Abstract
This study utilised a mixed culture of Chlorella vulgaris and bacteria from sludge to treat synthetic tannery wastewater (STWW) in modified stirred-tank photobioreactors (MSTPBRs). The MSTPBRs were fabricated locally and operated at irradiance value of 580 µmol/m2s supplied by red light-emitting diodes at 12:12 light–dark cycles and 100 ± 1 rpm continuous stirring. In each case, 50, 100 and 150 mg/L concentrations of STWW were inoculated with mixed culture of microalgae and bacteria in three MSTPBRs, with the control MSTPBR operating at 50 mg/L of STWW. Chromium concentrations were measured using colorimeter whilst Fourier transform infrared spectroscopy (FTIR) indicated possible Cr biosorption. Maximum Cr (VI) and total Cr removal efficiencies of 93 and 94% were achieved, with more than 78% total Cr recovery. Results from FTIR suggested involvement of Chlorella vulgaris in the Cr biosorption. The hybrid microalgae-bacteria system efficiently treated tannery wastewater with considerable Cr removal efficiencies. The potentials of the system in treating tannery wastewater in larger scale may require further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Huge amount of wastewater is produced during tanning operations and this generates about 85% of the initial water used as wastewater (Lofrano et al. 2013; Sawalha et al. 2019). The wastewater which is usually discharged into the environment causes serious environmental pollution which affects the entire ecosystem. The wastewater produced in tanning cycles usually contains considerable amounts of chemical oxygen demand (COD) or biochemical oxygen demand (BOD), tannins, inorganic and organic compounds such as sulphides, chlorides as well as heavy metals such as lead (Pb), zinc (Zn), copper (Cu), nickel (Ni) and chromium (Cr) especially in tanning operations utilising chrome salts (Genawi et al. 2020; Subashini & Rajiv 2018).
The use of chrome tanning processes over vegetable tanning approach is due to its advantages which include production of thinner and softer leather resisting darkening over time, formation of livid colours, automatability of the process and shorter time of operation. In view of these advantages, about 90% of tanneries prefer chrome over vegetable tanning (Dabai and Mohammed 2020; Dixit et al. 2015). Despite these advantages, however, chrome tanning is associated with the major setback of producing wastewater containing high amount of Cr in the effluent (Durai and Rajasimman 2011; Eris et al. 2020).
Although Cr occurs in a number of oxidation states, trivalent chromium (Cr III) and hexavalent chromium (Cr VI) are more stable. Cr (III) is the most stable, relatively immobile and important for its effects on metabolic activities at trace concentrations (Belay 2010; Dixit et al. 2015). However, Cr (VI) is highly mobile, about 100 times more toxic than Cr (III), and was found to be teratogenic, mutagenic with its toxicity having adverse effects to all forms of life, and persistent with detrimental effects to the environment (Dai et al. 2012; Aththanayake et al. 2022).
The toxicity of chrome tanned wastewater coupled with increasing stringent wastewater discharge regulations makes the handling of tannery effluents very challenging. In developing countries, some of the industries situated near rivers and streams discharge their heavily polluted effluents into neighbouring water courses prior to the required level of treatment, disregarding guideline limits for effluent discharge (Bernard & Ogunleye 2015; Hammouda et al. 2015). This may be due to high cost of effluent treatment approaches. Therefore, there is need for developing efficient, cost effective and sustainable tannery wastewater treatment approaches.
Numerous tannery wastewater treatment approaches are available including chemical precipitation (Dai et al. 2012), carbon adsorption (Eris et al. 2020; Mustapha et al. 2020), chemical coagulation (Ahmad et al. 2021; Urbina-Suarez et al. 2022) and electrochemical processes (Al-jabri et al. 2021; Mustapha et al. 2019). These treatment approaches were found to perform well, especially in terms of high metal ion handling capacity and can be achieved within short period of time. Despite these advances, however, these methods are associated with excessive use of chemicals, huge sludge production and high energy requirement.
Of the available treatment approaches employed to treat industrial effluents, hybrid microalgae-activated sludge system was found to be a good and promising alternative due to its efficient carbon capture capability and photosynthetic oxygen production. Some of its other credits include high biomass production, effective nutrient removal, heavy metals bioremediation potential and production of both renewable energy and non-energy source materials (Mohammed et al. 2014; López-rosales et al. 2016; Chen et al. 2019; Solé-bundó et al. 2019; Dabai & Mohammed 2021). Microorganisms utilise nutrients available in wastewater for growth thereby mitigating eutrophication (Donmez et al. 1999; Mohammed and Mota 2019), adsorption of metal ions by organic ligands on microalgal cell walls (Lage et al. 2018; Subashini & Rajiv 2018) and biotransformation of these pollutants to non-toxic or less toxic substances by cellular constituents (Hossan et al. 2020).
Furthermore, due to the huge variability in tannery wastewater quality within the tanning cycles and high selectivity of metal ions by biomass amongst the competing heavy metal ions in biological tannery wastewater treatment and the preference in removing metal ions with lower valencies by negatively charged cell components (Bilal et al. 2018; Mohammed and Mota 2019), the current study employed the use of synthetic tannery wastewater (STWW) in accordance with Donmez et al. (1999); Sibi (2016) Mahmoud & Mohamed (2017), to assess Cr (VI) bioremediation potentials using microalgal specie Chlorella vulgaris, in modified photobioreactors.
Materials and methods
Algal culture
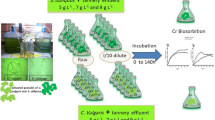
C. vulgaris was isolated and cultured in 1 L volumetric flask with Bold Basal Medium (BBM) in accordance with Fernández-Linares et al. (2017) in the Herbarium Laboratory of the Department of Plant Biology, Bayero University, Kano. This culture was illuminated under 20 W white fluorescent bulb and agitated using mechanical stirrer at an average speed of 200 ± 1 revolution per minute (rpm) and 25 °C, with initial pH adjusted to about 7. This was then up-scaled in modified stirred-tank photobioreactors (MSTPBRs) to reach 100 mg/L Cr (VI) concentration.
Synthetic tannery wastewater
The synthetic wastewater was prepared by diluting specific amount of industrial grade potassium dichromate to obtain specific concentrations of Cr (VI) of 50, 100 and 150 mg/L as described by Donmez et al. (1999); Mahmoud & Mohamed (2017) and Sibi (2016).
Modified stirred-tank photobioreactors (MSTPBRs)
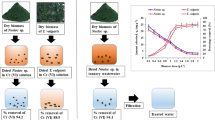
Three MSTPBRs were operated at optimum irradiance of 583 µmol/m2s, at 100 ± 1 rpm for 12:12 light–dark cycles in accordance with Mohammed et al. (2013) and wrapped with aluminium foil to minimise loss of light as shown in Fig. 1. The MSTPBRs were inoculated with a mixture of C. vulgaris and activated sludge based on 90:10 (weight/weight) microalgal culture-activated sludge ratio as suggested by Mohammed et al. (2013) and labelled as R2, R3 and R4 with the synthetic wastewater containing 50, 100 and 150 mg/L Cr (VI) concentration, respectively, whilst another reactor, R1, was supplied with 50 mg/L Cr (VI) concentration contained in the synthetic wastewater but with neither illumination nor aeration, serving as the control.
The central illumination chamber was made up of transparent Plexiglas (Globe Plastic Industries; Malaysia), and photobioreactors had maximum capacity of 21 and a 16 L working volumes (Dabai & Mohammed 2021). Samples were collected every 48 h for analyses.
Determination of Cr (VI)
Potassium dichromate solution was prepared by dissolving 141.4 mg of potassium dichromate solution in distilled water and diluted up to the 100 mL mark in a volumetric flask. Up to 250 mg of diphenylcarbazide (DPC) was dissolved in 50 mL acetone and stored in a dark brown bottle. Series of reference solutions were prepared at 0, 2, 4, 6, 8 and 10 mL. The stock Cr solution was pipetted into a volumetric flask and diluted with 95 mL of distilled water for calibration. The pH was adjusted with 0.25 mL phosphoric acid and 0.2 N sulphuric acid using colorimeter and then, 2 mL of DPC was added, mixed well, and allowed to stand for 10 min for colour development. The absorbance at 540 nm was red in 10 mm glass cuvette using a Lambda 35 UV/Vis Spectrophotometer (PerkinElmer, USA). The Cr stock solution was then replaced with an experimental sample (Heryanto et al. 2022; Hofmann et al. 2018; Sibi 2016).
Determination of total chromium (CrT)
To determine the CrT, the sample was oxidised with potassium permanganate prior to the reaction with DPC. The sample was digested with nitric-sulphuric acid solution and then oxidised with potassium permanganate. The excess permanganate was heated in a water bath at 100 °C for 30 min to decolourise it followed by reaction with 2 mL DPC and the above procedure was repeated (Sibi 2016; Hofmann et al. 2018; Heryanto et al. 2022;).
Cr recovery after biosorption
In desorption and biosorption regeneration process, the Cr was recovered from heavy metal-loaded C. vulgaris using solvent elution method. This was carried out by using a stripping agent (i.e. 0.1 M NH3O). A 0.1 g of dried Cr loaded C. vulgaris pellets was mixed with 50 mL of the stripping agent and centrifuged at 4000 × g for 5 min, and the supernatant was decanted and analysed for total Cr concentration in accordance with Dawodu et al. (2020) and Rezaei (2016).
FTIR spectroscopy
To determine the functional groups of C. vulgaris. Involved in Cr removal from the treated wastewater, about 0.1 g of the dried microalgal biomass was studied using Fourier transform infrared (FTIR) spectroscopy from each MSTPBR by Cary 630 FTIR Spectrophotometer (Agilent Technology, USA) with 32 scans each at a range of 650–4000 cm−1 (Han et al. 2021; Subashini & Rajiv 2018).
Results and Discussion
Cr (VI) removal
Results obtained from varying concentrations of the Cr stock solution were plotted against their corresponding optical densities for the calibration of the UV/Vis Spectrophotometer with correlation coefficient of 0.9967. It was observed that a remarkable Cr (VI) reduction was obtained in all of the bioreactors and the biosorption, in the form of percentage Cr (VI) removal, are shown in Fig. 2, with the following ranges: 8–92, 11–93, 6–89 and 7–83% for reactor A, B, C and D, respectively, during the bioremediation period.
Maximum Cr (VI) removal efficiency of 93% was achieved in reactor B, operated at 50 mg/L Cr (VI) concentration, followed by 91, 89 and 83% in reactors A, C and D, respectively. All the samples collected from the MSTPBRs satisfy discharge requirements for Cr (VI) except that from reactor D which operated at 150 mg/L and found to be at 0.8 mg/L concentration after 24 days.
In a related research, 23% of Cr (VI) was removed when 100 mg/L of synthetic tannery wastewater was treated with C. vulgaris under the influence of other competing heavy metal ions (Donmez et al. 1999), and 77% Cr (VI) removal efficiency was reported at 200 mg/L by Mahmoud & Mohamed, (2017). A slightly higher Cr (VI) removal efficiency of 81% was reported by Sibi, (2016) when heavy metal-loaded industrial effluent was treated using C. vulgaris under extreme conditions of contact time, algal dose, pH, and salinity.
Although reactor A was operated with neither illumination nor aeration, an efficient Cr (VI) removal efficiency was achieved at 50 mg/L Cr (VI) concentration reducing it to about 0.5 mg/L within the first 14 days. This satisfies the discharge requirement specified by National Environmental Standards and Regulations Enforcement Agency (NESREA; Mustapha et al. 2019). This higher removal efficiency in the control reactor might be due to the fact that increase in chlorophyll concentration does not always follow increase in light intensity. Some studies have linked variation of chlorophyll content from the surface to the bottom of lakes with nutrients availability (e.g. Desortova 1976; Felip & Catalan 2000).
Total Cr removal
Total Cr (CrT) removal efficiencies of 86, 94, 89 and 84% have been achieved in reactor A, B, C and D, respectively, as shown in Fig. 3. The CrT removal efficiency was observed in the control reactor with lowest CrT concentration in the effluent to be 0.26 mg/L. CrT removal efficiencies in all of the reactors satisfy the discharge limits except in reactor D with effluent concentration of 0.86 mg/L after bioremediation from the initial concentration of 150 mg/L which is above the 0.5 mg/L specified by NESREA. This initial total Cr concentration of 150 mg/L appears to be beyond the biosorption capacity of C. vulgaris. The highest CrT absorption from the bioreactors was in the order B > C > A > D.
Several attempts were made to remove Cr from wastewater using different treatment approaches. Pandi et al. (2009) reported 95% Cr removal by cyanobacteria in 200 mg/L initial Cr concentration of retan chrome liquor. Another study by Ajayan et al., (2015) reported 81 to 96% Cr removal efficiency using Scenedesmus sp. in tannery wastewater and up to 92% Cr was removed by Scenedesmus sp. in wet market wastewater. However, only about 40% of Cr was removed by Chlorella sp. under the influence of Cu, Zn and Fe and amongst these metals, Cr ions were the least absorbed (Subashini & Rajiv 2018).
Cr recovery
In the desorption and biosorption regeneration process, considerable amount of Cr was recovered after the bioremediation process, and the highest CrT recoveries were found to be 70, 78, 74 and 72% from reactors A, B, C and D, respectively (see Fig. 4).
The highest Cr recovery from microalgal biomass was found to be higher in reactors with medium concentrations of 100 mg/L followed by 150 mg/L. Much higher Cr recoveries of 72, 90 and 95% of Cr desorbed using EDTA, HCl and HNO3 as stripping agents were reported by Rezaei, (2016). In a similar study, Dawodu et al. (2020) recovered 89–96% Cr in three cycles using nitric acid as stripping agent.
Fourier Transform Infrared (FTIR) Spectroscopy of C. vulgaris
The spectra of C. vulgaris. (Fig. 5) revealed the availability of carboxylic, carbonyl, hydroxyl, and amino groups in the Cr bioremediation, as presence of OH together with carbonyl group confirmed the involvement of carboxylic acid group in C. vulgaris biosorption.
The presence of NH, OH and carboxylic groups in the biosorbent might be connected with the presence of amines as shown in Table 1. The shift in the stretching vibrations of NH, OH and C≡O groups to a certain extent after biosorption reaction indicates the possibility of involvement of these groups in Cr uptake.
The assignment of C. vulgaris functional groups before and after the Cr bioremediation was done with the help of FTIR chart, and the differences in peaks were more pronounced in the functional group region between 1200 and 4000 cm−1 than in the finger print region of 650 to 1200 cm−1. It was also observed that carboxyls, aldehydes, alkynes, amides, sulphonamides, alcohols, ethers, esters, and anhydrites might be involved in the Cr bioremediation process.
Conclusion
This study exploited the capability of Chlorella vulgaris for chromium bioremediation in synthetic tannery wastewater using a hybrid microalgae-activated sludge system. Maximum Cr (VI) removal efficiencies of 91, 93, 89 and 83% were achieved in reactors A, B, C and D, respectively, and 86, 94, 89 and 84% total Cr removal efficiencies were achieved in reactors A, B, C and D, respectively. Highest Cr recovery of 78% was achieved in reactor B. It was also found out that carboxyl, carbonyl, amino and hydroxyl groups of C. vulgaris were involved in Cr bioremediation. Although considerable removal efficiencies were obtained in this study, different microalgae–bacteria compositions should be tested in order to exploit the full potentials of the modified hybrid microalgae-bacteria system for biosorption and desorption of chromium from tannery effluents.
References
Ahmad W, Qaiser S, Ullah R, Jan BM, Karakassides MA, Salmas CE, Kenanakis G, Ikram R (2021) Utilization of tires waste-derived magnetic-activated carbon for the removal of hexavalent chromium from wastewater. Materials 14(34):1–19. https://doi.org/10.3390/ma14010034
Ajayan KV, Selvaraju M, Unnikannan P, Sruthi P (2015) Phycoremediation of tannery wastewater using microalgae scenedesmus species. Int J Phyt 17(10):907–916. https://doi.org/10.1080/15226514.2014.98931
Al-jabri H, Das P, Khan S, Thaher M, Abdulquadir M (2021) Treatment of wastewaters by microalgae and the potential applications of the produced biomass-a review. Water 13(27):1–26. https://doi.org/10.3390/w13010027
Almomani F, Bhosale RR (2021) Bio-sorption of toxic metals from industrial wastewater by algae strains Spirulina platensis and Chlorella vulgaris: application of isotherm, kinetic models and process optimization. Sci Total Environ 755:142654. https://doi.org/10.1016/j.scitotenv.2020.142654
Aththanayake AMKCB, Rathnayake IVN, Deeyamulla MP, Megharaj M (2022) Potential use of Chlorella vulgaris KCBAL01 from a freshwater stream receiving treated textile effluent in hexavalent chromium [Cr(VI)] removal in extremely acidic conditions. J Environ Sci Health 57(9):780–788. https://doi.org/10.1080/10934529.2022.2113281
Belay AA (2010) Impacts of chromium from tannery effluent and evaluation of alternative treatment options. J Environ Prot 1:53–58. https://doi.org/10.4236/jep.2010.11007
Bernard E, Ogunleye A (2015) Evaluation of tannery effluent content in Kano metropolis, Kano State Nigeria. Int J Phys Sci 10(9):306–310. https://doi.org/10.5897/IJPS2014.4240
Bilal M, Rasheed T, Eduardo J (2018) Biosorption: an interplay between marine algae and potentially toxic elements-a review. Mar Drugs 16(65):1–16. https://doi.org/10.3390/md16020065
Chen X, Hu Z, Qi Y, Song C, Chen G (2019) The interactions of algae-activated sludge symbiotic system and its effects on wastewater treatment and lipid accumulation. Biores Technol 292(92):122017. https://doi.org/10.1016/j.biortech.2019.122017
Chhikara S, Hooda A, Rana L, Dhankhar R (2010) Chromium (VI) biosorption by immobilized Aspergillus niger in continuous flow system with special reference to FTIR analysis. J Environ Biol 31(5):561–566
Dabai AI, Mohammed K (2020) Chromium removal from tannery wastewater: a review. Platform: J Sci Technol 3(1):63–73
Dabai AI, Mohammed K (2021) Pigments extraction of treated hybrid microalgae-activated sludge. Niger J Technol 40(3):534–539. https://doi.org/10.4314/njt.v40i3.19
Dai J, Ren F, Tao C (2012) Adsorption of Cr (VI) and speciation of Cr (VI) and Cr (III) in aqueous solutions using chemically modified chitosan. Int J Environ Res Public Health 9:1757–1770. https://doi.org/10.3390/ijerph9051757
Dawodu FA, Akpan BM, Akpomie KG (2020) Sequestered capture and desorption of hexavalent chromium from solution and textile wastewater onto low cost Heinsia crinita seed coat biomass. Appl Water Sci 10(1):1–15. https://doi.org/10.1007/s13201-019-1114-6
Desortova B (1976) Relationship between clrlorophyll concentratíon and phytoplankton biomass in several reservoirs in Czechoslovakia. Int Revue Ges Hydrobiol 66(2):153–169
Dixit S, Yadav A, Dwivedi PD, Das M (2015) Toxic hazards of leather industry and technologies to combat threat: a review. J Clean Prod 87:39–49
Dönmez GÇ, Aksu Z, Öztürk A, Kutsal T (1999) A comparative study on heavy metal biosorption characteristics of some algae. Proc Biochem 34(9):885–892
Durai G, Rajasimman M (2011) Biological Treatment of tannery wastewater: a review. J Environ Sci Technol 4(1):1–17. https://doi.org/10.3923/jest.2011.1.17
El-sheekh MM, El-kassas HY, El-din NGS, Eissa DI, El-sherbiny BA, El-kassas HY, El-din NGS, Doria I (2021) Green synthesis, characterization applications of iron oxide nanoparticles for antialgal and wastewater bioremediation using three brown algae. Int J Phytorem 23(14):1–15. https://doi.org/10.1080/15226514.2021.1915957
Eris J, Parañaque L, Ser C, Sci M (2020) Chromium removal from chrome-tannery effluent after alkaline precipitation by adsorption using municipal solid waste-derived activated biochar alkaline precipitation by adsorption using municipals. 26th Reg Symp Chem Eng. https://doi.org/10.1088/1757-899X/778/1/012134
Felip M, Catalan J (2000) The relationship between phytoplankton biovolume and chlorophyll in a deep oligotrophic lake: decoupling in their spatial. J Plankton Res 22(1):91–105
Fernández-linares LC, Barajas CG, Páramo ED, Corona JAB (2017) Assessment of Chlorella vulgaris and indigenous microalgae biomass with treated wastewater as growth culture medium. Biores Technol. https://doi.org/10.1016/j.biortech.2017.07.141
Genawi NM, Ibrahim MH, El-Naas MH, Alshaik AE (2020) Chromium removal from tannery wastewater by electrocoagulation: optimization and sludge characterization. Water 12(5):1374. https://doi.org/10.3390/w12051374
Hammouda O, Abdel-Raouf N, Shaaban M, Kamal M (2015) Treatment of mixed domestic-industrial wastewater using microalgae. J Am Sci 11(12):303–315. https://doi.org/10.7537/marsjas11121538.Keywords
Han Z, Guo N, Yan H, Xu Y, Wang J, Zhao Y, Zhao Y, Meng L, Chi X, Zhao H, Tucker ME (2021) Recovery of phosphate, magnesium and ammonium from eutrophic water by struvite biomineralization through free and immobilized Bacillus cereus MRR2. J Clean Prod 320:1–16. https://doi.org/10.1016/j.jclepro.2021.128796
Heryanto R, Rohaeti E, Wulansari L, Arif Z, Tri W, Riza B (2022) Potentiometric sensor for chromium ( VI ) using a composite of diphenylcarbazide-natural zeolite-modified membrane electrodes. ScienceAsia 48:434–442. https://doi.org/10.2306/scienceasia1513-1874.2022.068
Hofmann A, Sanchez-hachair A, Hofmann A (2018) Hexavalent chromium quantification in solution: comparing direct UV – visible spectrometry with 1, 5-diphenylcarbazide colorimetry comptes Rendus chimie hexavalent chromium quanti fi cation in solution : comparing direct UV e visible spectrometry with 1. Comptes Rendus-Chimie 21(9):890–896. https://doi.org/10.1016/j.crci.2018.05.002
Hossan S, Hossain S, Islam MR (2020) Bioremediation of hexavalent chromium by chromium resistant bacteria reduces phytotoxicity. Int J Environ Res Public Health 17(6013):1–19. https://doi.org/10.3390/ijerph17176013
Lage S, Gojkovic Z, Funk C, Gentili FG (2018) Algal biomass from wastewater and flue gases as a source of bioenergy. Energies 11(664):1–30. https://doi.org/10.3390/en11030664
Lofrano G, Meriç S, Emel G, Orhon D (2013) Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: a review. Sci Total Environ 462:265–281. https://doi.org/10.1016/j.scitotenv.2013.05.004
López-rosales L, García-camacho F, Sánchez-mirón A, Beato EM, Chisti Y (2016) Bioresource Technology Pilot-scale bubble column photobioreactor culture of a marine dinoflagellate microalga illuminated with light emission diodes. Biores Technol 216:845–855. https://doi.org/10.1016/j.biortech.2016.06.027
Mahmoud MS, Mohamed SA (2017) Calcium alginate as an eco-friendly supporting material for Baker ’ s yeast strain in chromium bioremediation. HBRC Journal 13(3):245–254. https://doi.org/10.1016/j.hbrcj.2015.06.003
Mohammed K, Mota C (2019) Microalgae and sustainable wastewater treatment: a Review. Bayero J Pure Appl Sci 11(1):408. https://doi.org/10.4314/bajopas.v11i1.65s
Mohammed K, Ahammad ZS, Sallis PJ, Mota CR (2013) Optimisation of red light-emitting diodes irradiance for illuminating mixed microalgal culture to treat municipal wastewater. WIT Trans Ecol Environ 178:263–270. https://doi.org/10.2495/WS130221
Mohammed K, Ahammad SZ, Sallis PJ, Mota CR (2014) Energy-efficient stirred-tank photobioreactors for simultaneous carbon capture and municipal wastewater treatment. Water Sci Technol 6(10):2106–2112. https://doi.org/10.2166/wst.2014.123
Mustapha S, Ndamitso MM, Abdulkareem AS, Tijani JO, Mohammed AK, Shuaib DT (2019) Potential of using kaolin as a natural adsorbent for the removal of pollutants from tannery wastewater. Heliyon 5:1–17. https://doi.org/10.1016/j.heliyon.2019.e02923
Mustapha S, Tijani JO, Ndamitso MM, Abdulkareem SA, Shuaib DT (2020) The role of kaolin and kaolin/ZnO nanoadsorbents in adsorption studies for tannery wastewater treatment. Sci Rep 10(13068):1–22. https://doi.org/10.1038/s41598-020-69808-z
Pandi M, Shashirekha V, Swamy M (2009) Bioabsorption of chromium from retan chrome liquor by cyanobacteria. Microbiol Res 164(4):420–428. https://doi.org/10.1016/j.micres.2007.02.009
Rezaei H (2016) Biosorption of chromium by using Spirulina sp. Arab J Chem 9(6):846–853. https://doi.org/10.1016/j.arabjc.2013.11.008
Roca M, Silva B, Tavares T, G M (2023) Biosorption of hexavalent chromium by bacillus megaterium. Processes. https://doi.org/10.3390/pr11010179
Sawalha H, Alsharabaty R, Sarsour S, Al-jabari M (2019) Wastewater from leather tanning and processing in palestine: characterization and management aspects. J Environ Manag 251:1–8
Sibi G (2016) Biosorption of chromium from electroplating and galvanizing industrial effluents under extreme conditions using Chlorella vulgaris. Green Energy Environ 1(2):172–177. https://doi.org/10.1016/j.gee.2016.08.002
Solé-bundó M, Garfí M, Matamoros V, Ferrer I (2019) Co-digestion of microalgae and primary sludge: effect on biogas production and microcontaminants removal. Sci Total Environ 660:974–981. https://doi.org/10.1016/j.scitotenv.2019.01.011
Subashini PS, Rajiv P (2018) Chlorella vulgaris DPSF 01: a unique tool for removal of toxic chemicals from tannery wastewater. Afr J Biotech 17(8):239–248. https://doi.org/10.5897/AJB2017.16359
Tegegn K, Yusuf Z, Sasikumar JM, Gorfu K (2022) Biosorbent efficacy of groundnut husk for the elimination of chromium from the effluent of mojo tannery industry, Ethiopia. Int J Biomater. https://doi.org/10.1155/2022/9997348
Urbina-Suarez NA, Ayala-González DD, Rivera-Amaya JD, Barajas-Solano AF, Machuca-Martínez F (2022) Evaluation of the light/dark cycle and concentration of tannery wastewater in the production of biomass and metabolites of industrial interest from microalgae and cyanobacteria. Water 14(3):346. https://doi.org/10.3390/w14030346
Acknowledgements
The authors are grateful to the Tertiary Education Trust Fund, Abuja, Nigeria, for funding this research. The efforts of Dr Aminu Mohammed, Department of Pure and Industrial Chemistry, Bayero University, Kano, for FTIR analysis and Mr Musa Garba Beli of Central Laboratory Complex, Bayero University, Kano, for Cr analysis are highly appreciated and acknowledged.
Funding
Funding for the current research was received from Tertiary Education Trust Fund (TETFund), Abuja, Nigeria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The compliance of ethical standard was ensured. The ethical conduct was observed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dabai, A.I., Mohammed, K. Biosorption and desorption of chromium using hybrid microalgae-activated sludge treatment system. Appl Water Sci 14, 16 (2024). https://doi.org/10.1007/s13201-023-02068-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-02068-0