bot 93:216–220. https://doi.org/10.1016/j.aquabot.2010.08.002
Cristina SC, Calheiros A, Rangel P (2008) Evaluation of different substrates to support the growth of Typhalatifolia in constructed wetlands treating tannery wastewater over long-term operation. Biores Technol 99:6866–6877. https://doi.org/10.1016/j.biortech.2008.01.043
Derradji M (2015) Contribution à l’étude de la tolérance des plantes épuratrices dans l’épuration des eaux usées : stratégie et application (Contribution to the study of the tolerance of purifying plants in wastewater purification: strategy and application). PhD Thesis.University Mohamed KhiderBiskra
El hachemi O, El halouani H, Meziane M, Torrens A, Salgot M, Sbaa M (2012) Étude des performances épuratricesdansune station de traitement des eauxusées parlagunage en climatdésertique (Oasis de Figuig-Maroc): Aspect bactérienetorganique [Study of purification performance in a wastewater treatment plant by lagooning in a desert climate (Oasis of Figuig Morocco): Bacterial and organic aspect]. Re. Microbiol Ind San et Environn 6(1):84–97
Greenway M, Woolley A (1999) Constructed wetlands in Queensland: performance efficiency and nutrient bioaccumulation. Ecol Eng 12:39–55. https://doi.org/10.1016/S0925-8574(98)00053-6
Jean-marie P, Lacina C, Pascal N, Germain G (2008) Traitement des EauxRésiduairesUrbaines par un Marais artificiel à Drainage vertical Planté Avec Panicum Maximum sous Climat Tropical [Treatment of urban wastewater by an artificial swamp with vertical drainage planted with Panicum maximum in a tropical climate]. J. Sci Res 23(1):25–40
Kendouci MA, Bendida A, Khelfaoui R, Kharroubi B (2013) The impact of traditional irrigation (Foggara) and modern (drip, pivot) on the resource non-renewable groundwater in the Algerian Sahara. Energy Procedia 36(2013):154–162
Kendouci MA, Kharroubi B, Mebarki S, Bendida A (2016) Physicochemical quality of groundwater and pollution risk in arid areas: the case of Algerian Sahara. Arab J Geosci 9:146. https://doi.org/10.1007/s12517-015-2221-9
Kendouci MA, Bendida A, Mebarki S, Kharroubi B (2019) Study of the management efficiency of the drinking water supply in arid areas: case of Bechar city (southwest of Algeria). Appl Water Sci 9:192. https://doi.org/10.1007/s13201-019-1081-y
Kendouci MA, Mebarki S, Kharroubi B (2023) Investigation of overexploitation groundwater in arid areas: the case of the lower Jurassic aquifer, Bechar province southwest of Algeria. Appl Water Sci 13:102. https://doi.org/10.1007/s13201-019-1081-y
Kendouci MA (2012) Contribution à la valorisation des matériaux locaux destinés au prétraitement des eaux usées (Contribution to the recovery of local materials intended for the pre-treatment of wastewater). Masters Thesis. University of Bechar
Kroer N, Barkay T, Soerensen S, Weber D (1998) Effect of root exudates and bacterial metabolic activity on conjugal gene transfer in the rhizosphere of a marsh plant. FEMS Microbiol Ecol 25:375–384. https://doi.org/10.1111/j.15746941.1998.tb00489.x
Maazouzi A (2010) Study of sand filtration processes (south of western Algeria): application to the production of drinking water in frontal filtration. Laboratory scale column case study. PhD Thesis. National Polytechnic School of Algiers
Mebarki S, Kharroubi B, Kendouci MA (2021) Physicochemical evolution and evaluation of groundwater quality in Mougheul area (southwest of Algeria). Appl Water Sci 11:40. https://doi.org/10.1007/s13201-021-01368-7
Mimeche L, Mancer H, Debabeche M (2010) Analyse du pouvoirépuratoire d’un filtreimplante de Phragmiteaustrals pour le traitement des eauxusées sous climat semi-aride—région de Biskra (Analysis of the purifying power of a filter implanted with Austral Phragmites for the treatment of wastewater in a semi-arid climate-Biskra region]. Int Netw Environ Manag Confl 1(1):10–15
Mimeche L (2014) Étude de faisabilité de l’installation de station d’épuration des rejetsurbains par les filtresplantés en milieu —Application à la région de Biskra (Feasibilitystudy of the installation of a treatment plant for urbanwaste by plantedfilters in an aridenvironment—Application to the Biskra region). PhD Thesis.University Mohamed KhiderBiskra
Rodier J (2009) L’analyse de l’eau, 9th edn. Dunod, Paris, p 1526
Saini G, Kalra S, Kaur U (2021) The purification of wastewater on a small scale by using plants and sand filters. Appl Water Sci 11(68):1–6. https://doi.org/10.1007/s13201-021-01406-4
Shu W, Ye Z, Lan C, Zhang Z, Wong M (2001) Acidification of lead/zinc mine tailings and its effect on heavy metal mobility. Environ Int 26:389–394. https://doi.org/10.1016/S0160-4120(01)00017-4
Tam N, Wong Y, Wong M (2009) Novel technology in pollutant removal at source and bioremediation. Ocean Coast Manag 52(2009):368–373. https://doi.org/10.1016/j.ocecoaman.2009.04.009
Tylova-Munzarova E, Lorenzen B, Brix H, Votrubova O (2005) The effects of NH4+ and NO3− on growth, resource allocation and nitrogen uptake kinetics of Phragmitesaustralis and Glyceria maxima. Aquat Bot 81(4):326–342. https://doi.org/10.1016/j.aquabot.2005.01.006
Zhang X, Liu P, Yang Y, Chen W (2007) Phytoremediation of urban wastewater by model wetlands with ornamental hydrophytes. J Environ Sci 19(2007):902–909. https://doi.org/10.1016/S1001-0742(07)60150-8
Zimmo O, Der DteenN V, Gijzen H (2003) Comparison of ammonia volatilisation rates in algae and duckweed-based waste stabilisation ponds treating domestic wastewater. Water Res 37:4587–4594. https://doi.org/10.1016/j.watres.2003.08.013
Zou C, Wang X, Wang Z, Zhang F (2000) Potassium and nitrogen distribution pattern and growth of flue-cured tobacco seedlings influenced by nitrogen form and calcium carbonate in hydroponic culture. J Plant Nutr 28:2145–2157. https://doi.org/10.1080/01904160500320624
Acknowledgements
The authors would like to express their gratitude to the ministry of higher education and scientific research (Algeria), for the support.
Funding
This study was funded by the author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors announce that they have no conflict of interest.
Ethical approval
Respect.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bendida, A., Kendouci, M.A., Mebarki, S. et al. Wastewater purification and recycling using plants in an arid environment for agricultural purposes: case of the Algerian Sahara. Appl Water Sci 14, 123 (2024). https://doi.org/10.1007/s13201-024-02148-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02148-9