Abstract
The effects of different perlite/biosolid compositions upon the uptake of Cd and Mn, and the growth of radish plants (Raphanus sativus L) was investigated by using inductively coupled plasma optical emission spectroscopy, and inductively coupled plasma mass spectrometry (ICP-OES and ICP-MS). Mn and Cd were added in soluble forms to perlite/biosolid compositions. Notably, Mn concentrations in different plant parts were found to increase with increase in biosolid compositions, in the order [Mn]leaves > [Mn]shoot > [Mn]roots. This is plausible for Mn, in conformity with the essential role Mn plays during photosynthesis, in metabolic processes, and oxidation–reduction processes in cells. Results indicate that Mn concentrations in plant parts increased up to ~ 50% (wt/wt) perlite/biosolid application rates. In contrast the Cd uptake concentrations in plant parts decreased in the order [Cd]roots > [Cd]shoots > [Cd]leaf. Thus, toxic Cd tends to be sequestered in the roots vis-à-vis Mn that is translocated to the leaves. These results suggest that radish plants sequester Cd in the roots. Biosolids therefore play an important role in sequestering and binding of Cd. The observed concomitant increase in biomass yields implicates the rich contribution of N and P from biosolids. The results from the greenhouse experiments lead to the conclusion on the role played by the biosolids in cleanup and remediations for Cd and Mn, which increased in plant parts with composted wastewater sludge—compositions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enormous amounts of wastewater sludge (also known as biosolids) are produced globally from wastewater treatment plants (Du et al. 2014; Onchoke et al. 2022). Wastewater treatment facilities in the USA produce approximately 6.2 × 106 tons (dry basis) of sludge annually (Federation 2018). Wastewater sludge contains toxic heavy metals (such as Pb, Cd, Hg, and Se), essential trace metals, organic and inorganic pollutants (Onchoke et al. 2018a). The use of biosolids free of toxic chemicals is an important issue of concern for agriculture, industry, and human health (Dichiara et al. 2015). The disposal of wastewater sludge to landfills, as land restoration projects, for fertilization of lands contributes to the increased metal concentrations in the environment. In addition, natural and anthropogenic sources, including weathering, agriculture, and industrialization, increase the metal concentrations in the atmosphere (Biddau and Cidu 2017).
The search and development for new methods to sequester toxic substances in wastewater is important and essential to controlling their concentrations in the environment. Among these include use of phytoremediation plants such as ferns (Singh and Ma 2006), use of nanomaterials such as carbon nanotubes (Huang and Keller 2020; Liné et al. 2021; Patel et al. 2021) and biosolids (or sludges). The choice of any one method depends upon the strong affinity of the material and sequestration of the target metals (Gong et al. 2021; Urasa and Macha 1996). Traditionally, total metal concentrations in plants or any material have been used for assessing health risks to plants or soil or organisms. However, not all plants or materials bioaccumulate metals to the same extent (Kandziora-Ciupa et al. 2017a). Importantly, such studies ignore the fact that metals exist in different forms and the extent of their bioavailability upon application to plants varies. Thus, uptake of metals by plants and translocation to various plant parts may be determined by dissolution kinetics of specific pollutants.
Heavy metal uptake by plants may be controlled by various mechanisms including the role of microorganisms, soil particle size (Ajjabi and Chouba 2009; Chen et al. 2008), and pH of biosolids (Szada-Borzyszkowska et al. 2022). Other mechanisms include metal transfer by the apoplasmic pathway or symplastic transport across the root cortex to plant storage tissues (Shahid et al. 2016).
In this study, the concentrations and transfer factors of Cd and Mn in radish plants (Raphanus sativus L.) were investigated at pH 6.70 and 7.30. The role of pH in metal uptake, at acidic and near neutral conditions, was investigated. In addition, the effects of different perlite/biosolid composition ratios upon Cd and Mn uptake by radish plants (Raphanus sativus L) were evaluated. Perlite is a hydroponics growth media that contains low amounts of metals and is useful as a bulking agent. R. sativus L. forms a good model in experiments over short periods study times. Finally, the influence of biosolids ratios upon uptake of Cd and Mn by R. sativus L (radish) plants was assessed.
Materials and methods
Chemicals and reagents
The high-purity analytical reagents used were purchased from Fisher (Fair Lawn, NJ). Ultrapure water obtained from a Milli-Q water filtration station (18.2 MΩ.cm at 20 °C) was used in the preparation of all standards. Nitric acid (70%, ACS reagent, from Flinn Scientific Inc., Batavia, IL, USA) and hydrogen peroxide (35% wt, Sigma Aldrich, St. Louis, MO) were used in digestion of sludge samples following USEPA Method 3050B (USEPA 1996). Hoagland solution was prepared following established protocols (Hewitt 1966; Onchoke et al. 2018b).
Characterization of soil therapy compost (STC) and perlite
Composted wastewater sludge (CWS, sold under the trade name Soil Therapy Compost, STC) were collected from Neches Compost treatment Facility (NCF) in East Texas. The CWS samples were air-dried, sifted through a mesh diameter of ≤ 2 mm, and analyzed with scanning electron microscopy/energy-dispersive X-ray analysis (SEM/EDX), inductively coupled plasma optical emission spectrometry (ICP-OES) and Fourier transform infrared spectroscopy (FTIR).
Elemental concentrations (P, K, Ca, Mg, Fe, Mn, Cu, and Zn) in STC and perlite were previously examined using ICP-OES or ICP-MS after digestion with 4 M nitric acid (Onchoke and Fateru 2021; Onchoke et al. 2022). Via ICP-OES, ICP-MS, and SEM/EDX analysis (Onchoke and Fateru 2021; Onchoke et al. 2022), perlite was shown to contain macroelements Al, Ca, Mg, Fe, K, Na, P, and S and microelements Ag, As, Ba, B, Cd, Cr, Co, Cu, Mn, Mo, Ni, Zn, and V. In addition, both STC and perlite contained 33.7 wt% C (weight percentages), % N (1.59 wt%), P, organic matter (67.40 mg/kg). Perlite contains low amounts of organic matter, P or N (Onchoke and Fateru 2021; Onchoke et al. 2022). The pH in perlite and STC was found in the range 5.3–7.2.
Experimental design
Plant materials, perlite, and composted wastewater sludge (CWS)
Raphanus sativus L. seeds were purchased from local stores (Burpee seed Company, https://www.burpee.com/). Commercial perlite was obtained from local stores and characterized for its morphology and metal content (Onchoke et al. 2022), and confirmed in agreement with previously published data. Composted wastewater sludge (CWS) and perlite were air-dried and mixed in different proportion ratios of 0% (wt/wt), 25% (wt/wt), 25% (wt/wt), 50% (wt/wt), 75% (wt/wt), and 100% (wt/wt). Although field amounts of 25—33% (wt/wt) are recommended and practiced in field samples for soil amendments (Onchoke et al. 2018b; Siedt et al. 2021), a full range of biosolid content up to 100% (wt/wt) CWS composition) was examined. Notably, perlite/CWS ratios in this study encompass appropriate field treatment ratios (Siedt et al. 2021).
Plant cultivation and harvest in pot experiments
Radish seeds (Raphanus sativus L.) were germinated and grown in pots under greenhouse conditions at temperatures 30–40 °C in Summer 2018. Plants were supplied with Hoagland nutrient solution as described in Ref. # (Onchoke et al. 2018b). The plants were harvested at maturity after three weeks. Harvested whole plants were washed with nanopure water (18.2 MΩ resistivity). Plants were weighed immediately after harvest to obtain their fresh biomass. Thereafter, plants were oven-dried at ~ 60 °C for 48 h. Subsequently, roots, shoots, and leaves were separated, and their biomasses weighed, then ground into a fine powder, and stored in plastic containers until digestion with nitric acid and metal concentration analysis.
Metal analyses in radish plants
Inductively coupled plasma spectroscopy/mass spectrometry (Perkin Elmer, Elan DRC-ICP-MS equipped with a dynamic reaction cell (DRCe) was used for the analysis of Mn in plant parts. Four to 5 mL of 4 M HNO3 and 2 mL of H2O2 were added to the plant samples and digested in a DigiPREP digestion block (SCP science, https://www.scpscience.com) at different temperature ranges as described in USEPA method 3050B (USEPA 1996). The Cd concentration in plant parts was analyzed by flame atomic absorption spectroscopy (Shimadzu AAS 6800) at a wavelength of 228.8 nm.
Quality assurance, quality control, and method validation
Standard Reference Material (SRM) from SCP science (SS-2) was used for quality assurance. ICP-MS was optimized daily using a tuning solution containing Li, Y, Tl, Ce, and Co. The instrument passed the mass calibration, cross-calibration, and daily performance reports for sensitivity, stability, oxide production ratio, and doubly charged production ratio prior to sample measurement. To ensure quality assurance and quality control measures, analysis of continuous calibration verification after every 10 runs was performed. Blanks were run for every batch of sample to check for any laboratory contamination. For quality control, a Certified Reference Material (CRM, EnvironMAT, Contaminated Soil SS-2 from SCP Science Granham, NY) was run for each analytical batch. All analyses were carried out in triplicate and results reported are the mean values of replicate analyses. Table S1 shows agreement between measured values and the CRMs as analyzed by ICP-OES in the range 80% to 115%.
A validation of the ICP measurements was performed for each analyte. The limit of detection (LOD) for Hg was 0.030 ppb and for other metals in ppm (mg/kg) was determined as follows: Ag (0.001868), Al (0.002812), As (0.008147), B (0.0257), Ba (0.000469), Ca (0.5028), Cd (0.000407), Co (0.00047), Cr (0.00126), Cu (0.0043), Fe (0.00260), Hg (0.00255), K (0.2356), Mg (0.008697), Mn (0.000200), Mo (0.000408), Na (0.8436), Ni (0.001508), P (0.006847), Pb (0.00667), S (0.00829), V(0.001626), and Zn (0.0003216).
Statistical analysis
Data presented herein is the average of at least three triplicates and is shown as mean ± standard deviations. Analyses were conducted in Excel version 22 (Microsoft Corporation, 2018. Microsoft Excel, Available at: https://office.microsoft.com/excel). Data at p ≤ 0.05 was deemed significant during analyses. All figures were plotted using Sigmaplot 12.5 (Systat Software Inc.)
Results
Analysis of certified reference materials
The accuracy and precision of the procedure was first evaluated by using certified reference materials, namely, contaminated soil SS-2 (CRM, SS-2, EnvironMAT, from SCP Science Granham, NY) as previously reported (Onchoke and Fateru 2021; Onchoke et al. 2022). Good agreement between measured and certified values to ± 20% is evident (Table S1). The measured concentrations for most metals are in agreement to within 100 ± 15% for K, Ca, Mg, S, P, Al, Fe, Mn, Zn, Cu, and Mo, V, Ni, and falls within accepted CRM values. Therefore, the analytical concentrations results were considered acceptable (Table S1).
Physicochemical and spectroscopic properties of Perlite and STC
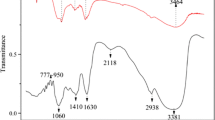
The physicochemical properties of STC and Fourier transform infrared (FTIR) data are compared in Table 1 and Fig. 1, respectively. Thus, the pH of the biosolids is in the range 5.74 to 6.77. This is comparable to previous reports (Onchoke et al. 2018a, 2018b). Whipkern et al. (1996) and Truong et al (2018) noted that a pH range of 5.5–6.5 is ideal for many plants to adequately absorb various mineral nutrients to levels that are not too high to prevent toxicity. Notably, the measured pH is favorable for the absorption of metals into the root system. Figure 1 shows STC absorption FTIR spectral peaks at 3398 cm−1, 2931 cm−1, 2856 cm−1, 1652 cm−1, 1541 cm−1, 1356 cm−1, 1374 cm−1, 1161 cm−1, 1010 cm−1, 916 cm−1, 815 cm−1, 702 cm−1. The broad bands at ~ 3400—3700 cm−1 correspond to the presence of amino ν(N–H), ν(O–H) of phenolic compounds, and carboxylic acid bands. The absorption bands in the range 1300–1375 cm−1 and 1500–1575 cm−1 may be attributed to absorption of ν(NO2). The ν(C–C) and ν(C-H) bands occur in the ranges 1600 and 1500–1430 cm−1, and ~ 2800–2700 (medium) cm−1, respectively, and 2000–1650 cm−1 (C–H bending in aromatic compounds) (Onchoke and Fateru 2021).
Concentrations of macro- and microelements in Perlite and STC Sludge
Previous studies (Onchoke et al. 2022) determined concentrations of macroelements and microelements (Ag, Al, As, B, Ba, Ca, Cd, Co, Cr, Cu, Fe, Hg, K, Mg, Mn, Mo, Ni, P, Pb, S, Se, Zn, V, Na, S, and P) in perlite (PER) and STC by using ICP-OES and ICP-MS. Perlite was found to contain low amounts of macro- and microelements. The total metal concentrations of macroelements in STC (Neches Composted Wastewater Sludge (CWS)) samples were higher than in perlite (PER) (Onchoke et al. 2018b). Figure 2 depicts an EDX spectrum and identifies elements in STC, namely, S, K, O, Fe, Mg, Al, Si, P, Ca, and Fe. Figure 3 depicts SEM micrographs of STC with particle sizes in the range 30.0 μm to 91.6 μm. In addition to its known advantages, sewage sludge also contains hazardous or potentially toxic metals that may constrain its use in agriculture such as phyto-toxicity, soil pollution, and bioaccumulation of toxic elements in food (Dar et al. 2023; Zulfiqar et al. 2022). In agreement with earlier studies, macro- and microelement concentrations were found below regulated USEPA guideline levels (Onchoke et al. 2018a).

Adopted from: Onchoke, KK, Fateru OO, Friedfeld RB, Weatherford PW (2022) Evaluation and analysis of perlite and municipal wastewater sludge (biosolids) from three wastewater treatment plants in East Texas, USA. Environmental Monitoring and Assessment. 194: 121. 10.1007/s10661-022–09794-z. Permission granted
SEM micrograph for STC at a magnification of X200, an accelerating voltage of 20 kV, and filament current of 200 A.
Effect of STC (Composted Wastewater Sludge) amounts on Plant Biomass
Figures 4a and b, and 5a and b show the total dried biomass plant biomass after harvest from 0%, 25%, 50%, 75%, and 100% (wt/wt) perlite/CWS compositions, with Mn or Cd metal amendments at pH values 6.70 and 7.30, respectively. Figure 4a and b shows increase in plant biomass vis-à-vis the control; in accord with a previous study (Onchoke et al. 2018b). Plant biomass increased up to 75% (wt/wt) compost composition. The plant biomass increased by 32.23% and 12.94% in plants grown in 25% (wt/wt), 50% (wt/wt) compost treatment vis-à-vis the control, respectively. In particular, the root, shoot, and leaf increased by 23.81%, 45.95%, and 56.32% vis-à-vis control plants at pH 6.7 (Fig. 4a). The biomass of 100% (wt/wt) CWS composition was noted to have increased as well. These results show that increasing amounts of compost treatments influenced increases in plant biomass. This is attributable to increase in nitrogen (N) and phosphorus (P) content with concomitant increased amounts of applied biosolids. This is in agreement with reported research findings (Reddy and Crohn 2018) where biomasses of okra, tomato, and chili peppers increased in plant biomass with increased use of sludge.
Average dry masses of rapid radish plants (Raphanus sativus, L.) after harvest for radish plants grown in various perlite/biosolids compositions (wt/wt) at pH 7.30. Plants harvested from biosolid/perlite treatment (wt/wt) with a 100 ppm Mn, b 100 ppm Cd treatment. Plants were harvested after 3 weeks
Distribution of Cd and Mn in Raphanus sativus L. and transfer Factors
Uptake of Cd in plant parts grown in different perlite/biosolid ratios at pH 6.70 and 7.30
Figure 6a and b displays Cd amounts in plant parts cultivated at pH 6.70 and 7.30. With increase in percent biosolid amounts (Fig. 6), Cd concentrations decreased in the order [Cd]root > [Cd]shoot > [Cd]leaf in plants. The Cd uptake decreased by 18.75%, 12.77%, 9.0%, 2.74–18.75 (25%wt/wt, 50% (wt/wt), 75% (wt/wt), and 100% wt/wt) CWS composition. This contrasts with 2.23%–4.00% CWS vis-a-vis 0% wt/wt CWS compositions in shoots, and by 0.66%–72.51% % in leaves compared to the control (0% (wt/wt)). In general, more Cd is stored in roots compared to shoot or leaves; concomitant with increase in biosolid ratios. Kandziora-Ciupa et al. (Kandziora-Ciupa et al. 2017a) found Vaccinium myrtillus L. and Vaccinium vitis-idaea L plants stored more Cd in roots in comparison with metal amounts translocated to shoots or leaves.
Cadmium (Cd) is a toxic metal with no known role in the physiology of the plant. It is thus plausible that Cd uptake and translocation to above ground parts is selective. The ability for biosolids to preferentially withhold Cd may be related to its high affinity for metals (Urasa and Macha 1999)(Onchoke et al. 2018b). A possible synergistic interaction of Cd with other metal ions may be envisaged and/or prevalent in CWS. In the present study, there is possible risk to the environment as a result of the roots’s ability to bioaccumulate Cd vis-à-vis other plant parts. On the other hand, sequestration of Cd in radish roots can be viewed as environmentally beneficial. Therefore, radish plants may be used to clean up toxic Cd from soils.
Apart from hyperaccumulator plants such as lettuce (Tang et al. 2016), Cd is known to be less readily up-taken by plants (Brown et al. 1995, 1996; He et al. 2017; Zare et al. 2018). Further, evidence from scanning and/or transmission electron microscopy (SEM, TEM) studies (Qi et al. 2020; Yang et al. 2020) shows that Cd accumulates in the nodules of the root system due to the presence of carboxylic acids including butyric acids (Adeleke et al. 2017; Choudhary et al. 2021), which enhance sorption of metals into the root system. Such findings imply that wastewater sludges contribute to suppress the uptake of Cd from being translocated to the stem or leaves.
Uptake of Mn and Transfer Factors
Concomitant with increase in biosolid amendments, Mn concentrations in plant parts increased in the order [Mn]leaf > [Mn]shoot > [Mn]root at pH 6.70 and 7.30 (Fig. 7a and b) This is plausible given Mn’s essential role for photosynthesis in photosystem II (Carmona et al. 2010; Keren et al. 2002; Maiga et al. 2005). The Mn concentrations in leaves and shoot were about 1.61-fold to three-fold vis-à-vis roots, respectively (Table 2). The transfer factors (TF) of Mn from the soil to the plant parts were calculated as 1.6–4.6 and 1.8–3.4 in plants grown at pH 6.70 and 7.30, respectively. The transfer factors obtained are within the low to moderate contamination factor of 1 < CF < 3 (Sagagi et al. 2022). Notably, higher transfer factors are evident for plants grown at lower pH 6.70 vis-à-vis pH 7.30 (Table 3). A linear relationship was observed (Fig. 7) in plants grown in perlite/compost ratios at pH 6.70 composition with highest Mn amounts observed in 25–50% (wt/wt) CWS treatments.
Influence of pH upon Mn and Cd uptake by plants
Cd Concentrations in plants cultivated in biosolids treated with 100 ppm Cd at pH 6.70 and 7.30
The influence of pH upon uptake of Cd and Mn metals was investigated at pH 6.70 and 7.30. Initially, seeds were sown in various perlite/CWS (wt/wt) ratios. Prior to planting seeds, CWS/perlite materials were watered with Hoagland solution—a plant nutrient solution which supplies essential minerals, needed for growth three days prior to sowing seeds.
The chosen pH (6.70) is comparable to pH 6.50 recommended for fertilizers for growing plants (Liu et al. 2021). Increase in amounts of applied CWS influences Cd or Mn uptake in plant parts at pH 6.70 and 7.30 (Fig. 6). Figure 6a further shows Cd concentrations in the root, shoot, and leaves of radish cultivated in 100 ppm Cd treatment at pH 6.70. Notably, there is decrease in the concentration of Cd in the roots upon addition of biosolids. The highest Cd concentrations were found in roots at 0% (wt/wt) CWS treatment. Concomitant with increase in CWS amounts was decreased Cd concentrations in the order [Cd]root > [Cd]shoot > [Cd]leaf. Radish plants grown at pH 7.30 show Cd concentration in roots < 0.3 mg Cd/kg. In general, [Cd] in plant parts decreased in the order [Cd]root > [Cd]shoot > [Cd]leaf with the highest [Cd] in the root and shoot at 0% (w/w) CWS. The Cd concentration in the leaves was below 0.04 mg/kg plant−1. Clearly, increase in pH from 6.70 to 7.30 results in decreased Cd concentration in plant parts.
Mn Concentrations in plants cultivated in biosolids treated with 100 ppm Mn at pH 6.70 and 7.30
Figure 7a depicts Mn concentrations in root, shoot, and leaves of radish plants cultivated with 100 ppm Mn treatment at pH 6.70. It is noted that Mn concentration in the root and shoot of radish increased from 0 to 25% (wt/wt) CWS treatment. The highest Mn concentration in the root and shoot was found at 75% (wt/wt) and 100% (wt/wt) CWS treatment. The highest Mn concentrations were found in the leaves at CWS 75% (wt/wt).
Figure 7b shows the concentration of Mn in the root, shoot, and leaves of radish plants cultivated in 100 ppm manganese treatment at pH 7.30. The [Mn] in the root, shoot, and leaves of radish plants increased from 0 to 75% (wt/wt) CWS treatment. An increase in the Mn concentration in the root is observed upon addition of biosolids. In similar trend with pH 6.70, concentrations of the Mn in radish parts were found in the order [Mn]leaf > [Mn]shoot > [Mn]root. These experiments are supported by recent reports which show that low molecular acids are produced by roots at lower pH values than at neutral pH values (Tazawa et al. 2021).
Discussions
Influence of sludge amendments on plant biomass
Findings from this investigation relate to the biosolid’s (wastewater sludge) influence on Mn and Cd uptake by Raphanus sativus L. plants. CWS and perlite (a hydroponic material) were used in varying weight ratios, thus enabling assessment of the influence of wastewater sludge upon Mn and Cd uptake.
In general, it is observed that increasing application of CWS amounts resulted in increased biomass to maximum growth at 50%–75% (wt/wt) CWS composition (Figs. 4 and 5). These findings are consistent with previous studies which evaluated effects of sludge and compost on the growth of tomatoes, corn, pepper, and okra (Naz et al. 2019). Compared to controls, use of CWS resulted in increase in plant biomass at applications of 25–50% (wt/wt) by up to 40%. This growth can be attributed to higher nutrients in the compost and/or sludge, and improved aeration of the perlite (Cui et al. 2021). The effect of increased sludge amounts implicates the nutrient availability and concentrations. Studies show increased application of sludge to soils increases crop yields in corn, and barley (Agegnehu et al. 2016).
Zubillaga and Lavado (2002) performed experiments to determine heavy metal content in lettuce plants cultivated in composted biosolid. Under greenhouse conditions, lettuce plants were cultivated in varying amounts of compost biosolids (0–100% wt/wt). Notably, the use of composted biosolid resulted in a 20%–40% increment in the biomass accumulation. Experimental examination further showed Cd concentrations below detection in lettuce leaves in all treatments (Zubillaga and Lavado 2002). Garrido et. al., (2005) investigated the influence of sewage sludge in soils upon uptake of heavy metals by broad bean seeds (Vicia faba L.). Results showed that Cd was not detectable in the broad bean seeds. It was thus concluded that cultivation of broad beans in biosolids signified lower health or environmental risks (Garrido et al. 2005).
Bioconcentration and translocation factors of Mn and Cd
Translocation factor (TF) is the ratio of metal concentration in the shoot to the root. This ratio explains the ability of a plant to translocate heavy metals from the roots to the stem and leaves. Tables 2 and 3 show calculated translocation factors for Mn and Cd in experiments in which radish plants were cultivated with/and without (control) 100 ppm Mn and 100 ppm Cd treatments at pH 6.70 and 7.30, respectively. The translocation factors for Mn in all treatments at both pH values were greater than 1.0, while Cd TFs were less than 1.0. The high Mn TFs implicates a selective Mn transport system in radish plants to the leaves. On the other hand, comparatively low TFs for Cd indicate differential selective mechanisms for Cd translocations in the radish parts.
Implications of uptake mechanisms of metals
The pH changes in the area of the soil around the plant root (rhizosphere) are the most documented chemical reactions taking place at the soil–root interface (Darrah 1993; Hinsinger 2001). Research investigations on pH changes in the soil by cultivating root of beans on the surface of a marble polished plate showed that acid secretion in the roots in beans was strong enough to dissolve calcium carbonate, leaving behind visible imprints on the rock (Paul 2007). The acidic secretion of beans root was attributed to carbonic and organic acids generated by the rhizosphere microflora and roots through root respiration and exudation. Changes in pH of rhizosphere have been attributed to the release of H+ or OH− ions. Hinsinger et al. (2003) found that release of charges caused by hydrogen ions (H+) and hydroxyl ions (OH−) counterbalances for the unbalanced cation–anion uptake at the soil–root interface as the major factor that causes root-induced pH changes in the rhizosphere. In addition, ions passing through the plasma membrane of the root cells such as organic anions released by plants also play a role in root-induced pH changes (Hinsinger et al. 2003).
The different uptake of cations and anions by plant roots is the main source of the flow of H+ in the rhizosphere (Haynes 1990; Hinsinger 1998; Tang and Rengel 2003). The need to compensate for the electrical charges and regulation of cellular pH in the root cell is a major cause of uptake of cations and anions in the root cell. The pH of the aqueous part of the cytoplasm is usually maintained with a range of values around 7.30 with an efficient pH–stat system. The pH–stat system consists of both biochemical and biophysical H+ exchange (Hinsinger et al. 2003). The biochemical components involve the generation and utilization of H+ as a result of carboxylation and decarboxylation of organic acids in the root cell (Hinsinger et al. 2003; Tang and Rengel 2003). The pH of both the apoplasm and the cystol cannot be controlled by ATPs. The ATPs are considered to mainly act through energizing the transport of ions across the membrane which results in significant changes in pH (Gerendás and Schurr 1999). The uptake of cations is better understood with the mechanisms of ATPs (Haynes 1990). When more cations are up-taken than anions, hydrogen ion is released into the apoplasm to balance for the excess positive charges entering the cell. This results in an increase in the pH of the cytoplasm (cytosol) (54, 55). For instance, a larger uptake of K+ exists than SO42− when a plant is supplied with a K2SO4 solution (Hinsinger 1998). But if more anions are up-taken than cations, hydroxyl ion, OH−, will be released or hydrogen ion, H+, will be taken up from the apoplasm to balance for the excess negative charge entering the cell, leading to a decrease in the pH of the cytosol. For instance, there is less uptake of Cd2+ than Cl− when a plant is supplied with a CaCl2 solution (Hinsinger 1998; Hinsinger et al. 2003). This results in a strong relationship that occurs between H+ release and cation–anion balance.
Conclusions
This study showed that radish plants (Raphanus sativus L.) exposed to Mn and Cd cultivated in CWS/perlite compositions tend to differentially accumulate these metals in the roots or leaves. The following conclusions are presented from this research. Firstly, increasing application biosolids up to ~ 25–50% (wt/wt) CWS composition results in increases of plant biomass. Secondly, the SEM/EDX studies show that biosolids contain elemental trace and major metals necessary for plant growth. Although the metal concentrations in biosolids are lower than the USEPA maximum ceiling concentrations, continued usage of biosolids may lead to the bioaccumulation of Mn or Cd in the environment. Thirdly, FTIR spectra shows biosolids contain –COOH in carboxylic acids, which are important in binding of metal ions—and thus contribute to the high affinity for metals. Fourthly, to avoid any contamination to the environment continuous studies are needed to ascertain the extent of heavy metal uptake from various metals.
The novelty of the conducted experiments is related to the effects of biosolid amendments on radishes (Raphanus sativus L.) and the uptake of Mn ad Cd into the roots, shoot, or plant parts. Despite the findings, further analysis and investigation would be needed to explain the biochemical basis of the translocation of Mn into the leaves vis-à-vis Cd in the roots.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Adeleke R, Nwangburuka C, Oboirien B (2017) Origins, roles and fate of organic acids in soils: a review. S Afr J Bot 108:393–406. https://doi.org/10.1016/j.sajb.2016.09.002
Agegnehu G, Nelson PN, Bird MI (2016) Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Tillage Res 160:1–13. https://doi.org/10.1016/j.still.2016.02.003
Ajjabi LC, Chouba L (2009) Biosorption of Cu2+ and Zn2+ from aqueous solutions by dried marine green macroalga Chaetomorpha linum. J Environ Manage 90:3485–3489. https://doi.org/10.1016/j.jenvman.2009.06.001
Biddau R, Cidu R (2017) Metals and Metalloids in Wild Asparagus at Uncontaminated and Mining-Contaminated Sites. J Environ Qual 46:320–329. https://doi.org/10.2134/jeq2016.09.0354
Brown SL, Chaney RL, Angle JS, Baker AJM (1995) Zinc and cadmium uptake by hyperaccumulator thlaspi caerulescens grown in nutrient solution. Soil Sci Soc Am 59:125–133. https://doi.org/10.2136/sssaj1995.03615995005900010020x
Brown SL, Chaney RL, Lloyd CA, Angle JS, Ryan JA (1996) Relative uptake of cadmium by garden vegetables and fruits grown on long-term biosolid-amended soils. Environ Sci Technol 30:3508–3511. https://doi.org/10.1021/es9601797
Carmona A, Deves G, Roudeau S, Cloetens P, Bohic S, Ortega R (2010) Manganese accumulates within golgi apparatus in dopaminergic cells as revealed by synchrotron x-ray fluorescence nanoimaging. ACS Chem Neurosci 1:194–203. https://doi.org/10.1021/cn900021z|
Chen W, Chang AC, Wu L, Zhang Y (2008) Metal uptake by corn grown on media treated with particle-size fractionated biosolids. Sci Total Environ 392:166–173. https://doi.org/10.1016/j.scitotenv.2007.11.019
Choudhary A, Kumar A, Kaur H, Balamurugan A, Padhy AK, Mehta S (2021) Plant Performance and Defensive Role of β-Amino Butyric Acid Under Environmental Stress. In: Husen A (ed) Plant Performance Under Environmental Stress : Hormones, Biostimulants and Sustainable Plant Growth Management. Springer International Publishing, Cham, pp 249–275
Cui X, Yang J, Cui M, Zhang W, Zhao J (2021) Comparative experiments of two novel tubular photobioreactors with an inner aerated tube for microalgal cultivation: enhanced mass transfer and improved biomass yield. Algal Res 58:102364. https://doi.org/10.1016/j.algal.2021.102364
Dar ZA, Bhat JIA, Qazi G, Ganie SA, Amin A, Farooq S, Nazir A, Rasool A (2023) Municipal sewage sludge, aquatic weed compost on soil enzymatic activity and heavy metal accumulation in Kale (Brassica oleracea L.). Appl Water Sci 13:60. https://doi.org/10.1007/s13201-022-01855-5
Darrah PR (1993) The rhizosphere and plant nutrition: a quantitative approach. Plant Soil 155:1–20. https://doi.org/10.1007/BF00024980
Dichiara AB, Weber MR, Gorman WR, Rogers RE (2015) Removal of copper ions from aqueous solutions via adsorption on carbon nanocomposites. ACS Appl Mater 7:15674–15680
Du F, Freguia S, Yuan Z, Keller J, Pikaar I (2014) Enhancing toxic metal removal from acidified sludge with nitrite addition. Environ Sci Technol 49:6257–6263. https://doi.org/10.1021/es504507m
Federation, WE (2018). "Activated Sludge and Nutrient Removal, Manual of Practice No. OM-9," 3rd/Ed.
Garrido S, Campo GMD, Esteller MV, Vaca R, Lugo J (2005) Heavy metals in soil treated with sewage sludge composting, their effect on yield and uptake of broad bean seeds (Vicia faba L.). Water Air Soil Pollut 166:303–319. https://doi.org/10.1007/s11270-005-5269-4
Gerendás J, Schurr U (1999) Physicochemical aspects of ion relations and pH regulation in plants—a quantitative approach. J Exp Bot 50:1101–1114
Gong X, Huang D, Liu Y, Zou D, Hu X, Zhou L, Wu Z, Yang Y, Xiao Z (2021) Nanoscale zerovalent iron, carbon nanotubes and biochar facilitated the phytoremediation of cadmium contaminated sediments by changing cadmium fractions, sediments properties and bacterial community structure. Ecotoxicol Environ Saf 208:111510. https://doi.org/10.1016/j.ecoenv.2020.111510
Haynes R (1990) Active ion uptake and maintenance of cation-anion balance: A critical examination of their role in regulating rhizosphere pH. Plant Soil 126:247–264
He H, Tam NFY, Yao A, Qiu R, Li WC, Ye Z (2017) Growth and Cd uptake by rice (Oryza sativa) in acidic and Cd-contaminated paddy soils amended with steel slag. Chemosphere 189:247–254. https://doi.org/10.1016/j.chemosphere.2017.09.069
Hewitt, EJ (1966). Sand and water culture methods used in the Study of Plant Nutrition, Technical Communication No. 22. Commonwealth Agricultural Bureau London.
Hinsinger P (1998) How do plant roots acquire mineral nutrients? Chemical processes involved in the rhizosphere. Adv Agron 64:225–265
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195. https://doi.org/10.1023/A:1013351617532
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Huang Y, Keller AA (2020) Remediation of heavy metal contamination of sediments and soils using ligand-coated dense nanoparticles. PLoS ONE 15(9):e0239137. https://doi.org/10.1371/journal.pone.0239137
Kandziora-Ciupa M, Nadgórska-Socha A, Barczyk G, Ciepał R (2017) Bioaccumulation of heavy metals and ecophysiological responses to heavy metal stress in selected populations of Vaccinium myrtillus L. and Vaccinium vitis-idaea L. Ecotoxicology 26:966–980. https://doi.org/10.1007/s10646-017-1825-0
Keren N, Kidd MJ, Penner-Hahn JE, Pakrasi HB (2002) A light-dependent mechanism for massive accumulation of manganese in the photosynthetic bacterium synechocystis sp. PCC 6803. Biochemistry 41:15085–15092
Liné C, Manent F, Wolinski A, Flahaut E, Larue C (2021) Comparative study of response of four crop species exposed to carbon nanotube contamination in soil. Chemosphere 274:129854. https://doi.org/10.1016/j.chemosphere.2021.129854
Liu, G, Simonne EH, Morgan KT, Hochmuth GJ, Agehara S, Mylavarapu R, Williams P (2021). "Chapter 2. Fertilizer Management for Vegetable Production in Florida. Vegetable Production Handbook for Florida, 2020–2021 Edition. https://edis.ifas.ufl.edu/," University of Florida Institute of Food and Agricultural Sciences, Gainesville: University of Florida Institute of Food and Agricultural Sciences.
Maiga A, Diallo D, Bye R, Paulsen BS (2005) Determination of some toxic and essential metal ions in medicinal and edible plants from mali. J Agric Food Chem 53:2316–2321. https://doi.org/10.1021/bi100303f
Naz S, Anjum MA, Haider STA (2019) Effect of different irrigation sources on growth, yield and heavy metals accumulation in tomato and okra. J Hortic Sci 2:10–19. https://doi.org/10.46653/jhst190201010
Onchoke KK, Fateru OO (2021) Evaluating bioavailability of elements in municipal wastewater sludge (Biosolids) from three rural wastewater treatment plants in East Texas (USA) by a sequential extraction procedure. Results in Chem 3:100211. https://doi.org/10.1016/j.rechem.2021.100211
Onchoke KK, Franclemont CM, Weatherford PW (2018a) Structural characterization and evaluation of municipal wastewater sludge (Biosolids) from two rural wastewater treatment plants in East Texas, USA. Spectrochim Acta A 204:514–524. https://doi.org/10.1016/j.saa.2018.06.096
Onchoke KK, Urasa IT, Shipes BG (2018b) Influence of composted wastewater sludge (CWS) on lead and copper uptake by radish (Raphanus sativus L.). Compost Sci Util 26:244–255. https://doi.org/10.1080/1065657X.2018.1496044
Onchoke KK, Fateru OO, Friedfeld RB, Weatherford PW (2022) Evaluation and analysis of perlite and municipal wastewater sludge (biosolids) from three wastewater treatment plants in East Texas, USA. Environ Monit Assess 194:121. https://doi.org/10.1007/s10661-022-09794-z
Patel A, Enman J, Gulkova A, Guntoro PI, Dutkiewicz A, Ghorbani Y, Rova U, Christakopoulos P, Matsakas L (2021) Integrating biometallurgical recovery of metals with biogenic synthesis of nanoparticles. Chemosphere 263:128306. https://doi.org/10.1016/j.chemosphere.2020.128306
Paul EA (2007) 1 - Soil Microbiology, Ecology, and Biochemistry in Perspective. In: Third Edition)" (E. A. Paul, (ed) "Soil Microbiology, Ecology and Biochemistry. Academic Press, San Diego, pp 3–24
Qi X, Tam NF-Y, Li WC, Ye Z (2020) The role of root apoplastic barriers in cadmium translocation and accumulation in cultivars of rice (Oryza sativa L.) with different Cd-accumulating characteristics. Environ Poll 264:114736. https://doi.org/10.1016/j.envpol.2020.114736
Reddy N, Crohn DM (2018) Effect of composted greenwaste and rockwool on plant growth of Okra. Tomato, and Chili Peppers. https://doi.org/10.1080/1065657X.2018.1463878.CompostSciUtil26:217-224
Sagagi BS, Bello AM, Danyaya HA (2022) Assessment of accumulation of heavy metals in soil, irrigation water, and vegetative parts of lettuce and cabbage grown along Wawan Rafi, Jigawa State. Nigeria Environ Monit Assess 194:699. https://doi.org/10.1007/s10661-022-10360-w
Shahid M, Dumat C, Khalid S, Schreck E, Xiong T, Niazi NK (2016) Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J Hazard Mater 325:36–58. https://doi.org/10.1016/j.jhazmat.2016.11.063ff.ffhal01436218f
Siedt M, Schäffer A, Smith KEC, Nabel M, Roß-Nickoll M, van Dongen JT (2021) Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci Total Environ 751:141607. https://doi.org/10.1016/j.scitotenv.2020.141607
Singh N, Ma LQ (2006) Arsenic speciation, and arsenic and phosphate distribution in arsenic hyperaccumulator Pterris vittata L. and non-hyperaccumulator Pterris ensiformis L. Environ Poll 141:238–246. https://doi.org/10.1016/j.envpol.2005.08.050
Szada-Borzyszkowska A, Krzyżak J, Rusinowski S, Starzewska-Sikorska A, Ratman-Kłosińska I, Pogrzeba M (2022) The effect of amendments on Lolium perenne roots arbuscular mycorrhizal fungi colonization when cultivated in contaminated soil. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03783-4
Tang X, Pang Y, Ji P, Gao P, Nguyen TH, Ya T (2016) Cadmium uptake in above-ground parts of lettuce (Lactuca sativa L.). Ecotoxicol Environ Saf 125:102–106. https://doi.org/10.1016/j.ecoenv.2015.11.033
Tang, C, Rengel Z (2003). Role of plant cation/anion uptake ratio in soil acidification. In "Handbook of soil acidity", CRC Press, pp 71–96
Tazawa J, Aoki D, Hayakawa H, Matsushima K-i, Nozoe T, Uchino A, Miura S (2021) Suppressive activity of volatile fatty acids and aromatic carboxylic acids on the germination of Monochoria vaginalis. Plant Prod Sci 24:505–511. https://doi.org/10.1080/1343943X.2021.1896953
Truong HD, Wang CH, Kien TT (2018) Effect of Vermicompost in Media on Growth, Yield and Fruit Quality of Cherry Tomato (Lycopersicon esculentun Mill.) Under Net House Conditions. Compost Sci Util 26:52–58. https://doi.org/10.1080/1065657X.2017.1344594
Urasa IT, Macha SF (1996) Speciation of heavy metals in soils, sediments, and sludges using D.C. plasma atomic emission spectrophotometry coupled with ion chromatography. Intern J Environ Anal Chem 64:83–95. https://doi.org/10.1080/03067319608028338
Urasa IT, Macha SF (1999) Investigation into heavy metal uptake by wastewaster sludges. Water Air Soil Pollut 109:207–218. https://doi.org/10.1023/A:1005093600196
USEPA (1996). EPA Method 3050B: Acid Digestion of Sediments, Sludges, and Soils. https://www.epa.gov/homeland-security-research/epa-method-3050b-acid-digestion-sediments-sludges-and-soils
Whipker BE, Bailey DA, Nelson PV, Fonteno WC, Hammer PA (1996) A novel approach to calculate acid additions for alkalinity control in greenhouse irrigation water I. Commun Soil Sci Plant Anal 27:959–976. https://doi.org/10.1080/00103629609369610
Yang L, Chen Y, Shi L, Yu J, Yao J, Sun J, Zhao L, Sun J (2020) Enhanced Cd accumulation by Graphene oxide (GO) under Cd stress in duckweed. Aquat Toxicol 229:105579. https://doi.org/10.1016/j.aquatox.2020.105579
Zare AA, Khoshgoftarmanesh AH, Malakouti MJ, Bahrami HA, Chaney RL (2018) Root uptake and shoot accumulation of cadmium by lettuce at various Cd: Zn ratios in nutrient solution. Ecotoxicol Environ Saf 148:441–446. https://doi.org/10.1016/j.ecoenv.2017.10.045
Zubillaga MS, Lavado RS (2002) Heavy metal content in lettuce plants grown in biosolids compost. Compost Sci Util 10:363–367. https://doi.org/10.1080/1065657X.2002.10702099
Zulfiqar U, Jiang W, Xiukang W, Hussain S, Ahmad M, Maqsood MF, Ali N, Ishfaq M, Kaleem M, Haider FU, Farooq N, Naveed M, Kucerik J, Brtnicky M, Mustafa A (2022) Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils. A Comprehensive Review Front Plant Sci 13:773815. https://doi.org/10.3389/fpls.2022.773815
Acknowledgements
The authors acknowledge financial support from Stephen F. Austin State University Department of Chemistry and Biochemistry and Robert A. Welch Foundation Grant Number AN-0008. O. Fateru was supported through the Welch and SFASU Chemistry Department. Special thanks go to Nacogdoches Wastewater Treatment Plant facility personnel (David Wolfgang, and Gary Barton). We thank Professors Jared Barnes (Agriculture) and Robert Friedfeld for the use of SEM/EDX and X-ray diffractometer. Any opinions expressed in this paper are those of the authors, and therefore, no official endorsement should be inferred. We thank the Editors and anonymous reviewers whose helpful comments improved the manuscript.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
KKO was involved in project administration, conceptualization, resources, methodology, investigation, data curation, formal analysis, validation, writing—first draft, writing—original draft, writing—reviews and editing, visualization, supervision, funding acquisition. OOF helped in formal analysis, writing—first draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Onchoke, K.K., Fateru, O.O. Influence of perlite/biosolid composition on growth and uptake of Cd and Mn by radish (Raphanus sativus L.) under greenhouse conditions. Appl Water Sci 14, 7 (2024). https://doi.org/10.1007/s13201-023-02059-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-02059-1