Abstract
In this study, the removal efficiency of chemical oxygen demand (COD), color, turbidity, phosphate, and nitrate from wet coffee processing wastewater by pulsed-electrocoagulation process (PECP) was examined with various factors such as pH: 3–11, reaction time: 15–75 min, current: 0.150–0.750 Amp, and electrolyte concentration: 0.25–1.25 g/L. Several operational parameters for the treatment of wet coffee processing wastewater utilizing the PECP have been optimized through the application of the surface response design technique, which is based on the central composite design. A quadratic model helped estimate the percentage removal of COD, color, turbidity, phosphate, and nitrate with power consumption under various situations. It also evaluated the significance and their interaction with independent variables using analysis of variance (ANOVA). Through the use of statistical and mathematical techniques, optimum conditions were determined in order to remove the maximum pollutant and nutrient while using the minimum of power. The results showed that the removal of COD—98.50%, color—99.50%, turbidity—99.00%, phosphate—99%, and nitrate—98.83%, with a power consumption of 0.971 kWh m−3 were achieved at pH-7, NaCl dose of 0.75 g/L, electrolysis duration of 45 min, and current of 0.45 Amp. Therefore, under the different operating conditions, the PECP demonstrated to be a successful technique for pollutant removal from wastewater and industrial effluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid population growth, urbanization, unreliable and costly treatment options, and poor water quality are all contributing factors to the growing worldwide concern about water pollution (Rajkumar and Palanivelu 2004; Bhagawan et al. 2018). Industries, such as distilleries, wet coffee processing, oil mills, textile productions, processing of food and chemical, soft drinks, papers, and metals production, were producing a large amount of wastewater (Gebeyehu et al. 2018). Waste containing different pollutant is becoming a major environmental threat as a result of the fast expansion of industries like wet coffee processing industries and other pesticide industries in Ethiopia (Mosivand et al. 2018). Chemical compounds are produced and used on a far larger scale now than ever before, which implies that many of them end up in the environment and are not biodegradable. Industries generate a lot of pollutants, such as bio-refractory organic compounds, which have negative effects on the aquatic ecosystem and environment due to their toxicity, color, and odor. An increasing volume of organic/inorganic wastewater from different industries deals about different business, but on the other hand, there are strict legal restrictions on how to dispose of wastewater (Vinicius et al. 2022).
Seven million tons of coffee are consumed worldwide each year, making it one of the most popular drinks in the world (Villanueva-Rodríguez et al. 2014). For nations that produce coffee, it is a significant agricultural crop. The estimated 5.5 million tons of coffee produced annually worldwide, or 6.4%, come from Ethiopia. 55.35% of the nation’s annual total output is produced in the west, south, and east, respectively. The agricultural land in the nation has roughly 600,000 hectares of coffee plantations. One of the most important issues confronting developing nations is the improper management of enormous volumes of waste produced by various human activities. Even more challenging is the risky dumping of these harmful chemicals and contaminates such as high organic concentrations, nutrients, suspended matter, and highly acidic wastewater into the environment (Ali et al. 2021; Mohyudin et al. 2022; Rasheed et al. 2022). In particular, freshwater reservoirs are quite susceptible. In Africa, social and economic underdevelopment usually contributes to organic contamination of inland water systems. One of the most environmentally destructive agro-based industries is the coffee processing industry. The disposal of effluent from pulping, fermenting, and washing coffee beans poses a variety of problems for the receiving environment, especially aquatic bodies, in many coffee processing nations. Wet processing is considered to produce coffee of a better quality than dry processing. Currently, Ethiopia has more than 400 wet coffee processing facilities, many of which are situated close to rivers (Woldesenbet et al. 2014).
A significant amount of organic compounds that might harm the environment are present in coffee wastewater. During the wet coffee processing process, the pulp and mucilage of coffee cherries are eliminated. Coffee effluent contains a lot of organic chemicals, which might harm the environment. Wet coffee processing wastewater is made up of caffeine-rich coffee fruits, sugars, phenolic compounds, fatty acids, lignin, cellulose, pectic chemicals, and other macromolecules that are undesirable for disposal in soil and water bodies (Sahana et al. 2018). Excess phosphate and nitrate concentrations in coffee wastewater effluents directed to the environment create eutrophication, which is one of the most common problems in the monitoring of environmental water sources today (Chen et al. 2014). A significant amount of acidic waste is created as a result of the high proportion of organic materials used in this process, which is especially hazardous to the environment.
As a consequence, prior to discharge into water bodies, treatment is necessary (Tacias-Pascacio et al. 2018). The physiochemical process is not economical, necessitates excessive chemical usage, and simultaneously produces a sizable volume of sludge. The biological treatment approach, which is a long and drawn-out process, requires high dilution (Shahedi et al. 2020). Finding a method to manage industrial waste that is affordable is crucial.
The process of electrochemistry has received a great deal of attention for the treatment of different forms of industrial waste, such as wet coffee processing, paper, distiller organic fertilizer, automotive industry, potentially toxic metals and tanner metal, metal plating wastewater, and real industrial waste by using pulsed electrochemical process (PECP) (Vasudevan 2012; Mahesh et al. 2016; Payami Shabestar et al. 2021). Electrochemical treatment, or electro-oxidation, has been proposed as a viable option for a variety of industrial effluents (AlJaberi 2019; AlJaberi et al. 2020). The treatment of pesticide processing industrial waste was carried out in the current study by utilizing an electrochemical method as well as a combination of other advanced technologies (Science 2018; Mena et al. 2019; Akter and Islam 2022).
The utilization of electrochemical treatment methods for the removal of organic and inorganic components from wastewater is a promising approach, due to its affordability and user-friendly technology (Bernal-Martínez et al. 2013; Sahana et al. 2018; AlJaberi et al. 2020; Ebba et al. 2022). Pulsed electrochemical wastewater treatment method is one of the most widely used electrochemical procedures in wastewater treatment is electrocoagulation. The electrochemical dissolution of sacrificial metal electrodes (iron or aluminum) into soluble or in soluble species, depending on the pH, is the basis of electrocoagulation (EC) (Sher et al. 2021). The electrochemical process involves using sacrificial soluble iron (Fe) and aluminum (Al) as anodes and/or cathodes to release metal ions by anodic oxidation. These ions combine with the cathode's produced aluminum hydroxide, resulting in flock formation. Under the application of alternating current to minimize the electrode passivation, it is one of the main disadvantages of electrocoagulation technique under direct current. The use of alternating current allows a more efficient process, with lower power consumption and sludge generation, in comparison with the process of EC (Kamaraj et al. 2013). As a result, the pulsed electrochemical process is more efficient at removing color and COD while using less energy than the (direct current electrocoagulation) DCE technique. However, for removing contaminants from industrial effluent, the pulsed electrochemical process is the best option. One of these techniques is electrochemical coagulation, which is the electrochemical reaction of destabilizing agents that cause charge neutralization for pollutant removal and has been employed in wastewater treatment (Vasudevan 2012). Because of their availability and inexpensive cost, iron and aluminum are the most commonly utilized electrode materials in the EC process (Zhang et al. 2021).
Pulsed-electrocoagulation treatment alone is an interesting and preprocessing method for removing hazardous and organic compounds using a technology that is effective, adaptable, cost-effective, simple, and clean (Bernal-Martínez et al. 2013; Tahreen et al. 2020; Ren et al. 2011). As a result, pulsed electrochemical (PEC) was successful in eliminating % color removal, % COD removal while using less electricity (Asaithambi et al. 2021). (Bian et al. 2019) The study conducted an investigation on the treatment of high salinity bilge water, employing both direct and alternating current-powered electrocoagulation processes. The results revealed that the utilization of constant alternating current exhibited superior performance in terms of sustaining consistent output levels while consuming less energy compared to the operation in direct current mode. (Othmani et al. 2017) added that, in comparison with the direct current electrocoagulation method, the alternating-current electrocoagulation technique had the greatest elimination of the colors methylene blue and indigo carmine while consuming less energy. (Eyvaz 2016) found that, in comparison with the direct current electrocoagulation technique, the alternating pulse current had better clearance rates in shorter working times.
Pulsed-electrocoagulation process (PECP) was employed to clean wet coffee processing wastewater (WCPWW) and repurpose it for a number of uses. This may ease the strain on groundwater and bodies of surface water. The goal of the wastewater treatment process is to produce cleaned effluent and sludge that can be disposed of without harming the environment (Hassen and Asmare 2018). The main objective of this study to investigate the effect of PECP to treat wastewater generated from wet coffee processing. To determine the removal efficiency of color, COD, turbidity, phosphate, nitrate, and power consumption. To analyze effects of independent variables on removal efficiency of color, COD, turbidity, phosphate, and nitrate by using RSM. To optimize and select the best removal efficiency of the pulsed electrochemical process for the treatment of coffee processing wastewater.
Material and methods
Chemical and reagents
Chemicals used in this investigation are mercury sulfate (HgSO4), ferrous ammonium sulfate (Fe(NH3) SO4), sulfuric acid (H2SO4), silver sulfate (Ag2SO4), ferroin indicator (Fe(o-phen)3 SO4), potassium dichromate (K2Cr2O7), hydrogen per oxide (H2O2), sodium sulfate (Na2SO4), KOH, NaOH, NaCl, sodium hydrogen carbonate (NaHCO3), phenolphthalein, stannous chloride, ammonium molbidate, phenol, buffer solution, and distilled water.
Experimental setup
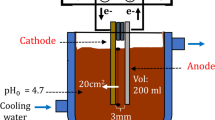
The goal of this experiment is to determine the contribution of pulsed-electrocoagulation processes in wastewater treatment, as well as to assess the efficiency of color, COD, turbidity, nitrate, and phosphate removal from coffee processing wastewater and by using aluminum (Al) electrode. The schematic arrangement of the experimental setup is shown in Fig. 1. The experiments were carried out in a 1 L batch mode electrochemical reactor with a pair of rectangular-shaped Al electrodes with a total area of 90 cm2. The electrodes were connected vertically with an adjustable gap of 1 cm between them and were powered by a DC/AC power supply in monopolar mode. The electrodes were thoroughly cleaned and washed with HCl and water for about 15 (min) before and after each run. The solution was continuously stirred using a magnetic stirrer at a constant speed. The pH of the wastewater was measured using pH meter and it was adjusted using a 0.1 N NaOH and H2SO4 solution. With required experimental conditions, the samples were collected from EC reactor and filtered using Whatmann 42 filter paper. The color, COD, turbidity, nitrate, and phosphate were determined to examine the behavior of PECP process for treatment of wastewater.
The experimental design and statistical analysis
Response surface methodology (RSM) is a technic tool for analyzing the relationship between the process variables and the responses (Sadaf et al. 2022). As a result, response is used to describe a performance metric or quality attribute. The input variables are also known as independent variables, and they are under the scientist’s or engineer’s control. The response surface approach is a set of strategies for using experimental methods to find the best operating conditions. Typically, this requires conducting a series of experiments and using the results of one to guide the following steps (Bähre et al. 2013; Akter and Islam 2022). In this study, laboratory experiments were carried out using a PECP by varying parameters in their interval pH (3–11), electrolyte concentration (0.3–0.6 g/L) of NaCl, current (0.5–1 Amp), and reaction time (30–60 min). Those parameters were considered to determine the removal efficiency of COD, color, turbidity, phosphate, and nitrate. The order of experiments was arranged randomly (Darvishmotevalli et al. 2019; AlJaberi et al. 2020; Sharma and Simsek 2020).
As shown in Table 1, these inputs give number of experimental runs, range of pH, time, current, and electrolyte which were generated by using RSM software.
Analysis
% COD removal
Equation (1) used to determine % of COD removal efficiency (Asaithambi et al. 2021).
where CODi and COD0 are the COD (mg/L) at initial (i = 0) and at any reaction time (t), respectively.
% color removal
where Absi and Abst are absorbance of sample for corresponding weave length (ƛ = 420 nm) at initial (i = 0) and at any reaction time (t), respectively.
% turbidity removal
where Turi and Turt are the turbidity of the sample (NTU) at initial (t = 0) and at any reaction time (t), respectively.
% nitrate removal
where NO43i and NO43t are concentration of nitrate before and after treatment, respectively.
% phosphate removal
where PO43i and PO43t are the concentration of phosphate before and after treatment, respectively.
Power consumption, (kWhr/m3)
On the practical and economic perspective, the consumption of electrical energy is an important factor in the alternating-current electrocoagulation (ACE) process and it was calculated using equation.
where V cell voltage (volt), I applied current (amp), t—ACE treatment time (hr), and VS, volume of wastewater used (L).
Result and discussion
Characteristics of wastewater
Chemical characteristics of wet coffee processing, such as nitrate, phosphate, and COD, were analyzed, before treatment as well as physical parameters such as color and turbidity. According to the analysis, the color is very black with 2.042 abs, turbidity of 631NTU, pH of 4.5, COD of 2980 mg/L, phosphate of 17.45 mg/L, and nitrate of 14.491 mg/L.
Removal efficiency of Pulsed electrochemical process
Wastewater generated from coffee processing industry was treated in laboratory by considering effects of independent variables and using electrolytes with different charges as below.
Pulsed-electrochemical process by using NaCl
Sodium chloride (NaCl) is an electrolyte that is used to increase conductivity and decrease the amount of voltage supplied to wastewater during the treatment process by forming Na+ and Cl−. The addition of various concentrations of NaCl as a supportive electrolyte increases the conductivity of wastewater (Asaithambi et al. 2020).
Effect of operating parameters on % of removal efficiency
The operating parameter that has the greatest influence on PECP, such as solution pH, electrolyte concentration (NaCl), electric current, and reaction time, was investigated in the terms of percent COD, color, turbidity, NO3, and PO3 removal at room temperature. The removal efficiency of % color, COD, and turbidity, nitrates and phosphate with an energy consumption using PECP are shown in Table 2, which is based on the Al–Al electrode combination with its respective predicted values from RSM.
Optimization of response surface methodology
Response surface methodology is a type of regression analysis that predicts the value of a dependent variable based on the controlled values of the independent variables (Sadaf et al. 2022). RSM was used to optimize an experimental parameter for another process, which included electrochemical oxidation. It is a highly efficient procedure because it not only determines the best operating conditions to maximize system performance, but it also generates a response surface model that predicts a response based on a combination of factor levels. It also depicts the magnitude and impact of various factors on the response, as well as their interactions. As a result, they have been used to simulate a variety of water and wastewater treatment systems and processes (Asaithambi and Matheswaran 2016).
Estimation models were used to optimize the responses in order to determine optimal points for operational conditions and achieve the highest removal percentage. COD, color, turbidity, nitrate, and phosphate removal percentages were set to their maximum values to achieve the best removal performance under operational conditions and the results are given in Table 3. In the in-range state, the target values of four independent variables, including reaction time, solution pH, electric current, and electrolyte, are observed. The following were the optimal conditions for independent variables: pH = 7, reaction time of 45 min, electric current of 0.45 Amp, salt concentration of 0.75 g/L. Under these conditions, the model’s degree of desirability was equal to one, while the removal % of COD, color, and turbidity.
Analysis of variance
Analysis of variance (ANOVA) was used to determine the interaction between the process factors and the response in graphical data analyses. The R2 value was used to describe the quality of the fit polynomial model, and the F test was used to determine its statistical significance. With a 95% confidence level, the P value (probability) was used to evaluate model terms. The data was examined using the analysis of variance (ANOVA), which includes descriptive statistics and statistical tests. This test is used to determine the effect of all variables on the intended response. ANOVA is a statistical method for testing hypotheses about model parameters by dividing total variance in a set of data into smaller groups and component portions that are linked to specific sources of variation (Bui 2017). ANOVA was used to determine significant values between input factors and responses. To determine whether the recommended design is consistent with the test data, statistical factors such as R2, Adj. R2, F-test value (F value), and probability value must be examined (p value). The higher the F value and the lower the P value, the greater the significance of the corresponding term in the recommended correlation for response, so a P value less than 0.05 is considered significant. The mean squares values were calculated by dividing the sum of each variation source’s squares. To avoid systemic error, the experiments were conducted at random. The coefficients of the second-order model, which interpret the amount of removal of the investigated parameters (responses), determine the performance of independent variables (factors). To identify key terms in the model, surface response analysis looks for low p values. The ANOVA results for responses with p < 0.05 probability values show that the second-order model is significant. The mean squares values were calculated by dividing the sum of the squares of each variation source by their degrees of freedom, and the statistical significance was determined in all analyses using a 95 percent confidence level (0.05).
According to Table 4, ANOVA for the % removal of COD by quadratic model is observed. The Model F value of 41.04 implies that the model is significant. There is only a 0.01% chance that an F value this large could occur due to noise. P values less than 0.0500 indicate that model terms are significant. In this case, A, B, C, D, A2, B2, and D2 are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. The lack of fit F value of 2.59 implies that the lack of fit is not significant relative to the pure error. There is a 15.22% chance that a lack of fit F value this large could occur due to noise. Nonsignificant lack of fit is good.
According to Table 5, ANOVA for the % removal of turbidity by quadratic model is observed. The Model F value of 451.28 implies the model is significant. There is only a 0.01% chance that an F value this large could occur due to noise. P values less than 0.0500 indicate model terms are significant. In this case, A, B, C, D, A2, B2, C2, and D2 are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. The lack of fit F value of 4.18 implies that there is a 6.40% chance that a lack of fit F value this large could occur due to noise.
According to Table 6, ANOVA for the % removal of nitrate by quadratic model is observed. The Model F value of 221.87 implies the model is significant. There is only a 0.01% chance that an F value this large could occur due to noise. P values less than 0.0500 indicate model terms are significant. In this case, A, B, C, D, AC, AD, A2, and D2 are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. The lack of fit F value of 4.18 implies that there is a 6.38% chance that a lack of fit F value this large could occur due to noise.
According to Table 7, ANOVA for the % removal of phosphate by quadratic model is observed. The Model F value of 562.48 implies the model is significant. There is only a 0.01% chance that an F value this large could occur due to noise. P values less than 0.0500 indicate model terms are significant. In this case, A, B, C, D, A2, B2, C2, and D2 are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. The lack of fit F value of 4.06 implies that there is a 6.77% chance that a lack of fit F value this large could occur due to noise.
According to Table 8, ANOVA for the % removal of color by quadratic model is observed. The Model F value of 31.54 implies the model is significant. There is only a 0.01% chance that an F value this large could occur due to noise. P values less than 0.0500 indicate model terms are significant. In this case, A, B, C, D, A2, and B2 are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. The lack of fit F value of 3.58 implies there is a 8.61% chance that a lack of fit F value this large could occur due to noise.
Fit statistics
The statistical significance of the model equation and model terms was determined using ANOVA. According to (Darvishmotevalli et al. 2019), model fitting quality was controlled using determination coefficients (R2 and Adj. R2), while statistical significance was controlled using the Fischer test (F-test). The model was validated using predicted R-squares (R2), which employs the leave-one-out technique to assess the model’s prediction power in the face of new observations. Coefficients are used to quantify the relationship between experimental and expected responses (R2). The coefficient of determination (R2) is defined as the ratio of total changes in the expected response caused by the model variables. Show the ratio of sum of squares regression to total sum of squares. R2 should be large and close to 1, and there should be a desired correspondence with adjusted R2 (Adj.R2). R2 expresses the fitness quality of a second-order polynomial model. According to (Mirhosseini et al. 2009), the model’s goodness of fit was also assessed using coefficients of determination R2 (correlation coefficient) and adjusted coefficients of determination R2adj. The high value of the correlation coefficient R2 = 0.9707 indicated that the model was highly reliable in predicting removal percentages, with the model explaining 97.07 percent of the response variability. In this study, all of the R2 values were greater than 0.9. According to (Thirugnanasambandham et al. 2015) for a satisfactory model fitness, R2 should be at least 0.8. R2 values greater than one indicate a high level of agreement between experimental and model-estimated data. As a result, in this study, high R2 values and their agreement with Adj are examined. The value of R2 indicates that the model is highly significant. Table 9 show the “signal-to-noise ratio” index as adequate precision (AP). In other words, AP compares the predicted range of values at design points to the mean prediction error.
The predicted R2 of 0.8712 is in reasonable agreement with the Adjusted R2 of 0.9508; i.e., the difference is less than 0.2. Adeq precision measures the signal-to-noise ratio. A ratio greater than 4 is desirable. Ratio of 28.624 indicates an adequate signal. This model can be used to navigate the design space.
Effect of model parameter and their interactions
The most useful method for revealing reaction system conditions is to use 3D surfaces and 2D contour plots, which are graphical representations of the regression equation for optimizing reaction conditions. They are also used to see how each variable influences the results. In such quadratic model plots, the response functions of two elements are depicted by varying within the experimental ranges, while all other factors are held constant at their values. According to the findings, all of the combined process variables had a significant effect on color, COD, turbidity, nitrate, and phosphate removal with their power consumption in the treatment process. The three-dimensional response surface analysis of the independent variables and the dependent variable was used to estimate the optimal values of the operation parameters. A series of three-dimensional (3D) response surface graphs were created and are shown in Figure below to demonstrate the relationship between removal efficiency and factors.
Interaction of pH and current
The removal COD efficiency was low at pH 3 and increased too much at neutral pH, as indicated by the red color and when the current was around 0.45Amp, according to the 3D graph in Fig. 2. As a result, there was a significant interaction between pH and current. The initial pH and current interacted significantly in the removal of COD in the PEC process. Figure 2 shows that increasing the current density from 0.3 to 0.6 Amp and increasing the pH from acidic 3 to neutral 7 have a positive effect on COD removal. The mutual interaction effect of current density, on the other hand, was critical in the removal of COD and color in the PEC process (Shahedi et al. 2020; Sharma and Simsek 2020).
Interaction of current and time
The color removal efficiency was high at 0.45Amp, which is indicated by red color, and when the time is 45 min. Increasing time up to the center of the graph and increasing time in the same way, it comes to the red color where it shows the high removal efficient; terminal points indicated by green color shows lower removal efficiency, indicating that the interaction effect of current and time was significant. Through contour plots and 3D response surface plots, the mutual effects of current density and time as an estimate of response removal efficiency are shown Fig. 3 EC processes. Current density and time interacted in the PEC process to achieve significant color and COD removal. Because of anodic dissolution in accordance with Faraday’s law, the removal efficiency was increased as the current density was increased. The impact of applied current on the quantity of Fe ions discharged from electrodes, as well as the release of gas bubbles and the formation of flocs. The effect of applied current should be considered in any EC approach for wastewater treatment (Shahedi et al. 2020; Sharma and Simsek 2020). The quantity of Al released from electrodes is directly influenced by electrolysis time, in which turn the effects amount of Al released from the anode and determined the COD, color, and nutrient removal efficiency (Othmani et al. 2022).
Interaction of pH and electrolyte
Figure 4 graph shows that color removal efficiency increased as pH increased from lower to higher; maximum removal at neutral was indicated by more red color, but it decreased after and before neutral pH. It also shows a small increase in removal efficiency from a low electrolyte dosage to the maximum, followed by a decrease at the maximum dosage. The increase in NaCl concentration enhances the generation of oxidizing agents, resulting in increased pollutant removal. Even if the concentration of NaCl varies, the removal efficiency of pollutants is increased with increase in the concentration of NaCl (Othmani et al. 2022). The interaction effect of pH and electrolyte is only marginally significant in the color removal process.
Regression equation
Regression analysis is one of the most commonly used statistical techniques in social and behavioral sciences as well as physical sciences, and it entails identifying and evaluating the relationship between a dependent variable and one or more independent variables, also known as predicator or explanatory variables. It is especially useful for assessing and adjusting for confounding variables. The relationship model is hypothesized, and parameter estimates are used to develop an estimated regression equation. Various tests are then used to determine whether or not the model is satisfactory (Akter and Islam 2022). (Mirhosseini et al. 2009) If the model is satisfactory, the estimated regression equation can be used to predict the value of the dependent variable given the independent variable values. Regression analysis is used to explain variability in dependent variable by means of one or more of independent or control variables and to analyze relationships among variables to answer; the question of how much dependent variable changes with changes in each of the independent’s variables, and to forecast or predict the value of dependent variable based on the values of the independent’s variables.
The experimental results were analyzed using multiple regressions, and the empirical relationship between the response and independent variables was expressed using a second-order polynomial equation. Two empirical models were created to better understand the interactive relationship between responses and process variables (Thirugnanasambandham et al. 2015; Akter and Islam 2022). As a result, the experiment was evaluated in terms of pH (A), reaction time (B), applied current (C), and supporting electrolytes (D). All influencing factors were optimized in order to determine optimal operating conditions for maximum color, COD, nitrate, phosphate, and turbidity removal efficiency with minimum power consumption in the pulsed electrochemical process. NaCl was used to achieve an empirical relationship between the response and the independent variables, which could be approximated by a quadratic polynomial as follows:
The equation in terms of coded factors can be used to predict response for different levels of each factor. The high levels of the factors are coded as + 1 by default, while the low levels are coded as − 1. By comparing the factor coefficients, the coded equation can be used to determine the relative importance of the factors.
The models’ terms are organized using a coding system. The ANOVA test was used to determine the model’s suitability. As shown in the tables above, the lack of fit test was used to confirm model validity. The ANOVA for lack of fit was insignificant (P < 0.05) on the regression model. All of the results show that this model fits the experimental data well. Due to the higher percentage COD, color, nitrate, phosphate, and turbidity removal value and better extraction yield in the shortest time, these findings indicate that PECP was the most effective method for wastewater treatment. Significant differences (P ≤ 0.05) are indicated by means with different letters within the same row.
The desired optimum condition for response
Figure 5 shows that at the optimum levels, the prediction and experimental findings are in good agreement and in line with the straight line, indicating that the model is very valid. According to RSM, the expected R2 for this initial model was 98.91 percent. The backward elimination method was used to construct a parsimonious model with meaningful predictors. The coefficient of determination of the anticipated model revealed a quadratic relationship between responses and parameters with a good regression coefficient.
The optimum PECP conditions were determined as a practical optimum using Design Expert 11.1.2.0 software: electrolysis time of 45 min, salt concentration of 0.75 g/L, and pH of 7. To further validate the predictability of the theoretical model, verification experiments were carried out under optimal conditions. The results showed that experimental removal efficiencies were very close to predicted values, with a difference of less than 0.2 and no significant differences (P > 0.05). As a result, it was possible to conclude that the established model in this study was appropriate and valid. The diagrams a, b, c, and d show the actual and predicted values of NO43, color, PO43, and COD which are not dispersed from each other and in line with the straight line.
Furthermore, the model’s adequacy can be evaluated using diagnostic diagrams including normal probability distribution diagram of residuals, the diagram of predicted values versus real values. Figures 6, 7 and 8 show the distribution of normal probability percentage versus student zed residuals for COD, turbidity, and phosphate removal levels. The points in these diagrams are arranged in a relatively straight line, indicating that the variance and normal distribution are constant. The points in the normal probability distribution diagram of residuals are almost straight line aligned. Some of the scattered points are even expected in the data’s normal distribution. The figures show that there are no outliers that cross the red line.
The results of removal efficiency were closer to the straight line on the distribution of probability percentage and residuals graphs for pollutant removal, as shown in shown in Figs. 8a, b. The number of externally studentized residuals on the second graph run varied uniformly, where the experiment results are valid.
Conclusion
Pulsed-electrocoagulation process for the treatment of coffee processing wastewater was the newest technology with consumed low power consumption. By using the PECP, operating parameters such as pH, electrolyte concentration, electrolysis time, and current play a major role on pollutant and nutrient removal efficiency. As a result, by using the NaCl as an electrolyte concentration, the maximum removal efficiency of color, COD, turbidity, phosphate, and nitrate was 99.92%, 98.75%, 99.00%, 99.02%, 98.83% obtained, respectively, with the pH of 7, reaction time of 45 min, electrolyte concentration of 0.75 g/L, and current of 0.45Amp with total power consumption is 0.972 kWh/m3.
The response surface methodology was used to analyze a large number of experimental runs while producing multiple outputs based on variables such as pH, reaction time, current, electrolyte concentration, and responses such pollutant and nutrient removal. The analysis of variance (ANOVA) with 95 percent confidence limits was used to test the significance of independent variables and their interactions. The quadratic regression equation was recommended as a good model for predicting color, COD, turbidity, phosphate, and nitrate removal efficiency. The model’s goodness of fit was validated with high values of squared correlation coefficient for each of color, COD, turbidity, phosphate, and nitrate. This study suggests that the PECP process could be a successful alternative treatment process for coffee processing wastewater when compared to traditional treatment methods such physicochemical process. As a result, it is concluded that this treatment technology can be used on a large scale to treat the coffee processing wastewater and industrial effluent.
Data availability
Data will be made available on request.
References
Akter S, Islam MS (2022) Effect of additional Fe2+ salt on electrocoagulation process for the degradation of methyl orange dye: an optimization and kinetic study. Heliyon 8:e10176. https://doi.org/10.1016/j.heliyon.2022.e10176
Ali N, Hassan M, Bilal M et al (2021) Chemosphere Adsorptive remediation of environmental pollutants using magnetic hybrid materials as platform adsorbents. Chemosphere 284:131279. https://doi.org/10.1016/j.chemosphere.2021.131279
AlJaberi FY (2019) Operating cost analysis of a concentric aluminum tubes electrodes electrocoagulation reactor. Heliyon 5:e02307. https://doi.org/10.1016/j.heliyon.2019.e02307
AlJaberi FY, Ahmed SA, Makki HF (2020) Electrocoagulation treatment of high saline oily wastewater: evaluation and optimization. Heliyon 6:e03988. https://doi.org/10.1016/j.heliyon.2020.e03988
Asaithambi P, Matheswaran M (2016) Electrochemical treatment of simulated sugar industrial effluent: optimization and modeling using a response surface methodology. Arab J Chem 9:S981–S987. https://doi.org/10.1016/j.arabjc.2011.10.004
Asaithambi P, Govindarajan R, Yesuf MB, Alemayehu E (2020) Removal of color, COD and determination of power consumption from landfill leachate wastewater using an electrochemical advanced oxidation processes. Sep Purif Technol 233:115935. https://doi.org/10.1016/j.seppur.2019.115935
Asaithambi P, Govindarajan R, Yesuf MB et al (2021) Investigation of direct and alternating current-electrocoagulation process for the treatment of distillery industrial effluent: studies on operating parameters. J Environ Chem Eng 9:104811. https://doi.org/10.1016/j.jece.2020.104811
Bähre D, Weber O, Rebschläger A (2013) Investigation on pulse electrochemical machining characteristics of lamellar cast iron using a response surface methodology-based approach. Procedia Soc Behav Sci 6:362–367. https://doi.org/10.1016/j.procir.2013.03.028
Bernal-Martínez LA, Barrera-Díaz C, Natividad R, Rodrigo MA (2013) Effect of the continuous and pulse in situ iron addition onto the performance of an integrated electrochemical-ozone reactor for wastewater treatment. Fuel 110:133–140. https://doi.org/10.1016/j.fuel.2012.11.067
Bhagawan D, Chandan V, Srilatha K, et al (2018) Industrial wastewater treatment using electrochemical process. In: IOP conference series: earth and environmental science, vol 191https://doi.org/10.1088/1755-1315/191/1/012022
Bian Y, Ge Z, Albano C et al (2019) Oily bilge water treatment using DC/AC powered electrocoagulation. Environ Sci Water Res Technol 5:1654–1660. https://doi.org/10.1039/c9ew00497a
Bui HM (2017) Optimization of electrocoagulation of instant coffee production wastewater using the response surface methodology. Polish J Chem Technol 19:67–71. https://doi.org/10.1515/pjct-2017-0030
Chen S, Shi Y, Wang W et al (2014) Phosphorus removal from continuous phosphate-contaminated water by electrocoagulation using aluminum and iron plates alternately as electrodes. Sep Sci Technol 49:939–945. https://doi.org/10.1080/01496395.2013.872145
Darvishmotevalli M, Zarei A, Moradnia M et al (2019) Optimization of saline wastewater treatment using electrochemical oxidation process: prediction by RSM method. MethodsX 6:1101–1113. https://doi.org/10.1016/j.mex.2019.03.015
Ebba M, Asaithambi P, Alemayehu E (2022) Development of electrocoagulation process for wastewater treatment: optimization by response surface methodology. Heliyon 8:e09383. https://doi.org/10.1016/j.heliyon.2022.e09383
Eyvaz M (2016) Treatment of brewery wastewater with electrocoagulation: Improving the process performance by using alternating pulse current. Int J Electrochem Sci 11:4988–5008. https://doi.org/10.20964/2016.06.11
Gebeyehu A, Shebeshe N, Kloos H, Belay S (2018) Suitability of nutrients removal from brewery wastewater using a hydroponic technology with Typha latifolia. BMC Biotechnol 18:1–13. https://doi.org/10.1186/s12896-018-0484-4
Hassen EB, Asmare AM (2018) Predictive performance modeling of Habesha Brewery’s wastewater treatment plant using artificial neural networks. J Environ Treat Tech 6:15–25. https://doi.org/10.31221/osf.io/cjv7p
Kamaraj R, Ganesan P, Lakshmi J, Vasudevan S (2013) Removal of copper from water by electrocoagulation process-effect of alternating current (AC) and direct current (DC). Environ Sci Pollut Res 20:399–412. https://doi.org/10.1007/s11356-012-0855-7
Mahesh S, Garg KK, Srivastava VC et al (2016) Continuous electrocoagulation treatment of pulp and paper mill wastewater: operating cost and sludge study. RSC Adv 6:16223–16233. https://doi.org/10.1039/c5ra27486a
Mena VF, Betancor-Abreu A, González S et al (2019) Fluoride removal from natural volcanic underground water by an electrocoagulation process: parametric and cost evaluations. J Environ Manage 246:472–483. https://doi.org/10.1016/j.jenvman.2019.05.147
Mirhosseini H, Tan CP, Taherian AR, Boo HC (2009) Modeling the physicochemical properties of orange beverage emulsion as function of main emulsion components using response surface methodology. Carbohydr Polym 75:512–520. https://doi.org/10.1016/j.carbpol.2008.08.022
Mohyudin S, Farooq R, Jubeen F et al (2022) Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ Res 204:112387. https://doi.org/10.1016/j.envres.2021.112387
Mosivand S, Monzon LMA, Kazeminezhad I et al (2018) Pulsed electrochemical and electroless techniques for efficient removal of Sb and Pb from water. Environ Sci Water Res Technol 4:2179–2190. https://doi.org/10.1039/c8ew00645h
Othmani A, Kesraoui A, Seffen M (2017) The alternating and direct current effect on the elimination of cationic and anionic dye from aqueous solutions by electrocoagulation and coagulation flocculation. Eur-Mediterr J Environ Integr 2:1–12. https://doi.org/10.1007/s41207-017-0016-y
Othmani A, Kadier A, Singh R et al (2022) A comprehensive review on green perspectives of electrocoagulation integrated with advanced processes for effective pollutants removal from water environment. Environ Res 215:114294. https://doi.org/10.1016/j.envres.2022.114294
Payami Shabestar M, Alavi Moghaddam MR, Karamati-Niaragh E (2021) Evaluation of energy and electrode consumption of acid red 18 removal using electrocoagulation process through RSM: alternating and direct current. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15345-9
Rajkumar D, Palanivelu K (2004) Electrochemical treatment of industrial wastewater. J Hazard Mater 113:123–129. https://doi.org/10.1016/j.jhazmat.2004.05.039
Rasheed T, Kausar F, Rizwan K et al (2022) Chemosphere two dimensional MXenes as emerging paradigm for adsorptive removal of toxic metallic pollutants from wastewater. Chemosphere 287:132319. https://doi.org/10.1016/j.chemosphere.2021.132319
Ren M, Song Y, Xiao S et al (2011) Treatment of berberine hydrochloride wastewater by using pulse electro-coagulation process with Fe electrode. Chem Eng J 169:84–90. https://doi.org/10.1016/j.cej.2011.02.056
Sadaf S, Kumar A, Iqbal J et al (2022) Chemosphere Advancements of sequencing batch biofilm reactor for slaughterhouse wastewater assisted with response surface methodology. Chemosphere 307:135952. https://doi.org/10.1016/j.chemosphere.2022.135952
Sahana M, Srikantha H, Mahesh S, Mahadeva SM (2018) Coffee processing industrial wastewater treatment using batch electrochemical coagulation with stainless steel and Fe electrodes and their combinations, and recovery and reuse of sludge. Water Sci Technol 78:279–289. https://doi.org/10.2166/wst.2018.297
Science E (2018) Industrial wastewater treatment using electrochemical process.https://doi.org/10.1088/1755-1315/191/1/012022
Shahedi A, Darban AK, Taghipour F, Jamshidi-Zanjani A (2020) A review on industrial wastewater treatment via electrocoagulation processes. Curr Opin Electrochem 22:154–169. https://doi.org/10.1016/j.coelec.2020.05.009
Sharma S, Simsek H (2020) Sugar beet industry process wastewater treatment using electrochemical methods and optimization of parameters using response surface methodology. Chemosphere 238:124669. https://doi.org/10.1016/j.chemosphere.2019.124669
Sher F, Iqbal SZ, Rasheed T et al (2021) Coupling of electrocoagulation and powder activated carbon for the treatment of sustainable wastewater. Environ Sci Pollut Res Int 28:48505–48516. https://doi.org/10.1007/s11356-021-14129-5
Tacias-Pascacio VG, Cruz-Salomón A, Castañón-González JH, Torrestiana-Sanchez B (2018) Wastewater treatment of wet coffee processing in an anaerobic baffled bioreactor coupled to microfiltration system. Curr Environ Eng 6:45–54. https://doi.org/10.2174/2212717806666181213161302
Tahreen A, Jami MS, Ali F (2020) Role of electrocoagulation in wastewater treatment: a developmental review. J Water Process Eng 37:101440. https://doi.org/10.1016/j.jwpe.2020.101440
Thirugnanasambandham K, Sivakumar V, Maran JP (2015) Response surface modelling and optimization of treatment of meat industry wastewater using electrochemical treatment method. J Taiwan Inst Chem Eng 46:160–167. https://doi.org/10.1016/j.jtice.2014.09.021
Vasudevan S (2012) Effects of alternating current (AC) and direct current (DC) in electrocoagulation process for the removal of iron from water. Can J Chem Eng 90:1160–1169. https://doi.org/10.1002/cjce.20625
Villanueva-Rodríguez M, Bello-Mendoza R, Wareham DG et al (2014) Discoloration and organic matter removal from coffee wastewater by electrochemical advanced oxidation processes. Water Air Soil Pollut 225:1–11. https://doi.org/10.1007/s11270-014-2204-6
Vinicius C, Paschoa M, Kumar V et al (2022) Chemosphere Adsorptive remediation of naproxen from water using in-house developed hybrid material functionalized with iron oxide. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.133222
Woldesenbet AG, Woldeyes B, Chandravanshi BS (2014) Characteristics of wet coffee processing waste and its environmental impact in Ethiopia. Int J Res Eng Sci ISSN 2:1–05
Zhang J, Li J, Ma C et al (2021) High-efficiency and energy-saving alternating pulse current electrocoagulation to remove polyvinyl alcohol in wastewater. RSC Adv 11:40085–40099. https://doi.org/10.1039/d1ra08093h
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
KTA performed the experiments; wrote the paper. PA conceived and designed the experiments; analyzed and interpreted the data; wrote the paper. MT conceptualization; methodology; validation and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest regarding the publication of this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asefaw, K.T., Asaithambi, P. & Tegegn, M. Treatment of wet coffee processing wastewater using a pulsed-electrocoagulation process: optimization using response surface technique. Appl Water Sci 14, 54 (2024). https://doi.org/10.1007/s13201-024-02118-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02118-1