Abstract
This study focused on the chemistry and isotopes of sulfur in lakes. The bottom sediments and water columns of lakes were found to contain reduced forms of sulfur, including hydrogen sulfide ions, elemental sulfur, and thiosulfate ions, along with sulfate ions. It was determined that elemental sulfur in lakes is present mainly in the form of suspensions and colloids, and the proportion of elemental sulfur in polysulfides increases with increasing water pH. It was shown that sulfate reduction results in the greatest isotope fractionation, with a light sulfur isotope accumulating in hydrogen sulfide ions and a heavy sulfur isotope accumulating in sulfate ions. It was confirmed that the abiotic reaction of hydrogen sulfide with oxygen yields a mixture of products that are depleted in 34S and enriched in 34S in hydrogen sulfide. In contrast, the microbial oxidation of HS− → S0 yields zerovalent sulfur, which is 2–4‰ heavier than the initial product. It was shown that the loss of sulfate ions due to bacterial reduction is most significant in subtype-I and subtype-III chloride and soda lakes. In contrast, in subtype-II sulfate and soda lakes, an increase in sulfate ions was noted due to the oxidation of hydrogen sulfides in water-bearing rocks and bacterial hydrogen sulfide. This finding indicated that in addition to evaporation, the formation of a particular type and subtype of saline lake involves the processes of aluminosilicate hydrolysis, sulfate reduction and hydrogen sulfide oxidation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, a significant number of studies have been conducted on the factors influencing biogenic metamorphism of the chemical composition of various waters to explain changes in the ratios of principal ions resulting from microorganism activity (notable works here include Bazilevich 1965; Kovda 1973; Posokhov 1987; Zheng 2014). Under these conditions, water salinity varies greatly, especially under the reducing geochemical conditions prevalent during the bacterial reduction of sulfates to hydrogen sulfide. This primarily refers to the organic-enriched modern bottom sediments of water bodies. These contain the entire range of reduced sulfur compounds, i.e., iron sulfides, elemental sulfur, polysulfides, thiosulfates, and residual sulfates. Due to their high reactivity, they play important roles in a wide range of biogeochemical processes.
The main process determining the biochemical transformation of sulfur is sulfate reduction, which is carried out by sulfate-reducing microorganisms (e.g., Fauque 1995; Loubinoux et al. 2002; Rabus et al. 2006; Neretin et al. 2006; Wang 2012; Borzenko and Zamana 2008). Hydrogen sulfide can also be formed during the anaerobic oxidation of methane by sulfate (Martens and Berner 1974; Barnes and Goldberg 1976; Boetius et al. 2000). Hydrogen sulfide, whether formed organoclastically, methanotrophically, or by disproportionation, is oxidized by a complex network of microbiological and geochemical processes, with sulfate being the most oxidized product and pyrite the predominant reduced product at the reaction sites (Zopfi et al. 2004; Kompantseva et al. 2009; Avetisyan et al. 2019). Elemental sulfur is the first stable product in the oxidation of hydrogen sulfide (Taylor and Wirsen 1997; Frigaard and Dahl 2008). Among the intermediate compounds, only elemental sulfur accumulates in appreciable concentrations in most natural environments (Sorokin et al. 1995). An alternative explanation for the formation and turnover rate of elemental sulfur in oxygen-free hydrogen sulfide environments was recently proposed by Milucka et al. (2012). Their data suggest that archaea involved in the oxidation of methane reduce sulfate ions to elemental sulfur (and possibly further to hydrogen sulfide). Bacteria, in turn, are not sulfate ion reducing agents but disproportionate elemental sulfur. These results suggest that the reduction of sulfate ions under anaerobic conditions in the studied lakes may lead to the formation of elemental sulfur.
Moreover, the relative importance of abiotic and biotic processes in the formation of elemental sulfur may vary depending on the environment (Li et al. 2008a, b). This change is important because elemental sulfur produced abiotically and biotically differs in its crystal structure, reactivity, and bioavailability (Kleinjan et al. 2005a, b, c). In systems where elemental sulfur occurs in the absence of hydrogen sulfide, such as in the suboxic zone of the Black Sea and the Cariaco Basin or in the sediments of the Black Sea and the Baltic Sea, all sulfur is present in the particulate (colloidal and rhombic) or dissolved form of S8 (Kamyshny 2009a, b).
Elementary sulfur reacts with hydrogen sulfide to form polysulfides (Maronny 1959; Giggenbach 1972; Boulegue and Michard 1978; Kamyshny et al. 2003, 2004). Polysulfides are involved in the anaerobic microbial oxidation of methane combined with sulfate reduction (Milucka et al. 2012) and serve as intermediate products in both the phototrophic (Dahl et al. 2008) and chemotrophic (Wilmot and Vetter 1990) oxidation of sulfides. Polysulfides and sulfides are believed to be involved in the formation of pyrite under neutral and alkaline conditions, which are known to form complexes with metals (Rickard and Luther 2007). This process affects the speciation and mobility of sulfur (Jacobs and Emerson 1982; Wang and Tessier 2009).
The next most stable sulfur compound in natural water bodies is thiosulfate ions. It is formed from the oxidation of hydrogen sulfide with dissolved oxygen and is the result of numerous disproportionation reactions (Jørgensen and Bak 1991; Zhang and Millero 1993) or is formed from the chemical and bacterial oxidation of pyrite with oxygen Fe(III) and Mn(IV) (Pyzik and Sommer 1981; Yao and Millero 1995; Rimstidt and Vaughan 2003).
Despite numerous studies and data explaining the nature of various sulfur compounds, there are still uncertainties in the scale of sulfate reduction and hydrogen sulfide oxidation in the sulfur cycle. In this regard, the main objective of this study was to identify the main mechanisms determining the behaviour of sulfur, namely, the transformation and stability of its forms in different types and subtypes of saline lakes and the influence of these processes on their geochemical diversity.
Research objects
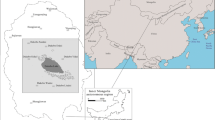
Southeast Zabaikalsky Krai has numerous shallow saline lakes. They are the northernmost saline lakes of the semiarid and arid zone of Central Asia. All the studied saline lakes are confined to the forest-steppe and steppe climatic and landscape zones and are located in the basins of the Ingoda, Onon and Argun rivers, as well as within the drainless Torey basin (Fig. 1).
The physical and geographical features of the region, geological structure, shallow relief, dry and sharp continental climate and perennial and seasonal permafrost all contribute to the formation of numerous saline lakes in the studied region, which differ in size, depth and hydrochemical regime. The vast majority of the lakes (72%) are so-called small water bodies with water areas between 1.0 and 10.0 km2. There are relatively few (4%) larger lakes (more than 10 km2), but their total area is relatively large and constitutes 82% of the total area of all lakes in the region. The largest are the Barun-Torey and Zun-Torey soda lakes, which have water areas of 500 and 380 km2, respectively, in the high-water period. While the lakes vary considerably in terms of their water areas, they all have relatively shallow depths, typically not exceeding a few metres. The deepest lakes are soda lakes, notably Bain-Tsagan Lake (7.5 m), Lake Nozhyi (5.0 m) and Lake Doroninskoye (6.0 m). All the lakes are endorheic. They are located in closed depressions, which are mostly endorheic in nature and collect groundwater (Borzenko 2021).
Research methods
This work is based on the results of the hydrogeochemical sampling of lakes conducted from 2013 to 2021 during the summer periods. Ninety-nine lakes were sampled during the surveys. Water samples, various forms of sulfur and their isotopes were collected from the central parts of the lakes for general chemical analysis. In lakes with a depth > 2 m, water samples were taken 1 m from the surface to the bottom, while in lakes with a depth < 2 m, samples were taken from the middle part of the water column. It is important to note that 95% of the sampled water bodies were shallow lakes with water column heights < 2 m.
The most rapidly changing parameters—pH, Eh, O2, temperature, water TDS and bottom sediment Eh—were determined at the sampling site using AMTAST AMT03 (USA) and pH Cond 340i, WTW (Germany) equipment. Ca and Mg concentrations were determined by atomic absorption in a nitrous oxide-acetylene flame on a SOLAAR 6 M spectrophotometer. A flame emission method was used to determine Na and K. Trace elements were determined by inductively coupled plasma‒mass spectrometry (ICP‒MS). F− and Cl− were determined potentiometrically using ion-selective electrodes. Titration was used to determine the content of carbonic acid derivatives CO2 and CO32− and HCO3−.
SO42− was determined by the turbidimetric method in the form of BaSO4. Hydrogen sulfide HS− and elemental sulfur S0 were determined by preliminary precipitation with zinc acetate from 100 to 2000 ml of water on 0.45 μm filters at the sampling point. Thiosulfate ions S2O32−, sulfite ions SO32−, and polythionate ions SnO62− were precipitated from the remaining filtrate with silver nitrate AgNO3. Triplicate sets of samples were taken to analyse the concentrations of various forms of sulfur. The precipitate filters were frozen prior to analysis.
The methods for preparing reagents and the sequence of operations were described in Dubinin et al. (2019) and were practically unchanged. Released H2S was quantified spectrophotometrically (DR 2800, HACH Lange) (Cline 1969). The detection limit of this method for reduced forms of sulfur was 0.16 µmol/L. The mean and highest relative standard deviations for H2S concentrations in triplicate sets of samples were 6 and 11%, respectively.
It is important to note that after the initial treatment of the precipitated ZnS with hydrochloric acid, the polyhydrogen sulfide Sn2− decomposes into H2S and S0. Consequently, Sn2− cannot be analysed separately and is measured with H2S. In contrast, S0 and Sn0 derived from Sn2− are not reduced during acidification but are obtained by treating CrCl2 with hydrochloric acid (Dubinin et al. 2019). Therefore, the data on S0 presented in the article refer to the sum of the initial S0 allotropes (true-soluble, mechanical, and colloidal) and zerovalent sulfur derived from some fraction (n− 1) of the initial polyhydrogen sulfide Sn2−.
The dissolved forms of sulfur, defined in the text as S2O32−, are the total amount of SO32− + S2O32− + SnO62− (n = 2–5). During treatment with CrCl2 under acidic conditions, the latter is completely reduced to H2S (Dubinin et al. 2019).
It is important to note that samples of the upper layer of bottom sediments were taken in parallel, hermetically packed and frozen, and pressed in the laboratory. Furthermore, Cl− and SO42−, HS− and S0 in the silt water were quantitatively determined according to the scheme above.
Water samples of the water column and silt waters of bottom sediments were also analysed for the isotopic composition of 34/32S in SO42−, HS− and S0 after preliminary precipitation with appropriate reagents. Samples of silt water were analysed for the isotopic composition of 34/32S in HS− and 13 samples for S0.
In the first stage, appropriate reagents were added to the water samples to determine the isotopic composition of 34/32S in HS− and S0–Zn(CH3COO)2 and to determine the isotopic composition of 34/32S in SO42–BaCl2 (preacidifying the sample to pH = 3). In the second stage, the obtained solutions were filtered, and the resulting precipitate was analysed on a Flash EA-1112 analyser (Thermo Scientific, Germany) in S configuration according to the standard protocol for converting sulfate and hydrogen sulfide into SO2. The isotope ratios of 34S/32S were measured on a MAT-253 (Thermo Scientific, Germany) mass spectrometer relative to laboratory standard gas SO2 calibrated to international standards IAEA-S-1, IAEA-S-2, IAEA-S-3 and NBS-127. The aforementioned international isotope standards were used to calibrate the analytical system during the analyses. The measurement results are presented in the generally accepted form δ34S = (Rsample/Rstandard – 1) and expressed in (‰), where Rsample and Rstandard are the ratios of 34S/32S in the sample and standard, respectively. The reproducibility of the δ34S results was 0.1‰ (1σ) for standards (n = 10) and samples. The measurement results for δ34S are given in relation to the VCDT international standard. The coefficient of fractionation was calculated according to the formula ε = 1000 × (α – 1), where α is the fractionation factor: α = (δ34S(SO4) + 1000)/(δ34S(H2S) + 1000) and α = (δ34S(S0) + 1000)/(δ34S(H2S) + 1000).
According to the accepted classification (Borzenko and Shvartsev 2019), three types of lakes were identified: soda, with pH ≥ 9.0, which in turn were divided into 3 subtypes (I—Na-HCO3, II—Na-SO4, III—Na-Cl); chloride, for pH < 9.0 with anions dominated by Cl−, and sulfate, for pH < 9.0 with anions dominated by SO42−. The results are presented in Tables 1, 2, and 3.
Results of hydrogeochemical studies
Main hydrogeochemical characteristics of the atmospheric, river and groundwater of lake catchment areas
Atmospheric precipitation falling in lake catchment areas can be ultrafresh (TDS = 10–50 mg/L), slightly acidic (pH 5–6.5), and more often of the Ca–HCO3 type, with a sulfate coefficient value SO4/Cl > 1. River waters are characterized by low salinity (TDS = 10–47 mg/L) and a slightly alkaline pH (pH 7.4–8.2) driven by Ca–HCO3, with SO4/Cl > 1. The groundwater of the upper hydrodynamic zone has a wide range of pH and TDS values. There are two types of waters: (1) those with TDS = 0.1–1.8 g/L and pH 7.0–8.8, which are dominated by HCO3− and have complex cation composition (Ca, Na, Mg), with the value of SO4/Cl > 1; and (2) those with TDS = 0.9–4.9 g/L and pH = 8.5–9.2, which are dominated by Na–HCO3, with SO4/Cl ≥ 1 (Borzenko et al. 2019).
In the water balance of lakes, the share of atmospheric precipitation varies from 0.5 to 0.7%, surface runoff (rivers, streams) accounts for up to 0.1%, and groundwater runoff is 0.2–0.5%. The average estimates of SO42− and Cl− content in atmospheric precipitation are 5 and 3 mg/L, 57 and 35 mg/L in rivers, 61 and 43 mg/L in type-1 groundwater, and 742 and 882 mg/L in type-2 groundwater, respectively. It was established that even with the maximum share of water coming from atmospheric precipitation in the saline lakes, groundwater is the main source of SO42− and Cl−. Therefore, due to evaporation, these ions accumulate in lake waters in a proportion similar to that in the groundwater feeding the lakes, at least up to the sulfate mineral precipitation stage. It was established that sulfate lakes form at a certain stage of water body salinization. However, within the studied region, they are extremely rare (3%).
Main physical and chemical characteristics of lake waters
Subtype-I soda lakes are characterized by relatively low TDS (average 14.5 g/L) and pH (9.3) values in their waters. The average content of HCO3− + CO32− is 55 eq %, SO42− is 15 eq %, and Cl− is 30 eq %. The few subtype-II soda lakes present are more saline (TDS = 19.4 g/L) and alkaline (average pH = 9.4). SO42− has an average of 42 eq %, and the proportions of Cl− and HCO3− + CO32− are 34 and 24 eq%, respectively. Subtype-III soda lakes are characterized by waters with relatively high TDS (31.9 g/L) and pH (9.5). The main anion is Cl−, with a proportion of 60 eq%. The amount of HCO3− + CO32− equals 25 eq%. As the average estimates indicate, the fraction of SO42−, as in subtype-I, is equal to 15 eq%. In general, in soda-type lakes, the transition from subtype I to the subsequent subtype is accompanied by an increase in TDS and pH values. Then, the Na + content increases (from 87 to 95 eq%); in contrast, the Ca2+ and Mg2+ contents remain relatively low. Soda lakes are generally distinguished by high concentrations of F, Si, As, U, Th, REEs, Mo, B, Al, Sc, Ti, Cr, Mn, Fe, etc. Subtype II contains high concentrations of U, As, Mo, Cu, Ni, and Co (Borzenko 2021).
Sulfate type waters are characterized by high water salinity (average TDS = 30.4 g/L) and low pH value (average 8.0). SO42− accounts for 52 eq% of the ions. The second most important ion is Cl−, which accounts for only 32 eq%. Sulfate lakes accumulate large amounts of Li, Mn, and Zn. There are slightly lower concentrations of Cu, Co and Ni in the latter than in subtype-II soda lakes. Chloride lakes have the highest salinity (TDS = 33.8 g/L) of the samples. The average pH value is 8.3. Cl− accounts for 80 eq % on average. SO42− accounts for 12 eq%. Carbonic acid derivatives, as in sulfate lakes, are present mainly in the form of HCO3− and account for 16 and 8 eq%, respectively. The proportion of Na+ remains high, averaging 82 and 91 eq%, respectively. Compared with other types of lakes, Ca2+ and Mg2+ are found in higher amounts in chloride lakes, and Br, Sr and Ba are among the trace elements present.
Analysis of the data obtained showed that in all the selected types and subtypes of lakes, in chloride and sulfate lakes, with an increase in TDS, HCO3− + CO32− does not accumulate, but its content in soda lakes remains high. The increase in Cl− content, with a few exceptions, significantly outstrips the increase in SO42−. The dependence of TDS on water pH is more complex, i.e., in soda lakes, the pH value increases with an increase in TDS, while in chloride and sulfate lakes, it decreases. With a rise in pH, the HCO3− + CO32− content increases (Borzenko 2021).
Composition of rocks of catchment areas and bottom sediments of lakes
In the coastal areas of the lakes, the rocks are composed of sandy and clayey deposits with a thickness of up to 100 m. These deposits are overlain by effusive-sedimentary chalky rocks that surface in the area of the Toreiskye Lakes. In this area, the rocks are mainly represented by clayey shales and sandstones, among which are intercalations of effusive basalts from the Cretaceous period. Metamorphic rocks of the Palaeozoic era are also widespread, consisting mainly of clayey shales, gneisses, limestones, argillites, and aleurolites, which are pierced by Mesozoic granites. Jurassic intrusive formations are rarely encountered (Fig. 2).
Most of the subtype-I and subtype-III chloride and soda lakes are concentrated within sandy–clay sediments overlying effusive sedimentary rocks, in some places reaching the daytime surface. Sulfate and subtype-II soda lakes are more often located in intrusive rocks. Intrusive rocks are represented by granites, granodiorites and gabbros. Sulfur in the rocks was detected mainly in the form of pyrite FeS2 and less often as sphalerite ZnS, chalcopyrite CuFeS2 and bravoite ((Fe,Ni,Co)S2).
The thickness of loose sediments under the lakes is insignificant (from a few centimetres to the first metres). X-ray phase analysis of lake bottom sediments revealed the presence of calcite, dolomite, clay minerals, feldspars (mainly albite), and quartz. A significant part of the clay material comprises hydromica, imperfect smectite, and mixed-layer minerals (chlorite and smectite), which account for an average of 30% of the total weight of the bottom sediments. All samples show an X-ray amorphous phase consisting of organic compounds, oxides and hydroxides, and iron sulfides. Mirabilite was detected in the sulfate lake Barun-Shivertui (TDS = 67.1 g/L).
Our data show that the HS content of silt water varies widely, from its complete absence in Khara-Nor Lake (sodic III) to 48.4 mmol/L in Lake Doroninskoye (soda I). A relatively low Eh value of − 423 mV is found in the latter. Average estimates show a higher HS− content in sulfate lakes. The less-reduced sediments of subtype-II soda lakes contain minimal amounts of HS−.
The S0 concentration varies over a range of 5 orders of magnitude: from minimum values < 0.16 µmol/L to the maximum values in Shuluta Lake (soda II). Within each type of lake, relatively high concentrations are found in Lake Barun-Shivertui (sulfate), Lake Shvartsivskoe (soda III), and Nizhny Kaltan Lake (chloride). The SO42− content also varies over a wide range, from the minimum in Doroninskoe (soda I) to the maximum in Lake Grishkino. Average estimates indicate that sulfur SO42− more often predominates in bottom sediments, with significant prevalence noted in sulfate and subtype-II soda lakes. These lakes also accumulate relatively large quantities of S0.
Hydrogen sulfide and its derivatives in the water column of lakes
In the water column of lakes, HS− concentrations are significantly lower than their contents in bottom-sediment silt waters. Relatively high HS− concentrations (mmol/L) are detected in the bottom water layer of the deepest meromictic lake of Doroninskoye (11.56) and in the water column of much shallower lakes (depth < 0. 5 m depth): the sulfate lake Barun-Shivertui (285.95) and the soda lakes Shvartsivskoe (90.94), Nozhi (10.5), Nizhny Mukey (70.02), Kujertai (24.45), Zhilino (20.41) and others. As average estimates show, large amounts of HS− are found in the water columns of sulfate lakes, while HS− is found in relatively low concentrations in lakes with an oxidizing environment (0 < Eh < 100 mV).
In general, elevated HS− concentrations are found in lakes with relatively large Eh (< 100 mV). The Eh values in the water columns of such lakes vary from negative values to 100 mV.
In addition to HS−, S0 and S(S2O32−) are detected in the water column of the lakes. The range of S0 concentrations varies from < 0.16 µmol/L to 0.78 mmol/L in Lake Barun-Shivertui (sulfate). Average estimates show that large amounts of S0 accumulate in sulfate lakes. Relatively high concentrations of S0 are detected in Shikhalin-Nuur Lake. S0 is found to be present in the form of tiny particles on the surface of bacterial films and directly on the water surface of the lakes Kudzhertay (Fig. 3), Khodatuy, Malye, Bolshiye Yakshi, Doroninskoye, etc.
The concentration of S2O32− in lake waters varies from < 0.04 µmol/L to 0.65 mmol/L in Lake Barun-Shivertui. Average estimates show relatively high concentrations of S2O32− in sulfate lakes. Average estimates also show that in the sulfate and subtype-II soda lakes, S0 dominates in the total reduced sulfur (∑Sred = S0 + S2O32− + HS−). In ∑Sred sodic subtype-I and subtype-III and chloride lakes, S0 is the second most abundant element after HS−.
Isotopic composition of sulfur
Chemical analyses of the composition of sulfur compounds alone are generally not sufficient to elucidate the relative importance of the various processes in the sulfur cycle. Chemical and microbial transformations of sulfur compounds result in measurable fractionations of sulfur isotopes between reactants and reaction products, and these fractionations provide insight into the sulfur reduction and oxidation pathways considered in the context of lake chemistry.
Variations in the sulfur isotopic composition of dissolved SO42− of the studied lakes (Tables 1, 2, 3) are within the 34S content observed in the waters of continental water bodies (e.g., Grinenko and Grinenko 1974; Ivanov 1989). According to the data obtained from the water columns of some lakes (Khodatuy, Kujertay, Baim-Bulak, Kuduk, Khujarnoye, Doroninskoye (soda I)), the δ34S(S6+) value of lake water (from 27.4 to 31.7‰) is greater than the δ34S(S6+) value of oceanic water (20.1 ± 0.8‰) and modern evaporites (24.3‰) as well as the upper limit of the range for acidic rocks (26.7 ‰), second only to the SO42− content of salt domes (62‰) (all values are taken from (Grinenko and Grinenko 1974)). Across all samples, the average δ34S(S6+) value in the water column is 12.2 ‰, but the range of values varies from − 8.4‰ in Lake Kharanor (soda III) to 31.7‰ in Lake Kujertai (soda I).
According to average estimates, SO42− in sulfate and subtype-II soda lakes is less enriched with 34S (δ34S(S6+) = 7.0 ‰). In subtype-II soda lakes, δ34S(S6+) values vary from 4.6 in Lake Sheluta to 8.2‰ in Lake Zhilino. SO42− is slightly isotopically heavier in sulfate lakes. In the latter, the minimum value (1.1‰) was found in Tsagan Torom Lake and the maximum value (16.0‰) in Barun Shivertui Lake. In chloride-type lakes, the minimum δ34S(S6+) (1.5‰) was found in Lake Bishihan and the maximum (13.2‰) in Lake Bilchir-Nur. According to average estimates, the most isotopically heavy SO42− was detected in the water columns of subtype-I soda lakes. In this subtype, the minimum value of δ34S(S6+) (− 0.9‰) was found in Lake Grishkino, and the maximum was found in Lake Kudzhertay (31.7‰). In subtype-III soda lakes, the minimum δ34S(S6+) was found in Lake Kharanor (− 8.4‰) and the maximum (28.4‰) in Lake Khodatuy (27.4).
The δ34S(HS−) values of silt water range from 20.3 in Lake Kujertai (soda I) to −48.8‰ in Lake Bolshoi Chindant (soda III). As average estimates show, 34S(HS−) accumulates in large quantities in subtype-I soda lakes (− 8.4‰) and is found in smaller quantities (− 31.6‰) in chloride-type lakes. The slightly isotopically heavier isotope of HS− is concentrated in subtype-III soda lakes (− 30.6‰). Subtype-III soda lakes have the largest scatter of δ34S(HS−) data, with an average of − 12.2. Isotope-light sulfur HS− was also found in sulfate-type lakes (− 27.1%).
The δ34S(HS−) value of the water column is on average 5‰ higher than that of silt water. The maximum difference between these values is noted in chloride lakes (7‰), and the minimum is noted in subtype-II soda and sulfate lakes (1‰). The latter (as well as bottom sediments) have the widest range of δ34S(HS−) values in the water column (from 21‰ in Kujertai Lake to − 40.3‰ in Shikhalin-Nuur Lake).
To better understand the processes influencing sulfur behaviour in silt water, the isotopic ratio of sulfur S0 was determined. The established values of δ34S(S0) vary over a wide range from − 49.3‰ in the sediments of the soda lakes Bolshoy Chindant (soda III) to 2.1‰ and Lake Ara-Torum (soda I).
Discussion of the results of hydrochemical studies of saline lakes
Sulfate reduction is an important step in the global sulfur cycle and is carried out by sulfate-reducing prokaryotes (Ollivier et al. 2007; Muyzer and Stams 2008; Barton and Fauque 2009; Barton et al. 2014). Sulfate-reducing prokaryotes are of great functional importance, are abundant in many ecosystems and can grow under various physicochemical conditions. Representatives of the genera Desulfosarcina, Desulfonatronum, Desulfobacterium, Desulfobacca, Desulfuromusa, Desulfurivibrio and Desulfobulbus belonging to the sulfate-reducing bacterial group responsible for the bacterial reduction of sulfates to form hydrogen sulfide were found in the surface layers of bottom sediments. Moreover, the isolated alkalophilic sulfate-reducing bacteria Desulfonatronum lacustre and Desulfonatronumaceae δ-subclass Proteobacteria (Zaharyuk 2010; Kozyreva et al. 2014; Dagurova et al. 2013) used S0, S2O32− and SO32− as alternative electron acceptors. According to (Namsaraev 2009), the sulfate reduction rates varied from 0.886 to 69.04 mgS/kg/day and occurred both in summer and during the frozen period.
The sulfate reduction reaction is often presented as follows:
Such reactions are characteristic of almost all water bodies with delayed water exchange. A characteristic feature of such water bodies is the presence of hydrogen sulfide in bottom sediments and in water. In turn, the formed hydrogen sulfide gives rise to a number of reduced sulfur compounds, the relationships between which are determined by many factors (content of O2 dissolved in water, kinetic parameters of each process stage, presence and composition of organic matter, etc.). Hence, the scale of such processes is different in each lake, which means that the proportion of reduced forms of sulfur also differs.
The results obtained demonstrate that S0 is the main product of H2S oxidation. This observation is consistent with the results of previous studies (Burdige and Nealson 1986; Kamyshny 2009a, b). Under standard conditions, the stable form of sulfur can be orthorhombic cyclooctasera S80 (Steudel and Eckert 2003), which, according to Kamyshny et al. (2003) and Strohl et al. (1981), is most suited for biogeochemical processes. However, it has very low solubility. Seawater at pH 8.2 and a temperature of 25 °C can dissolve only 1.15 mol of S80 per 1 mol of HS− (Kamyshny et al. 2007a, b). According to Kamyshny et al. (2004), Kamyshnyy (2009), and Wang and Tessier (2009), the solubility of S80 increases in the presence of HS− at neutral and alkaline pH due to the formation of soluble polysulfides Sn2–. It is known that inorganic Sn2– and its protonated forms contain (at least formally) one sulfur atom in the divalent oxidation state and one or more sulfur atoms with a zero oxidation state (Sn2− = [Sn0 – S(−2)]2−). The expected concentrations of Sn2− were calculated using the thermodynamic values of the constants (Kamyshny 2007) based on the following reaction:
based on the consideration that lake waters are saturated with S80.
According to thermodynamic calculations, S52− ([S52−] > [S42−] > [S62−] > [S72−] > [S32−] > [S82−] > [S22−]) prevails under the given conditions (Fig. 4a). The total [S42−] + [S52−] + [S62−] content averages 87% of Sn2− and 4% of the gross S0. With increasing pH, the Sn2− content increases and can reach 6% at pH = 10 (Fig. 4b). Suspended and colloidal sulfur SSM remains the dominant form of sulfur under the considered conditions, with a total proportion of 64–99%. The proportion of dissolved S8 is less dependent on pH.
Sn2− are highly geochemically important because they participate in the pyritization reaction (Rickard 1975; Rickard and Luther 2007; Luther and Rickard 2005). In turn, pyrite FeS2 is deposited in the bottom sediments and is eliminated for some time from the sulfur cycle until it is oxidized by oxygen or a community of bacteria. The oxidation process in the presence of dissolved oxygen is often represented by the following reaction:
In the absence of dissolved O2 in water, the pyrite oxidation process may proceed along a pathway involving autotrophic denitrification (Zhang et al. 2012):
S0 formation is often explained by the oxidation of HS− by dissolved O2. However, concentrations of the latter in the lakes in question are often either very low or absent. In this regard, the chemical oxidation of HS− to S0 may occur through two-electron oxidation by iron and manganese oxides (Yamanakaa et al. 2007):
The Fe2+ content in the lakes varies from 10–7 to 10–5 mol/L, a quantity that can allow for the S0 concentrations observed in the water column. The potential oxidation of HS− with the involvement of FeOOH is also confirmed by the presence of lepidocrocite (FeO(OH)) and goethite (FeO(OH)) on the filters obtained after lake water was filtered from Lake Doroninskoye (Borzenko 2021). Mn2+, which is at a relatively low content in the waters (from 10–9 to 10–7 mol/L), can allow for the S0 concentrations observed. In addition, no oxidation of HS− driven by MnO2 occurs in sulfate and chloride lakes, as this reaction is not possible at pH < 9 (Stumm and Morgan 1996).
HS− oxidation can also occur as a result of biological processes with the help of hydrogen sulfide-oxidizing bacteria, among which chemolithoautotrophic microaerobic thionic thiobacteria Thioalkalivibrio and purple serobacteria Thioalkalicoccus limnaeus, Ectothiorhodospira variabilis, Ectothiorhodospira Magna and Ectothiorhodospira shaposhnikovii were detected in the studied lakes (Namsaraev 2009; Gorlenko et al. 2010; Medová et al. 2011; Buryukhaev et al. 2017). These bacteria produce S0 as an HS− oxidation product. A new species of alkalophilic spore-forming bacteria, Alkaliphilus namsaraevii, capable of reducing iron in highly alkaline conditions was recently isolated (pH 10.7) (Zakharyuk 2010).
The oxidation of reduced sulfur by phototrophic sulfur-oxidizing bacteria proceeds according to the proposed reaction
These organisms (primarily green and purple sulfur bacteria) are also capable of reoxidizing S0 and several other reduced sulfur compounds (including S2O32−) to SO42−. Bacteria can mediate the disproportionation of S0, as well as S2O32−- and SO32− (Bak and Cypionka 1987; Cypionka et al. 1998), by the reactions
It is known that the microbiological reduction of SO42− to HS− combined with the oxidation of organic matter followed by the conversion of HS− back to SO42− leads to a dynamic sulfur cycle in lakes, and each reaction is associated with a certain range of isotopic fractions. Analysis of δ34S(S0) and δ34S(HS−) in silt water data showed that 3 variants of the S0 formation mechanism could be distinguished.
-
(1)
In the lakes Zhilino (soda II), Shikhalin-Nuur (sulfate), Maliy Chindant (chloride) and Dunda-Nur, Tsagan-Nur (U.A.) (soda I), the values of δ34S(S0) > δ34S(HS−). The value of the enrichment factor ε is 1–4‰, which is consistent with the equilibrium isotopic effects observed between S0 and HS− in equilibrium with Sn2− (Amrani et al. 2007). This finding may also reflect either the abiotic formation of S0 at the redox barrier or intracellular equilibrium isotopic effects associated with HS− oxidation by anoxygenic phototrophs. This separation of sulfur isotopes leads to the enrichment of 34S for S0 and simultaneously to the depletion of 34S for HS− due to oxidation by Fe-oxides < 1‰ (Goldhaber and Kaplan 1975; Fry et al. 1986) and anoxygenic phototrophs from 2 to 4‰ (Fry et al. 1988; Zerkle et al. 2009).
-
(2)
In the lakes Bolshoy Chindant, Shvartsivskoe and Borzinskoe (soda III), Malye Chindant (chloride), Nizhny Kaltan (soda III) and Barun-Shivertui (sulfate), the values of δ34S0 < δ34HS-− and ε values vary from − 1 to − 5‰. It can be assumed that oxygen penetrated the silt water at some point, for example, during wind agitation, since it is the abiotic reaction of HS− with O2 that produces a mixture of 34S-depleted products, resulting in an increase in δ34S in HS− up to 5‰ (Fry et al. 1988).
-
(3)
In lakes Ara-Torum, Tolutai and Bezymyannoye (soda I), δ34S (S0) > δ34S(HS−), and the ε values are 18 and 22‰, respectively; an explanation for this may be repeated multiple sulfur reduction, oxidation and disproportionation reactions.
One of the main compounds involved in the sulfur cycle is S2O32−, the nature of which is determined by the numerous processes taking place in different reactions, such as (Giggenbach 1974):
S2O32− can also be formed via reactions with iron or manganese oxides (Luther 1991):
The importance of H2S oxidation by MnO2 has been suggested in oxygen-free areas of Chesapeake Bay and the Black Sea. According to our calculated data, only 10–16-10–13 mol/L S2O32− can be formed at the established concentrations of Mn2+ and Fe2+, which is significantly lower than the established concentrations.
It is impossible to ignore the importance of S2O32− formation that occurs due to biological processes carried out by strains of alkalophilic aerobic chemoorganotrophic bacteria of the genus Halomonas, which can oxidize sulfur in the presence of organic substrates, as well as grow anaerobically by denitrification using substrates in the water column surface layer (Matyugina 2018). In turn, S2O32− is oxidized to SO42− by bacteriochlorophyll a-containing bacteria (ABC) of the genus Roseinatronobacter, which are found in the aerobic zone and are capable of chemolithoheterotrophic growth (Boldyreva 2008).
For the bacterial reduction of SO42−, we will try to provide at first approximation of the quantitative assessment of this process. It is known that the microbial reduction of SO42− leads to isotope fractionation characterized by a difference in δ34S values between HS− and SO42−- from 3 to 40‰ (Kaplan and Rittenberg 1964; Habicht and Canfield 2001). The degree of sulfur isotope fractionation by SO42− reduction depends on substrate type and the substrate concentration. SRs capable of oxidizing organic matter to acetate fractionate below 18‰, while SRs oxidizing organic substrate to CO2 stably fractionate above 18‰ (Detmers et al. 2001).
In the soda lakes of Balyktui, Kugertai, Tsagan-Nor (soda I) and Bolshiye Yakshi, Malaya Bulugunda, (soda III), the value of 34S(HS−) varies in the range of 13.0–21.0 ‰ at ≤ 8. It can be assumed that hydrogen oxidation or incomplete oxidation to acetate (as shown above) led to relatively minimal fractionation of sulfur. Hence, the HS− in the silt water has a similar isotopic composition as the sulfur of the original SO42− in the water. It is also impossible to rule out the small fractionation of sulfur that occurs due to the limited amount of SO42− present and its repeated bacterial reduction (Fry et al. 1986; Habicht et al. 1998).
However, there are large numbers of lakes (Borzinskoe, Shikhalin-Nuur, Bilchir-Nur, Gorbunka and others) in which significant fractionation of sulfur (40 ≤ ε≤ 54 ‰) between the SO42− and HS− in the water columns has been observed. This separation of sulfur isotopes may indicate additional fractionation during the oxidation of HS−, followed by the disproportionation of sulfur intermediates to produce large amounts of HS−, which is further depleted in 34S by 5–7‰, and SO42−, which is enriched in 34S by 17–21‰ (Canfield and Thamdrup 1994; Canfield and Teske 1996; Habicht and Canfield 2001; Fry 1991; Canfield 2001; Neretin et al. 2003).
Comparative assessment of the SO4/Cl values of the water column and silt water showed greater losses of SO42− in the soda lakes of subtype-III (80%); this was found in a smaller number of chloride (41%) and subtype-I soda lakes (39%). Among the subtype I soda lakes, an increase in SO42− was observed (Tsagan-Nur (H.A.), Exe-Nor, Noziy, Khantsagaytui-Nur, Grishkino, etc.). These lakes were distinguished by SO4/Cl > 1. In contrast, the silt waters of the sulfate and soda lakes of subtype II showed a marked increase in SO42− (48 and 9%, respectively). The latter two obviously had an additional source of SO42−, which may be pyritized rocks.
It is obvious that the presence of an additional source of HS− and the oxidative environment of the lake water columns allow SO42− to become concentrated, leading to the formation of sulfate lakes or soda lakes. Other evidence for the participation of rocks in the formation of the chemical composition of these lakes is the relatively high concentrations of Co, Ni, and Cu in the waters, which are a part of pyrite and related sulfides (Borzenko 2021). The presence of an additional source of sulfate ions and/or the absence of their bacterial reduction process leads to an increase in the content of isotopically light sulfur in sulfates and allows them to concentrate, leading to the formation of sulfate lakes or soda lakes of the second subtype.
It was established (Borzenko and Shvartsev 2019) that the interaction of water with aluminosilicates, intensive sulfate reduction, and relatively minimal evaporation lead to the formation of subtype-I soda lakes. An increase in water evaporation leads to Cl− accumulation and a transition from subtype-I to subtype III. Introduction of an additional hydrogen sulfide source leads to the formation of subtype-II soda lakes. Sulfate lakes also require an additional sulfur source. The degree of interaction of such waters with aluminosilicates is relatively small; as a result, the water pH remains low. The geochemical environment in this case contributes to the concentration of SO42−, leading to mirabilite precipitation. The formation of chloride lakes occurs under conditions in which a high degree of water evaporation occurs and sulfate-reduction processes dominate.
Conclusions
-
1.
The data obtained indicate that most of the brackish and saline lakes of southeastern Transbaikalia lack an accumulation of SO42− that is proportional to the Cl− content. Our studies showed that the water columns and silt water of bottom sediments of 84% of the studied lakes have a value of SO4/Cl < 1. As the average estimates show, subtype-III soda lakes and chloride lakes have the lowest value for this coefficient. A high sulfate coefficient (SO4/Cl > 1) is observed in subtype-I single lakes and in all sulfate and soda lakes of subtype-II.
-
2.
The presence of HS− and its derivatives S2O32− and S0 was detected in most of the studied lakes. The water columns of sulfate and soda subtype II lakes are dominated by S0 in ∑Sred; lakes of other types and subtypes are dominated by HS−. Thermodynamic calculations using equilibrium constants for polysulfides, hydrogen sulfide, and elemental sulfur revealed that the sulfur content of the polyhydrogen sulfide chain length shifted towards higher values (S42−, S52−, S62−). Calculations revealed that colloidal and suspended forms of sulfur make up the main stocks of S0 in the lakes. The amount of sulfur S2O32− is relatively low, which indicates its rapid turnover in the biogeochemical sulfur cycle.
-
3.
The formation of S0 and S2O32− is associated with numerous chemical and bacterial oxidation reactions of sulfides and/or hydrogen sulfide. The isotopic ratios of 34/32S in S0 confirm that the oxidation of HS− occurs under the anaerobic conditions in bottom sediments and in the water column of lakes as a result of both chemical and chemophototrophic oxidation. Different oxidation pathways for HS− conversion into S0 were confirmed for different isotopic ratios of sulfur.
-
4.
In addition to sulfate reductions, HS− can enter lakes due to hydrolysis of host rocks, which predominantly contain pyrite. It was established that the formation of sulfate and soda lakes with relatively high SO42− contents requires an additional source of sulfur and an oxidizing environment in the water column. Low concentrations of SO42− in lake water are associated with bacterial reduction. The difference in \(\varepsilon\) values between SO42− and HS− confirms the fact that bacterial reduction in lakes proceeds via different pathways. A slight fractionation of sulfur is explained by hydrogen oxidation or incomplete oxidation to acetate. Intensive dissociation of sulfur isotopes may indicate that additional fractionation occurs during the oxidation of HS−, followed by the disproportionation of sulfur intermediates. Large-scale losses of SO42− in silt waters were generally found in all chloride and subtype-I and subtype-III soda lake samples. In contrast, sulfate and subtype-II soda lakes show an increase in SO42−.
-
5.
The behaviour of sulfate ions in the studied lakes is complex because, in addition to evaporation, it is controlled by two processes that have opposite effects: sulfate reduction and oxidation of sulfides in mountain rocks. Bacterial sulfate reduction occurs in most lakes and is characterized by high values of δ34S in its oxidized form, the presence of H2S and other reduced forms of sulfur. For additional sources of sulfates, the values of δ34S decrease, and their content increases until a sulfate-type lake is formed.
-
6.
The chemical composition of lakes, along with evaporite concentration and evaporite sedimentation, is determined by the processes of sulfate reduction, hydrogen sulfide oxidation, and hydrolysis of host aluminosilicate rocks, the dominance of which leads to particular lake types and subtypes.
References
Amrani A, Turner JW, Ma Q, Tang Y, Hatcher PG (2007) Formation of sulfur and nitrogen cross-linked macromolecules under aqueous conditions. Geochim Cosmochim Acta 71:4141–4160
Avetisyan R, Eckert W, Findlay AJ, Kamyshny A Jr (2019) Diurnal variations in sulfur transformations at the chemocline of a stratified freshwater lake. Biogeochemistry 146:83–100
Bak F, Cypionka H (1987) A novel type of energy-metabolism involving fermentation of inorganic sulfur-compounds. Nature 326(6116):891–892
Barnes RO, Goldberg ED (1976) Methane production and consumption in anoxic marine sediments. Geology 4(5):297–300
Barton LL, Fardeau ML, Fauque GD (2014) Hydrogen sulfide: a toxic gas produced by dissimilatory sulfate and sulfur reduction and consumed by microbial oxidation. The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment, pp 237–277
Barton LL, Fauque GD (2009) Biochemistry, physiology and biotechnology of sulfate-reducing bacteria. Adv Appl Microbiol 68:41–98
Bazilevich NI (1965) Geochemistry of soils of soda salinization. Nauka, Moscow, p 351
Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, Gieseke A, Amann R, Jørgensen BB, Witte U, Pfannkuche O (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407:623–626
Boldyreva EN (2008) Aerobic anoxygenic phototrophic bacteria of alkaline habitats. Extended Abstract Dissertation. M, 26 p.
Borzenko SV (2021) The main formation processes for different types of salt lakes: evidence from isotopic composition with case studies of lakes in Transbaikalia, Russia. Sci Total Environ 782:146782. https://doi.org/10.1016/j.scitotenv.2021.146782
Borzenko SV, Shvartsev SL (2019) Chemical composition of salt lakes in East Transbaikalia (Russia). Appl Geochem 103:72–84. https://doi.org/10.1016/j.apgeochem
Borzenko SV, Zamana LV (2008) Sulfate reduction as a factor in the formation of soda waters of Lake Doroninskoye (Eastern Transbaikalia). TSU Bull 312:188–193
Borzenko SV, Zamana LV (2011) Reduced forms of sulfur in the brine of saline-soda Lake Doroninskoye. Eastern Transbaikal Region Geochem Int 49(3):253–261
Borzenko SV, Drebot VV, Fedorov IA (2019) Main formation conditions of soda-type groundwater: a case study from south-eastern Transbaikal region (Russia). Appl Geochem 123:104763
Boulegue J, Michard G (1978) Constantes de formation des ions polysulfures S62-, S52- et S42- en phase aqueuese. J Fr Hydrol 9:27–33
Burdige DJ, Nealson KH (1986) Chemical and microbiological studies of sulfide-mediated manganese reduction. Geomicrobiol J 4(4):361–387
Buryukhaev SP, Bazarov SM, Borzenko SV, Dambaev VB (2017) Ecological conditions and activity of microbial community in mineralized lakes of South-Eastern Transbaikalia. In: Yu. Abidueva E, Barhutova DD, Khakhinov VV (eds) Anthology: 13th International Conference on Salt Lake Research (ICSLR 2017). Book of abstracts. Ulan-Ude, 77.
Canfield D (1994) The production of s-34-depleted hydrogen sulfide during bacterial disproportionation of elemental sulfur. Science 266(5193):1973–1975
Canfield DE (2001) Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochim Cosmochim Acta 65:1117–1124
Canfield DE, Teske A (1996) Late Proterozoic rise in atmospheric oxygen concentration inferred from phylogenetic and sulfur-isotope studies. Nature 382:127–132
Cline JD (1969) Spectrophotometric determination of hydrogen hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Cypionka H, Smock AM, Bottcher ME (1998) A combined pathway of sulfur compound disproportionation in Desulfovivrio desulfuricans. FEMS Microbiol Lett 166(2):181–186. https://doi.org/10.1016/S0378-1097(98)00330-9
Dagurova OP, Garankina VP, Dambaev VB, Namsaraev BB (2013) Biogeochemical processes of the formation of methane and hydrogen hydrogen sulfide in the coastal sediments of Lake Baikal. BSU Bull 3:36–38
Dahl C, Schulte A, Shin DH (2008) Structural and molecular genetic insight into a widespread sulfur oxidation pathway. J Mol Biol 384(5):1287–1300
Detmers J, Brüchert V, Habicht KS, Kuever J (2001) Diversity of sulfur isotope fractionations by sulfate-reducing prokaryotes. Appl Environ Microbiol 67:888–894
Dubinin AV, Demidova TP, Rimskaya-Korsakova MN, Semilova LS, Ocherennik OA (2019) Determination of reduced sulfur species in the water of anaerobic basins. Mar Hydrophys J 35(1):32–46. https://doi.org/10.22449/0233-7584-2019-1-37-51
Fauque GD (1995). In: Bacteria S-R (ed) Biotechnology handbooks, vol 8. Plenum Press, L.L. Barton, New York, pp 217–241
Frigaard N-U, Dahl C (2008) Sulfur metabolism in phototrophic sulfur bacteria. Adv Microb Physiol 54:103–200
Fry B, Cox J, Gest H, Hayes JM (1986) Discrimination between 34S and 32S during bacterial metabolism of inorganic sulfur-compounds. J Bacteriol 165:328–330
Fry B, Gest H, Hayes JM (1988) 34S/32S Fractionation in sulfur cycles catalyzed by anaerobic bacteria. Appl Environ Microbiol 54:250–256
Giggenbach W (1972) Optical spectra and equilibrium distribution of polyhydrogen sulfide ions in aqueous solutions at 20 °C. Inorg Chem 11:1201–1207
Giggenbach WF (1974) Equilibria involving polyhydrogen sulfide ions in aqueous hydrogen sulfide solutions up to 240 C. Inorg Chem 13:1724–1730
Goldhaber MB, Kaplan IR (1975) Controls and consequences of sulfate reduction rates in recent marine sediments. Soil Sci 119(1):42–55
Gorlenko VM, Namsaraev ZB, Bryantseva IA, Boldareva EN, Sorokin DYu, Buryukhaev SP, Namsaraev BB, Matyugina EB, Borzenko SV (2010) Microbial communities of the stratified soda Lake Doroninskoye (Transbaikal Region). Microbiology 79(3):390–401. https://doi.org/10.1134/2FS0026261710030161
Grinenko VA, Grinenko LN (1974) Geochemistry of sulfur isotopes. Nauka, Moscow, 385 p. (in Russian)
Habicht KS, Canfield DE (2001) Isotope fractionation by sulfate-reducing natural populations and the isotopic composition of hydrogen sulfide in marine sediments. Geology 29:555–558
Habicht KS, Canfield DE, Rethmeier J (1998) Sulfur isotope fractionation during bacterial reduction and disproportionation of thiosulfate and sulfite. Geochim Cosmochim Acta 62:2585–2595
Ivanov MV (1989) The evolution of the global biogeochemical cycle. Nauka, Moscow, 200 p. (in Russian)
Jacobs L, Emerson S (1982) Trace-metal solubility in an anoxic fjord. Earth Planet Sci Lett 60(2):237–252
Jørgensen BB, Bak F (1991) Pathways and microbiology of thiosulfate transformations and sulfate reduction in marine sediment (Kattegat, Denmark). Appl Environ Microbiol 57:847–856
Kamyshny A Jr (2009a) Improved cyanolysis protocol for detection of zero-valent sulfur in natural aquatic systems. Limnol Oceanogr Methods 7:442–448
Kamyshny A Jr (2009b) Solubility of cyclooctasulfur in pure water and sea water at different temperatures. Geochim Cosmochim Acta 73:6022–6028
Kamyshny A Jr, Goifman A, Rizkov D, Lev O (2003) Kinetics of disproportionation of inorganic polysulfides in undersaturated aqueous solutions at environmentally relevant conditions. Aquat Geochem 9:291–304
Kamyshny A Jr, Ekeltchik I, Gun J, Lev OA (2004) Method for the determination of inorganic polyhydrogen sulfide distribution in aquatic systems. Anal Chem 78:2631–2639
Kamyshny A Jr, Gun J, Rizkov D, Voitsekovski T, Lev O (2007a) Equilibrium distribution of polyhydrogen sulfide ions in aqueous solutions at different temperatures by rapid single phase derivatization. Environ Sci Technol 41:2395–2400
Kamyshny A Jr, Gun J, Nrizkov DA, Voitsekovski T, Adialev OV (2007b) Equilibrium distribution of polyhydrogen sulfide ions in aqueous solutions at different temperatures by rapid single phase derivatization. Environ Sci Technol 41:2395–2400
Kaplan IR, Rittenberg SC (1964) Microbiological fractionation of sulfur isotopes. J Gen Microbiol 34:195–212
Kleinjan WE, de Keizer A, Janssen AJ (2005a) Equilibrium of the reaction between dissolved sodium hydrogen sulfide and biologically produced sulfur. Colloids Surf B 43:228–237
Kleinjan WE, de Keizer A, Janssen AJ (2005b) Kinetics of the chemical oxidation of polyhydrogen sulfide anions in aqueous solution. Water Res 39:4093–4100
Kleinjan WE, de Keizer A, Janssen AJ (2005c) Kinetics of the reaction between dissolved sodium hydrogen sulfide and biologically produced sulfur. Ind Eng Chem Res 44:309–317
Kompantseva EI, Komova AV, Rusanov II, Pimenov NV, Sorokin DYu (2009) Primary production of organic matter and phototrophic communities in soda lakes of the Kulunda Steppe (Altai Krai). Microbiology 78(5):709–715
Kovda VA (1973) Fundamentals of soil science. Nauka. Kn. I, Moscow, p 448
Kozyreva LP, Egorova DV, Namsaraev BB, Anan’ina LN, Plotnikova EA (2014) Microbial diversity of cellulolytic community of the sandy mat from lake Zun-Torey (Southern Transbaikalia). Inland Water Biol 7(2):134–140
Li X, Taylor GT, Astor Y, Scranton MI (2008a) Relationship of sulfur speciation to hydrographic conditions and chemoautotrophic production in the Cariaco Basin. Mar Chem 112:53–64
Li XN, Taylor GT, Astor YMI (2008b) Scranton Relationship of sulfur speciation to hydrographic conditions and chemoautotrophic production in the Cariaco Basin. Mar Chem 112:53–64
Loubinoux J, Bronowicki J-P, Pereira IAC, Mougenel J-L, LeFaou A (2002) Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol 40:107–112
Luther GW (1991) Pyrite synthesis via polyhydrogen sulfide compounds. Geochim Cosmochim Acta 55:2839–2849
Luther GW, Rickard DT (2005) Metal hydrogen sulfide cluster complexes and their biogeochemical importance in the environment. J Nanopart Res 7(4–5):389–407
Maronny G (1959) Constantes de dissotiation de l’hydrogene sulfure. Electrochim Acta 1:58–69
Martens CS, Berner RA (1974) Methane production in the interstitial waters of sulfate-depleted marine sediments. Science 185(4157):1167–1169. https://doi.org/10.1126/science.185.4157.1167
Matyugina E, Belkova N, Borzenko S (2018) Structure and diversity dynamics of microbial communities at day and night: investigation of meromictic Lake Doroninskoye, Transbaikalia, Russia. Chin J Oceanol Limnol 36(6):1978–1992
Medová H, Boldareva EN, Hrouzek P, Koblížek M, Borzenko SV, Namsaraev ZB, Gorlenko VM, Namsaraev BB (2011) High abundances of aerobic anoxygenic phototrophs in saline steppe lakes. FEMS Microbiol Ecol 76(2):393–400
Milucka J, Ferdelman TG, Polerecky L, Franzke D, Wegener G, Schmid M et al (2012) Zero-valent sulfur is a key intermediate in marine methane oxidation. Nature 491:541–546
Muyzer G, Stams AJM (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nature Rev Microbiol 6:441–454
Namsaraev BB (2009) Brackish and salt lakes of Transbaikalia: hydrochemistry, biology. Publishing House of the Buryat State University, Ulan-Ude, p 340
Neretin LN, Böttcher ME, Grinenko VA (2003) Sulfur isotope geochemistry of the Black Sea water column. Chem Geol 200:59–69
Neretin LN, Volkov II, Rozanov AG, Demidova TP, Falina AS (2006) Biogeochemistry of the Black Sea anoxic zone with a reference to the sulfur cycle. In: Neretin LN (ed) Past and Present Water Column Anoxia. Springer, Dordrecht, The Netherlands, pp 67–104
Ollivier B, Cayol J-L, Fauque G (2007) In: Barton LL, Hamilton WA (eds) Sulphate-reducing bacteria: environmental and engineered systems. Cambridge University Press, Cambridge, pp 305–328.
Posokhov EV (1987) The role of microorganisms in the formation of the chemical composition of natural waters. Hydrochemical materials. Issues of the chemistry of natural waters. Methods of their research. Leningrad: Gidrometeoizdat, pp 82–95 (in Russian)
Pyzik AJ, Sommer SE (1981) sedimentary iron monosulfides—kinetics and mechanism of formation. Geochim Cosmochim Acta 45(5):687–698
Rabus R, Hansen TA, Widdel F (2006) The Prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Scheifer K-K, Stackebrandt E (eds) 2, Ecophysiology and biochemistry. Springer, Berlin, pp 659–768
Rickard DT (1975) Kinetics and mechanism of pyrite formation at low-temperatures. Am J Sci 275(6):636–652
Rickard D, Luther GW (2007) Chemistry of iron sulfides. Chem Rev 107(2):514–562
Rimstidt JD, Vaughan DJ (2003) Pyrite oxidation: a state-of-the-art assessment of the reaction mechanism Topical Symposium on Advances in Oxide and Hydrogen sulfide Mineral Surface Geochemistry. Geochim Cosmochim Acta 67(5):873–880
Sorokin YI, Sorokin PY, Avdeev VA, Sorokin DY, Ilchenko SV (1995) Biomass, production, and activity of bacteria in the Black Sea, with special reference to chemosynthesis and the sulfur cycle. Hydrobiologia 308:61–76
Steudel R, Eckert B (2003) Aqueous sulfur sols. Top Curr Chem 230:1–79
Strohl WR, Geffers I, Larkin JM (1981) Structure of the sulfur inclusion envelopes from four Beggiatoas. Curr Microbiol 6:75–79
Stumm W, Morgan JJ (1996) Aquatic chemistry, 3rd edn. Wiley, New York
Taylor CD, Wirsen CO (1997) Microbiology and ecology of filamentous sulfur formation. Science 277:1483–1485
Wang R (2012) Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92:791–896
Wang FY, Tessier A (2009) Zero-Valent sulfur and metal speciation in sediment porewaters of freshwater lakes. Environ Sci Technol 43(19):7252–7257
Wilmot DB, Vetter RD (1990) The bacterial symbiont from the hydrothermal vent Tubeworm Riftia-Pachyptila is a hydrogen sulfide specialist. Mar Biol 106(2):273–283
Yamanakaa M, Nakanob T, Tasec N (2007) Sulfate reduction and hydrogen sulfide oxidation in anoxic confined aquifers in the northeastern Osaka Basin, Japan. J f Hydrol 335(1–2):55–67
Yao WS, Millero FJ (1995) The Chemistry of the Anoxic Waters in the Framvaren Fjord, Norway. Aquatic Geochem 1(1):53–88
Zakharyuk AG (2010). Distribution and activity of alkaliphilic sulfate and iron-reducing bacteria in soda lakes of Transbaikalia. Ulan-Ude., 22
Zerkle AL, Farquhar J, Johnston DT, Cox RP, Canfield DE (2009) Fractionation of multiple sulfur isotopes during phototrophic oxidation of hydrogen sulfide and elemental sulfur by a green sulfur bacterium. Geochim Cosmochim Acta 73:291–306
Zhang JZ, Millero FJ (1993) The products from the oxidation of H2S in seawater. Geochim Cosmochim Acta 57(8):1705–1718
Zhang Y-C, Slomp CP, Broers HP, Bostick B, Passier HF, Böttcher ME, Omoregie EO, Lloyd JR, Polya DA, Cappellen PV (2012) Isotopic and microbiological signatures of pyrite-driven denitrification in a sandy aquifer. Chem Geol 300:123–132. https://doi.org/10.1016/j.chemgeo.2012.01.024
Zheng M (2014) Saline lakes and salt basin deposits in China. Science Press, Beijing, p 321
Zopfi J, Ferdelman TG, Fossing H (2004) Distribution and fate of sulfur intermediates—sulfite, tetrathionate, thiosulfate, and elemental sulfur—in marine sediments. Special Paper Geol Soc Am 379:97–116. https://doi.org/10.1130/0-8137-2379-5.97
Funding
This study was supported by the Program for Basic Research of the Siberian Branch of the Russian Academy of Sciences; Project No. 121032200070-2.
Author information
Authors and Affiliations
Contributions
Authors have contributed significantly, and author is in agreement with the content of the manuscript. Author read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Consent for publication
Authors declare that the paper is being submitted for consideration for publication in Applied Water Science and that the content has not been published or submitted for publication elsewhere, in any language.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borzenko, S.V., Fedorov, I.A. Geochemical transformations of sulfur and their role in the formation of different types and subtypes of saline lakes in Southeastern Transbaikalia. Appl Water Sci 14, 32 (2024). https://doi.org/10.1007/s13201-023-02082-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-02082-2