Abstract
Adsorption properties of Cr(III) and Cr(VI) on natural (NCS), acid-activated (ACS) and base-activated (BCS) cherry stalks (CS) in Isparta were investigated in aqueous solutions. Batch adsorption studies had been completed with different initial chromium concentrations, pH, temperature, time and biosorbent dosage. Adsorption rapidly approached an equilibrium state between 10 and 60 min. The results displayed that the adsorption system was suitable for pseudo-second-order kinetics. Equilibrium isotherms (Langmuir, Freundlich and Dubinin–Radushkevich) were measured experimentally. The retention characteristics of Cr(III) onto NCS, ACS and BCS and Cr(VI) onto ACS were represented by Langmuir adsorption isotherms, while Cr(VI) ions onto NCS and BCS were compatible with Freundlich adsorption isotherms. The adsorption process was endothermic accompanied by a decrease in entropy and Gibbs energy. An artificial neural network (ANN) model was improved to estimate the efficiency of Cr(III) and Cr(VI) ion’s adsorption. The ANN model can predict the behaviour of the Cr(III) and Cr(VI) ion's adsorption under different circumstances. The results have shown that NCS, ACS and BCS biosorbents open up new possibilities and potential commercial uses in the cherry stalks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Waste biomass is receiving remarkable consideration in the whole world due to the fact it can be a form of renewable, extensively reachable, cheap and environment-friendly resource. The use of lignocellulose biomass as new carbon biosorbents has attracted attention in the last few decades. Water pollution is a major problem that affects humans internationally. Heavy metals and dyes are predominant pollutions, which pose a potential risk to the health of people and ecosystems. Chromium is liquid waste or sewage discharged in industrial effluents, mining, electroplating, steel industry, leather tanning, cloth manufacturing, wood preservation and processes like petroleum and oil refinery. Metals such as chromium, lead and mercury, etc. are generally toxic and non-biodegradable. Also, it is very easy for chromium ions to accumulate in living organisms and harmful side effects may occur on the entire ecosystem along the entire food chain.
Chromium is among the common heavy metals in an aqueous medium in the form of Cr(III) and Cr(VI). Cr(VI) has extreme toxicity and carcinogenic effect even in low concentrations in aqueous phases. Cr(VI) is one of the highly toxic metal types that were previously considered water and soil contaminants in the world. Leather tanning, steel manufacture, pigments in paints, inks, plastics, textile dyes, wood preservation, anti-corrosion coatings, electroplating and anodizing baths rinse waters, etc. are included in industrial sources of Cr(VI). When Cr(VI) is compared to Cr(III), it is known to be 500 times more toxic than Cr(III). Cr(VI) is generated mainly through anthropogenic sources (industrial waste water). However, the concentration of chromium was additionally detected in aquifers in EU above with a maximum discharge limit of 1 and 5 mg/L for Cr(VI) and Cr(total), respectively (Vaiopoulou and Gikas 2020).
According to characteristic clinical presentations of patients with acute exposure to Cr(VI) causes asthma, nausea, lung cancer, diarrhoea, bronchitis, liver and kidney damage, dermatitis, skin ulcers, internal bleeding and nasal septum perforation (Mohan et al. 2005). Inhalation may be a reason for acute toxicity, allergic and irritant dermatitis and ulceration of the nasal septum and respiratory problems (asthma) (Mohan et al. 2005, 2006). Ingestion can affect kidney and liver function.
To remove the leakage of chrome species into the water, companies have developed and implemented different treatment technologies and different types of biosorbents. But, plenty of those method's approaches are high-priced. They need extreme energy requirements and produce very toxic mud-like waste that wants to be cautiously disposed of. Addressing the one issue invoked the need for experienced methods which might be charge-effective, greener and surroundings-pleasant for water purification. Adsorption seems like a green, smooth and bendy technique for waste water treatment. Cellulose-based materials have attracted great interest in water treatment and have found different applications due to their abundance in nature, biodegradability and non-toxic structure.
Among the different waste water treatment methods, adsorption is considered a green method due to its easiness of application and biosorbent recovery. Common techniques consist of chemical precipitation, ion exchange (Rengaraj et al. 2003; Göde and Pehlivan 2007), membrane separation (Kozlowski and Walkowiak 2002), ultrafiltration (Ghosh and Bhattacharya 2006), flotation (Matis and Mavros 1991), solvent extraction (Salazar et al. 1992), sedimentation (Song et al. 2000), precipitation (Roundhill and Koch 2002) and electrochemical precipitation (Chen and Hao 1998).
Some different raw and modified lignocellulosic materials have been demonstrated to be suitable, low-cost biosorbent for Cr(VI) and Cr(III) removal (Miretzky and Cirelli 2010; Gogoi et al. 2018). Many studies have defended that there is a large potential stock in the preparation of carbon biosorbent material. Activated carbon has been produced from various biomass, i.e. rice husk (Kumagai et al. 2007) and cherry stones (Olivares-Marin et al. 2009). Agricultural wastes are known as suitable raw materials to be used as biosorbents by increasing their adsorption capacity (Rovani et al. 2014). They have the potential to get rid of special pollutant types by using them in adsorption processes due to their high lignocellulosic content. Besides, adsorption has proven to be an affordable, profitable, easy-to-apply methodology suitable for the treatment of industrial waste water containing inorganic and organic pollutants (Fernandez-Lopez et al. 2019).
Their use overcomes some of the drawbacks of conventional biosorbents (e.g. activated carbons) such as their high-cost ANN limitation by the nature and types of the pollutants existing in the waste water (De Gisi et al. 2016). Agro-industrial wastes are becoming a doable alternative as biosorbent because their cost is low, are quickly available and are easily removable due to the fact different microorganisms can use them as a carbon source (Abdolali et al. 2014).
Most of the waste produced in today's age has a wide range of carbon contents leading to the preparation of activated biosorbent (AB) from the main carbon source. AB is a high-grade carbon product, highly porous and adsorptive and is usually prepared by acid or base activation, carbonization and chemical activation (Kennedy et al. 2007). The adsorptive effectiveness of AB depends largely on the source material, activation method and temperature, activation time and impregnation processes (Cheenmatchaya and Kungwankunakorn 2014).
Recently, response surface methodology has been applied to model numerous processes in a chemical environment and biological sources, but artificial neural networks (ANNs) are a superior and more accurate modelling technique (Zhang et al. 2010). They offer a better alternative to statistical models due to the ability to predict a very quickly and accurate the ability to predict large number of data communities (Chelani et al. 2002). More specifically, the ANN model has been found to give better results to estimate the results from the adsorption database (Babu and Ramakrishna 2002), defining adsorption systems better than general ratio models (Du et al. 2007), and in addition, adsorption isotherm (Gao and Engell 2005) better represents. Thus, ANN is thought to be efficiently used to develop any facility containing toxic pollutants to monitor the model that can be combined with the model predictive control system for automatic or online monitoring.
Cherry stalks were chosen as a biosorbent material. CSs were collected from Isparta-Turkey. Cherry has been commonly used as a drink. The properties of AB are greatly influenced by physical and chemical methods (Tongpoothorn et al. 2011). High porosity, high adsorptive capacity, high mechanical strength, large surface area and high surface reactivity of AB are the prominent features. According to our literature survey, CSs have not been used as biosorbent for the removal of heavy metals from water and waste water. This research is related to the adsorption behaviours, kinetics and thermodynamics of chromium on NCS, ACS and BCS biosorbents. The adsorption efficiency of cellulose-based green biosorbents and their changed forms are compared. This research aims to develop a new sort of biosorbent with high Cr(III) and Cr(VI) adsorption ability by modifying CS with tartaric acid and sodium hydroxide. It is expected that such kind of biosorbent can efficiently adsorb chromium from the aqueous solution. The raw and modified biosorbents have been characterized with the help of numerous processes which include Fourier-transformed infrared spectroscopy (FTIR) and scanning electron microscopy (SEM) to discover their adsorption mechanisms. The batch adsorption trials were carried out with initial concentrations of Cr(III) and Cr(VI), contact time, pH and dosage changes.
Material and methods
Materials
CS belongs to the region of Isparta, and they were collected from the fruit gardens. Visible impurities on the surface of the CS were first washed with pure water. CS was air-dried after washing with pure water several times. They have then meshed with a blender. Sieve analysis was conducted to get them to a certain size (60 microns). All chemicals were purchased from Merck Company. By dissolving the required amount of K2Cr2O7 salt and Cr(NO3)3⋅9H2O salt in ultrapure water, the stock solution (1.0 × 10−3 mol/L) of Cr(VI) and Cr(III) was prepared. Standards were prepared by diluting for each experiment the suitable quantity of Cr(VI) and Cr(III) stock solution.
Methods
Modification of the cherry stalks
CS was rinsed in pure water and dried at 40 °C for 24 h. 3 g of CS were mixed and rinsed in a 0.1 M, 60 mL NaOH solution for 1 h and stirred with a magnetic stirrer and dried at 40 °C for 24 h to obtain a base-extracted cherry stalk (BCS).
0.2 g samples from CS were weighed, and 0.6 M 1.4 mL tartaric acid (TA) solution was added to the prepared biosorbent. This mixture was heated at 90 °C for 90 min, and then 30 mL of pure water was passed through each of these mixtures and dried in the oven. To neutralize the acidity in the mixture, 15 mL of 99.9% methanol and 1.2 mL of concentrated HCl were added, and the mixture was stirred and rinsed for 48 h in a magnetic stirrer. This mixture was dried at 40 °C for 48 h and prepared for analysis. The samples were stored in a desiccator and are called ACS.
Adsorption equilibrium measurements
Cr(III) and Cr(VI) concentrations were analysed by AAS (Perkin-Elmer-AA800 Model).
where C0 and Ce (mg/L) are the liquid-phase concentrations of Cr(III) and Cr(VI) at initial and equilibrium conditions, respectively; V (L) is the volume of the solution; and W (g) is the mass of biosorbent.
To investigate the saturation capacity of the biosorbent from the removal of chromium from the solution phase as a function of ion concentration, different adsorption isotherms (Langmuir, Freundlich and Dubinin–Radushkevich isotherm) were applied to obtain experimental data. The mechanism for the interaction of Cr(VI) and Cr(III) on the biosorbent site was based on the adsorption isotherms. It was characterized by certain constants providing information about the surface properties and the affinity of biosorbent toward Cr(VI) and Cr(III) in the solution phase.
Langmuir, Freundlich and Dubinin–Radushkevich isotherms were used using Eqs. (2), (3), (4), (5) and (6), respectively (Parlayici and Pehlivan 2017). Langmuir isotherm assumes that monolayer adsorption can occur equivalently in an equivalent finite number of localized regions. The linear form of the Langmuir isotherm equation (Langmuir 1918) is given as:
where As is the saturated adsorption capacity, and Kb (L/mg) is the Langmuir constant.
Freundlich isotherm is the oldest known relationship defining non-ideal and reversible adsorption. The well-known logarithmic form of Freundlich isotherm (Göde and Pehlivan 2006) is given as:
where Kf (mg/g) and 1/n are Freundlich adsorption constant and a measure of adsorption intensity, respectively.
where qm maximum capacity of biosorbent, k D–R isotherm constant, \(\varepsilon\) Polanyi potential and E (kJmol−1) sorption energy, respectively.
FT-IR studies
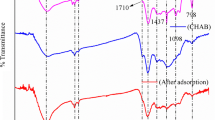
The FT-IR is a fundamental technique to evaluate functional groups. The FT-IR spectra of the biosorbent were recorded and showed in Fig. 1. The chemical structure of the biosorbent was analysed using FT-IR spectroscopy to decide the functional groups on the surface of the biosorbent (Fig. 1). A significant number of oxygen-functional groups (O–H, C=O, C–O) was observed. The main functional groups identified in the biosorbents belonged to the cellulose, hemicellulose and lignin polymers present in their structure. This truth was determined by an article (Parlayici and Pehlivan 2017). Furthermore, the presence of hydroxyl and carbonyl functional groups shows that the adsorption and binding of heavy metals are feasible, and the process is carried out efficiently (Reddy et al. 2011; Boonamnuavitaya et al. 2003; Kahraman and Pehlivan, 2017).
It is caused by hydroxyl tensile vibrations of broad-band hemicelluloses in the range of 3300–3400 cm−1 in CS fractions in NCS. When the FT-IR spectra of NCS and BCS biosorbents were compared, vibration occurred at 1550 cm−1 in BCS. This shows that C=C and C=O are double bonds. From the BCS spectrum, the shift to 3700 cm−1 shows that there is OH interaction. Furthermore, when the FT-IR spectra of the NCS and BCS biosorbents and the ACS biosorbents are compared, the sliding down of the ACS gives broadband at 1650–2900 cm−1, indicating that it is a carboxylic acid. This refers to the carboxylic acid present in tartaric acid. Thus, it indicates that the modification of BCS and ACS has been accomplished.
Scanning electron microscopy (SEM)
SEM (Fig. 2a–c) examined the morphologies of the NCS (Fig. 2a), BCS (Fig. 2b) and ACS (Fig. 2c) samples. Before adsorption, the NCS, ACS and BCS micrograph clearly shows the porous surface, and there are significant large breaks and channels in porous and microporous on the surface of the biosorbent. The SEM picture of NCS before adsorption (Fig. 2a) showed a relatively flat, smooth surface. When the SEM images in Fig. 2a are examined, it was seen that there were small particles in the image of CS. This is due to the amorphous tannins in the cherry stalk. In Fig. 2b and c, it is seen that these particles disappear when modified with base and acid and bind with base and acid addition.
Experimental design
The optimization of the adsorption method variables used to be completed through a Box–Behnken experimental design. Statistical techniques based totally on response surface methodology (RSM) have been extensively utilized for the optimization of different purification units. Box–Behnken format permits the optimization of the factors affecting the adsorption system and verifying its effect on the response. To optimize the Cr(VI) elimination, a Box–Behnken design for three factors was used, and the biosorbent showing the best performance was chosen. The experimental design was composed of 2 stages and three variables (23). The number of experiments was acquired as follows: A feed-forward ANN model with three layers of nodes is constructed as shown in Fig. 3. The logistic feature was applied as the activation function in a neural network. The training and trying out data sets must be uniformed into a range 0.1–0.9. The input and the output data set have been uniformed by the use of the following equation.
where XN is the normalized value of a variable (the network input or the network output), X is the original value of the variable, and Xmax and Xmin are the maximum and the minimum original values of the variables, respectively.
To produce sufficient data for training and testing of the model shown in Figs. 3 and 5, different standard solutions were prepared using 2.0–8.0 pH range, 20–80 °C temperature range, 0.0025– 0.025 mg biosorbent dosage range, 10–2880 min contact time range and 1.0 × 10−3–8.0 × 10−4 M concentrations range were subjected to neural network procedure. Randomly chosen 62 data pairs from these 90 data pairs have been used to sketch the neural network, and the rest of the data were used for testing. The root-mean-square (RMS) error values were calculated from the following equation (Eq. 8) to prove the accuracy of the testing results of neural network models quantitatively:
where N is the number of testing data, and \({X}_{1}{\prime}\) is the target value.
Results and discussion
Effect of contact time
In the concerned experimental work, the contact time was varied from 10 to 2880 min for the maximum adsorption of Cr(VI) and Cr(III). The effect of contact time on the adsorption of Cr(VI) and Cr(III) is shown in Fig. 4. During the adsorption phenomena, the adsorption of chromium ions steadily increased until the optimum value at equilibrium stage. This figure demonstrated that the adsorption of chromium ions from the aqueous medium can be done in two basic steps: Initially, the adsorption rate is very high at the beginning of the reaction between chromium ions and biosorbent in a contact time of about 10–60 min. Secondly, the progress of adsorption continued slowly over time until the equilibrium stage.
Effect of initial solution pH
The solution pH was an important parameter for the removal of Cr(VI) because of the surface charge and ionic competition. In the solution, Cr(VI) may occur in various anionic forms, such as chromate (CrO42−), dichromate (Cr2O72−) or hydrogen chromate (HCrO4−), with full chromate concentrations and pH dominating particular chromate species. The effect of initial pH varying from 2.0 to 8.0 for Cr(III) and Cr(VI) adsorption is shown in Fig. 5. The adsorption capacity of Cr(III) improved and Cr(VI) decreased dramatically with the increase in aqueous solution pH. The maximum adsorption potential for Cr(III) and Cr(VI) was reached at pH 6.0 and 2.0 respectively for all biosorbents.
At lower pH, the main species on the biosorbent surface are positive charges as a result of protonation. As a consequence, relating to stationary electric charges or fields between the positive charges on the biosorbent surface and the negatively charged (CrO42−), (HCrO4−) and (Cr2O72−) increased adsorption ability (Pehlivan and Altun 2008). The increase in solution pH resulted in the reduction of adsorption of Cr (VI) from the solution phase due to the strong competition between hydroxyl ions and (CrO42−), (HCrO4−) and (Cr2O72−). The forms of Cr (VI) in the solution were diversified with pH change in the waste water. Therefore, competition in the aqueous phase occurred not only between Cr(VI) ions in each form, but also between Cr(VI) ions and OH−. pH 2.0 was thus selected as the optimal pH to implement the consequent investigation (Parlayici et al. 2015; Pehlivan and Altun 2008).
The effect of pH on the adsorption capacity of Cr(VI) on the biosorbents is shown in Fig. 5. Results indicate that Cr(VI) adsorption by biosorbents is highly dependent on the pH of the solution, which is significantly reduced with the increase in the initial pH of the solution from pH 2.0–8.0. The maximum Cr(VI) adsorption by NCS, ACS and BCS was detected at pH 2.0. Lowering the pH of the solution leads to further protonation of the surface of the modified biosorbent, so that a strong attraction occurs between these oxyanions of Cr(VI) and the positively charged surface of the biosorbent in the solution medium. Negatively charged hydroxyl ions will be abundant in the aqueous phase, resulting in an obstacle between negatively charged ions and negatively charged biosorbent at high pH, leading to a decrease in adsorption. A similar trend was reported in the adsorption of Cr(VI) on a banana peel (Park et al. 2008) and rice straw (Gao et al. 2008). Nevertheless, as it has high redox potential value, it was concluded that Cr(VI) is reduced to Cr(III) in acidic solutions by contacting the electron donor groups (E° = 1.2 V) at standard conditions. When looking at the adsorption mechanism, it was revealed that both the hexavalent chromium (anionic) and trivalent chromium (cationic) adsorption on the biosorbent surface and the electron donor groups give to share the electrons with Cr (VI) on the biosorbent surface and reduced them to Cr (III).
Adsorption isotherms
Adsorption isotherms are essential within the planning of adsorption systems for cleaning the contaminated water and they give information for the adsorption behaviour of dissolvable substances on particular biosorbents. Many researchers have used isotherms to study the importance of different parameters in the adsorption equilibrium of toxic metals with biosorbents. Using the Langmuir model equation (Table 1), the maximum capacities of NCS, ACS and BCS for Cr(III) were identified as 97 mg/g CS, 95 mg/g BCS and 94 mg/g ACS, respectively, and the maximum capacities of NCS, ACS and BCS for Cr(VI) were identified as 32 mg/g CS, 25 mg/g BCS and 35 mg/g ACS, respectively (Fig. 6).
Effect of biosorbent dose on the adsorption of chromium
The impact of biosorbent dosages on Cr (VI) removal was assessed for the biosorbent doses of between 0.01 and 0.05 g. Experimental results (Fig. 7) showed that the adsorption process is highly dependent on biosorbent dosages. An optimum biosorbent dose is essential to maximize binding chromium ions. As a result, the optimum biosorbent dosage determined for the chromium ions removal adsorption is 0.05 g.
Adsorption kinetics
For the equilibrium system, the pseudo-first-order and pseudo-second-order reaction equations were applied to take into account the reactions formed (Fig. 4). Pseudo-first-order kinetic model and pseudo-second-order kinetic model for Cr (III) on NCS, ACS and BCS are demonstrated in Table 2. The boundary condition qt = 0 at t = 0 and the equation can be linearized as Eq. (8). The pseudo-second-order kinetic model was used based on the following differential equation:
where k2 is the rate constant of pseudo-second-order adsorption (g mg−1 min−1). Some parameters were given in Table 2 where qe-cal. (mmol/g) the calculated amount of adsorption equation, k2 (g/mg.min.) rate constant of pseudo-second-order adsorption, qe-exp (mmol/L) amount of chromium adsorbed at equilibrium, R2 correlation coefficient.
It was found that the pseudo-second-order kinetic model provided a concordance between the experimental and calculated data, as indicated by correlation coefficients obtained using these models of greater than 0.99. The pseudo-second-order model equation was thus considered a useful model for kinetic studies, showing that chemical adsorption is a rate-limiting step. Similar results are obtained when the studies for removing Cr(VI) from waters are examined (Enniya et al. 2018).
The regression coefficients (R2) have been evaluated for each model. The results are shown in Table 2 (for Cr(III)) and Table 3 (for Cr(VI)). The greater regression coefficients showed that the pseudo-second-order model was more suitable than the pseudo-first-order model, which showed the presence of chemisorption. This behaviour was also reported in some articles (Belaid et al. 2013; Parlayici and Pehlivan 2017). In this study, adsorption for Cr(III) and Cr(VI) was managed by a pseudo-second-order process.
Thermodynamic studies
Adsorption of Cr (VI) and Cr(III) ions onto NCS, ACS and BCS was carried out at different temperatures (20 °C, 35 °C, 50 °C, 65 °C and 80 °C) for thermodynamic analysis (Fig. 8a, b).
The value of ΔH° is positive, indicating that the adsorption reaction is endothermic. It is emphasized that the standard entropy change seen from the results in the thermodynamic study is negative, and there is a decrease in variability in the biosorbent/solution interface during the adsorption of Cr(VI). ΔG° values had been discovered to be positive (Table 4).
Experimental design studies
To provide the optimal model for the removal efficiencies of NCS, BCS and ACS with Cr(III) and Cr(VI) ions, the data were trained and tested for many times on 8 different models. These tests were performed with experimental data given for training and testing. Prediction values for each of these biosorbents were determined separately in training studies as the highest specificity coefficient and the lowest mean square error. Matlab R2017a software carries out linear regression between regression button network inputs in the training window and the outputs of these inputs. The results of NCS are indicated in Fig. 9, and RMS values for the performance measurement of the network are given in Fig. 10. It was observed in the figures that verification and test results were really good (Figs. 11 and 12). Experimental data not shown in the network can also be simulated with this network architecture, which has a quite high performance.
The RMS values obtained for training, validation and testing from the recommended artificial neural networks for the Cr(III) are given in Tables 5, 6 and 7. The RMS values obtained for training, validation and testing from the recommended artificial neural networks for the Cr(VI) are given in Tables 8, 9 and 10.
Conclusion
CSs (NCS, ACS and BCS), a new biosorbent, were produced and tested to remove Cr(III) and Cr(VI) ions from aqueous solutions. Both TA and NaOH serve as chemical activating agents for the formation of AB with a high mesoporous surface area as they are more effective and cheaper activators. The results showed that the initial pH and the temperature of the solution influence the adsorption capacity. Adsorption of chromium ions was found to increase with increasing time and initial concentration of chromium ions. The adsorption parameters had been explained for Langmuir, Freundlich and D–R isotherm models. The equilibrium data have been best described by way of the Langmuir isotherm, showing that the adsorption of Cr(III) on NCS, ACS and BCS was a monolayer with heterogeneous surface adsorption. It was observed that the Langmuir isotherm gave excellent agreement on the full range of adsorption and the corresponding correlation coefficients (R2) for chromium ions on biosorbents ≥ 0.9. For Cr(VI) ions adsorption, Freundlich adsorption isotherm best described the equilibrium. The adsorption kinetics were suitable for a pseudo-second-order kinetic model. The adsorption process of Cr(III) and Cr(VI) ions with CSs was endothermic and spontaneous. These investigations indicated that the use of low-cost biosorbents can be an effective way to take out Cr(III) and Cr(VI) ions from an aqueous medium.
One of the most important objectives of the study is to estimate the removal efficiency of the artificial neural network (ANN) model and Cr(III) and Cr(VI) ions. Although there are many studies on Cr(III) and Cr(VI) removal and modelling with ANN, there is no study on the use of biosorbent CSs in the adsorption process with ANN. For this purpose, the most appropriate model to estimate the efficiency of the efficiency was done and the most appropriate function was found to have a logsig transfer function. The performances of the artificial neural network created were determined by RMS errors. The proposed model can significantly predict adsorption efficiency and adsorption capacity and be successful for the efficient execution of an automatic process.
References
Abdolali A, Guo WS, Ngo HH, Chen SS, Nguyen NC, Tung KL (2014) Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Bioresour Technol 160:57–66
Babu BV, Ramakrishna V (2002) Applicability of regression technique for physical modeling: a case study on adsorption in wastewater treatment. In: Proceedings of ınternational symposium & 55th annual session of IIChE (CHEMCON-2002), Osmania Univeristy, Hyderabad, India
Belaid KD, Kacha S, Kameche M, Derriche Z (2013) Adsorption kinetics of some textile dyes onto granular activated carbon. J Environ Chem Eng 1:496–503
Boonamnuavitaya V, Sae-ung S, Tanthapanichakoon W (2003) Preparation of activated carbons from coffee residue for the adsorption of formaldehyde. Sep Purif Technol 42:159–168
Chen JMN, Hao OJN (1998) Microbial chromium(VI) reduction. Crit Rev Environ Sci Technol 28(3):219–251
Cheenmatchaya A, Kungwankunakorn S (2014) Preparation of activated carbon derived from rice husk by simple carbonization and chemical activation for using as gasoline biosorbent. Int J Env Sci Dev 5(2):171–175
Chelani AB, Rao CVC, Phadke KM, Hasan MZ (2002) Prediction of sulphur dioxide concentration using artificial neural networks. Environ Modell Softw 17:161–168
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40
Du X, Yuan Q, Zhao J, Li Y (2007) Comparison of general rate model with a new model-Artificial neural network model in describing chromatographic kinetics of solanesol adsorption in packed column by macroporous resins. J Chromatogr A 1145:165–174
Enniya I, Rghioui L, Jourani A (2018) Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain Chem Phar 7:9–16
Fernández-López JA, Angosto JM, Roca MJ, Doval Miñarro M (2019) Taguchi design-based enhancement of heavy metals bioremoval by agroindustrial waste biomass from artichoke. Sci Total Environ 653:55–63
Gao W, Engell S (2005) Estimation of general nonlinear adsorption isotherms from chromatograms. Comput Chem Eng 29:2242–2255
Gao H, Liu Y, Zeng G, Xu W, Li T, Xia W (2008) Characterization of Cr(VI) removal from aqueous solutions by a surplus agricultural waste-Rice straw. J Hazard Mater 150(2):446–452
Ghosh G, Bhattacharya PK (2006) Hexavalent chromium ion removal through micellar enhanced ultrafiltration. Chem Eng J 119(1):45–53
Göde F, Pehlivan E (2006) Chromium(VI) adsorption by brown coals. Energy Sources Part A 28:447–457
Göde F, Pehlivan E (2007) Sorption of Cr(III) onto chelating b-DAEG–sporopollenin and CEP–sporopollenin resins. Bioresour Technol 98:904–911
Gogoi S, Chakraborty S, Saikia MD (2018) Surface modified pineapple crown leaf for adsorption of Cr(VI) and Cr(III) ions from aqueous solution. J Environ Chem Eng 6:2492–2501
Kahraman Tutar H, Pehlivan E (2017) Cr6+ removal using oleaster (Elaeagnus) seed and cherry (Prunus avium) Stone biochar. Powder Technol 306:61–67
Kennedy LJ, Vijaya JJ, Kayalvizhi K, Sekaran G (2007) Adsorption of phenol from aqueous solutions using mesoporous carbon prepared by two-stage process. Chem Eng J 132(1–3):279–287
Kozlowski CA, Walkowiak W (2002) Removal of chromium(VI) from aqueous solutions by polymer inclusion membranes. Water Res 36:4870–4876
Kumagai S, Noguchi Y, Kurimoto Y, Takeda K (2007) Oil biosorbent produced by the carbonization of rice husks. Waste Manag 27:554–561
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Matis KA, Mavros P (1991) Recovery of metals by ion flotation from dilute aqueous solutions. Sep Purif Meth 20:1–48
Miretzky P, Cirelli AF (2010) Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: a review. J Hazard Mater 180:1–19
Mohan D, Singh KP, Singh VK (2005) Removal of hexavalent chromium from aqueous solution using low-cost activated carbons derived from agricultural waste materials and activated carbon fabric cloth. Ind Eng Chem Res 44:1027–1042
Mohan D, Singh KP, Singh VK (2006) Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J Hazard Mater 135:280–295
Olivares-Marin M, Del Prete V, Garcia-Moruno E, Fernandez-Gonzalez C, Macias-Garcia A, Gomez-Serrano V (2009) The development of an activated carbon from cherry stones and its use in the removal of ochratoxin A from red wine. Food Control 20:298–303
Park D, Lim SR, Yun YS, Park JM (2008) Development of a new Cr(VI)-biosorbent from agricultural biowaste. Bioresour Technol 99(18):8810–8818
Parlayici S, Pehlivan E (2017) Removal of metals by Fe3O4 loaded activated carbon prepared from plum Stone (Prunus nigra): kinetics and modeling study. Powder Technol 317:23–30
Parlayici S, Eskizeybek V, Avcı A, Pehlivan E (2015) Removal of chromium (VI) using activated carbon-supported-functionalized carbon nanotubes. J Nanostruct Chem 5(3):255–263
Pehlivan E, Altun T (2008) Biosorption of chromium(VI) ion from aqueous solutions using walnut, hazelnut and almond shell. J Hazard Mater 155(1–2):378–384
Reddy DHK, Ramana DKV, Seshaiah K, Reddy AVR (2011) Biosorption of Ni(II) from aqueous phase by Moringa oleifera bark, a low cost biosorbent. Desalination 268:150–157
Rengaraj S, Joo CK, Kim Y, Yi J (2003) Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H. J Hazard Mater 102(2/3):257–275
Roundhill DM, Koch HF (2002) Methods and techniques for the selective extraction and recovery of oxoanions. Chem Soc Rev 31:60–67
Rovani S, Censi MT, Pedrotti SL, Lima TC, Cataluña R, Fernandes AN (2014) Development of a new biosorbent from agro-industrial waste and its potential use in endocrine disruptor compound removal. J Hazard Mater 271:311–320
Salazar E, Ortiz MI, Urtiaga AM (1992) Equilibrium, kinetics of Cr(V1) extraction with aliquat 336. Ind Eng Chem Res 31:1516–1522
Song Z, Williams CJ, Edyvean RGJ (2000) Sedimentation of tannery wastewater. Water Res 34(7):2171–2176
Tongpoothorn W, Sriuttha M, Homchan P, Chanthai S, Ruangviriyachai C (2011) Preparation of activated carbon derived from Jatropha curcas fruit shell by simple thermo-chemical activation and characterization of their physico-chemical properties. Chem Eng Res Des 89:335–340
Vaiopoulou E, Gikas P (2020) Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 254:126876
Zhang Y, Xu J, Yuan Z, Xu H, Yu Q (2010) Artificial neuralnetwork-genetic algorithm based optimization for the immobilization of cellulase on the smart polymer Eu-dragit L-100. Bioresour Technol 101:3153–3158
Funding
This research project was supported by Süleyman Demirel University Research Fund (Project Number: SDU-4967-YL1-17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Göde, F., Yılmaz, A., Aktaş, A.H. et al. Artificial neural network approach to model Cr(III) and Cr(VI) adsorption by NCS, ACS and BCS. Appl Water Sci 14, 28 (2024). https://doi.org/10.1007/s13201-023-02054-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-02054-6