Abstract
In this research, magnetic nanocomposite (MGO@TiO2) consisting of Fe3O4, TiO2 and graphene oxide is synthesized for the photocatalytic removal of methyl orange, and its photocatalytic activity is compared with the nanohybrid (Fe3O4@TiO2) without graphene oxide. The crystalline phases of both photocatalysts are determined using X-ray diffraction patterns. The results show that the removal efficiency of methyl orange using the synthesized nanohybrid and nanocomposite is affected by the irradiation time and the pH of the suspension. Comparing the removal efficiency of methyl orange using synthesized photocatalysts shows that the photocatalytic activity of nanocomposite is much higher than that of nanohybrid. The statistical analysis of the experimental data using the response surface method led to the selection of the quadratic model as the best statistical model to estimate the removal efficiency of methyl orange. Also, the numerical and graphical methods confirmed the adequacy of the quadratic statistical model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most common and widely used methods of destroying organic pollutants that are discharged from industrial wastewater to water systems is photocatalytic removal using oxide semiconductors (Ma et al. 2013; Liu et al. 2012). Several semiconductors that are used for the oxidation process of hazardous pollutants must be chemically stable and environmentally friendly. Also, the valence layer electrons have the ability to be excited and transferred to the conduction layer. Titanium dioxide has attracted the attention of researchers as one of the inexpensive and non-toxic semiconductors (Tao et al. 2023). Titanium dioxide has three crystal structures, including anatase, rutile and brookite, which have different photocatalytic activity (Huang and Wey 2011). Irradiation of ultraviolet light on the surface of titanium dioxide nanoparticles excites electrons in the valence layer, which have the ability to transfer to the conduction layer (Abbasi 2018, 2019). The transfer of electrons between these two layers causes the creation of holes and electrons in the valence layer and conduction layer, respectively. The resulting electric charges are the main factor in the formation of oxidizing radicals. The rate of pollutant decomposition is influenced by the amount of produced active radicals. Therefore, the stability of the produced electron–hole pairs is effective on the pollutant decomposition rate. So far, different strategies have been investigated to separate electric charges and increase the stability of electron–hole pairs. The most common methods include the combination of semiconductors with different band gap energy (Ghaderi et al. 2018a) and the use of porous materials with a high volume-to-surface ratio in the photocatalyst structure (Abbasi 2020, 2021a). According to the band gap of titanium dioxide nanoparticles, their photocatalytic activity in the range of visible and infrared light is almost negligible (Abbasi 2022a, 2023a). Therefore, several techniques can be used to increase their band gap. Doping photocatalytic TiO2 nanoparticles with elements such as carbon (Park et al. 2006), sulfur (Kumar et al. 2016), and nitrogen (Ananpattarachai et al. 2009), or transition-metal capable of increasing the absorption area of TiO2 nanoparticles successfully improves the photocatalytic efficiency of nanoparticles. In recent years, graphene oxide (GO), which is in the form of thin sheets consisting of carbon atoms with sp2 hybridization, has been widely noticed by researchers. The importance of GO can be considered due to its significant electrical conductivity, excellent surface area and suitable thermal and chemical stability (Abbasi 2023b). Electrons and holes produced in TiO2 nanoparticles have the ability to transfer to the surface of GO, and this increases their mobility in single-layer graphene sheets. Therefore, the probability of the recombination of electrons and holes is significantly reduced; in other words, the combination of TiO2 nanoparticles with GO increases the separation of produced electric charges, which effectively increases the amount of active oxidizing radicals and also improves the photocatalytic activity of TiO2 nanoparticles (Ismail et al. 2013). Despite the fact that the photocatalytic hybrid consisting of GO and TiO2 nanoparticles has a significant photocatalytic activity; however, recovering the hybrid from the polluting mixture is very difficult and expensive, which limits its efficiency. Therefore, one of the effective methods to overcome this problem is to magnetize the hybrid and form a new composite. The most common magnetic nanoparticles are Fe3O4 nanoparticles. Therefore, by combining photocatalytic hybrid and magnetic nanoparticles, the separation of composite from the polluting mixture becomes very convenient and inexpensive (Lin, et al. 2012; Wang et al. 2022; Abbasi 2021b). In this study, a magnetic nanohybrid consisting of titania and Fe3O4 nanoparticles as well as a ternary magnetic nanocomposite consisting of graphene oxide, titania nanoparticles and Fe3O4 nanoparticles are synthesized, and the photocatalytic activity of the synthesized samples is investigated to remove methyl orange pollutant. Also, the effect of GO on the intensity of photocatalytic activity is investigated. X-ray diffraction analysis (XRD) is used to characterize the hybrid and nanocomposite. The dependence of the methyl orange removal efficiency on the irradiation time and the acidity of the suspension at a fixed concentration of the photocatalyst are investigated. The statistical analysis of the laboratory results is done using the response surface method (RSM) in order to provide the most efficient statistical model.
Materials and methods
Materials
Graphene oxide is obtained from Novin Nano Negasht Company, Iran. Other chemicals used for the synthesis of magnetic nanohybrid and nanocomposite such as ferric acetylacetonate (Fe(acac)3), tetrachloride titanium (TiCl4, 99%), ammonium acetate (NH4Ac, NH4CH3CO2, 99.9%), methyl orange (99%) and ethylene glycol (C2H6O2, 99.9%) are obtained from Merck and are used without further purification.
Experimental procedure
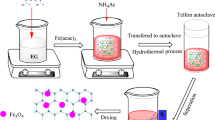
The synthesis of magnetic nanohybrid (Fe3O4@TiO2) for photocatalytic processes takes place in two steps. At first, Fe3O4 nanoparticles are synthesized using Fe(acac)3 as a precursor by hydrothermal method and inside an autoclave. The synthesis method of magnetic nanoparticles is fully and in detail presented in the previous reported study (Abbasi et al. 2020). After the synthesis of magnetic nanoparticles, TiO2 photocatalytic nanoparticles are synthesized using the hydrolysis method in the presence of TiCl4 (Abbasi et al. 2015). For this purpose, a suitable amount of synthesized magnetic nanoparticles is dispersed in 100 ml of distilled water and to ensure its uniform distribution in the water, the resulting suspension is placed in an ultrasonic bath for 30 min. Next, 8 ml of TiCl4 is added drop by drop to the suspension, and it is mixed for 5 h under ambient temperature on a stirrer. Then, the temperature of the mixture is increased to 60 °C, and it is kept at the same temperature for 12 h on a magnetic stirrer. At this stage, the increase in temperature causes nucleation and increases the number of nuclei of TiO2 nanoparticles. At the end of the heating process, the suspension is filtered and washed several times with distilled water, and after drying the resulting powder, it is calcined in the oven at 350 °C for 3 h. The calcined magnetic nanohybrids are considered as Fe3O4@TiO2. The synthesis of magnetic nanocomposites is completely similar to the synthesis of nanohybrids mentioned above. With the difference that in the first step for the synthesis of Fe3O4 nanoparticles, graphene oxide should be mixed with ethylene glycol with the same weight ratio, and then the rest of the steps should be done in the same way as mentioned. The obtained samples are considered as MGO@TiO2. The synthesized nanohybrid and nanocomposite are characterized by X-ray diffraction analysis to determine the phases of TiO2 nanoparticles as well as Fe3O4 nanoparticles.
Pollutant removal study
Photocatalytic activity of the synthesized nanohybrid and nanocomposite to remove methyl orange pollutant is investigated. Photocatalytic decomposition of the pollutant is carried out in a photoreactor equipped with a medium-pressure mercury lamp. (The applied irradiation intensity is 13,000 Lumen.) In order to increase the accuracy of the results, the UV lamp must be placed exactly in the center of the photoreactor so that the amount of radiation is uniform in all directions. 80 ml of methyl orange aqueous solution with a concentration of 10 ppm, which contains 0.1% by weight of each photocatalyst, is adjusted to the desired pH. The pH value of 3, 7 and 11 is adjusted using HCl and NaOH. Then, each of the prepared mixtures is placed on a magnetic stirrer for one hour inside the photoreactor whose lamp is off to establish the balance of absorption and desorption. Then, 3 ml of the suspension is drained from inside the photoreactor, and the photocatalyst is separated using a magnet. The light absorption intensity of methyl orange at wavelength of 464 nm, which is proportional to its concentration, is measured using a spectrophotometer (PerkinElmer Company) and is considered as the initial concentration (C0). Then, the mixture placed on the magnetic stirrer inside the photoreactor is irradiated using a UV lamp, and the pollutant concentration (Ct) is measured every 5 min by removing 2 ml of the suspension and separating the photocatalyst. Finally, the percentage of methyl orange removal efficiency (RE %) is calculated by Eq. 1 (Ghaderi et al. 2018b; Roozban et al. 2017a).
The photocatalytic efficiency of synthesized nanohybrid and nanocomposites is also statistically analyzed using Design-Expert version 7.0.0 software. In order to obtain high accuracy results, all measurements are repeated three times, and their average is reported. Response surface method (RSM) is used to investigate the mutual effect of irradiation time and pH. Also, different statistical models are used to fit the laboratory data, and the best statistical model is selected.
Results and discussion
XRD analysis of photocatalysts
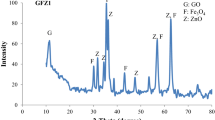
The X-ray diffraction patterns of the synthesized nanohybrid and nanocomposite, which is used to investigate the crystalline phases, is shown in Figs. 1 and 2, respectively. According to the characteristic peaks appearing in these two figures, the presence of crystalline nanoparticles of Fe3O4 and TiO2 can be confirmed with confidence. The cubic structure of Fe3O4 nanoparticles shows peaks in the range of 2\(\theta \)=29.65°, 2\(\theta \)=35.90°, 2\(\theta \)=57.35° and 2\(\theta \)=62.76°, so that each of the obtained peaks belongs to planes (220), (311), (511) and (440), respectively (Abareshi et al. 2010). By examining the peaks belonging to TiO2 nanoparticles that can be seen in Figs. 1 and 2, it is clear that TiO2 crystalline nanoparticles have both anatase and rutile phases. The characteristic peaks of anatase phase are observed at 2\(\theta \)=25.10°, 2\(\theta \)=38.10°, 2\(\theta \)=47.85° and 2\(\theta \)=62.90°, which are related to (101), (004), (200) and (204) lattice plane, respectively (Abbasi et al. 2021). The created characteristic peaks at 2\(\theta \)=27.25°, 2\(\theta \)=37.51°, 2\(\theta \)=54.15° and 2\(\theta \)=62.85° are attributed to the (110), (101), (211) and (002) planes of the rutile phase of TiO2 nanoparticles, respectively. Therefore, as can be seen, the used synthesis conditions lead to the presence of both anatase and rutile crystal phases. Comparing the peaks of Figs. 1 and 2 shows that the synthesized nanocomposite has a peak at 2\(\theta \)=10.85°, which is not present in the nanohybrid. This peak is due to the presence of graphene oxide sheets with the (002) Bragg reflection plane in the nanocomposite structure.
Investigating the efficiency of photocatalysts
The percentage changes of methyl orange removal efficiency with respect to irradiation time and pH in the presence of Fe3O4@TiO2 and MGO@TiO2 are presented in Figs. 3 and 4, respectively. Examining the results of these two figures shows that the irradiation time has a positive effect on the efficiency of both photocatalysts for the photodegradation of methyl orange, and with the increase in the irradiation time, the amount of methyl orange decomposition also increases. This behavior may be due to the change in the number of electrons in the valence layer of the photocatalyst. In fact, the exposure of the photocatalyst to UV radiation leads to the excitation of valence layer electrons, and if the excited electrons receive enough energy, they can be separated from the valence layer and transferred to the conduction layer (Abbasi et al. 2021; Abbasi 2022b). Electron transfer leads to the formation of a hole in the valence layer and an electron in the conduction layer. The created electron and hole are very reactive in the presence of oxygen and water and can produce oxidizing radical species such as \({O}_{2}^{\bullet }\) and \({OH}^{\bullet }\) (Abbasi et al. 2017a; Roozban et al. 2017b). The created active radicals are the main factor in the photodegradation of organic pollutants. Therefore, in order to increase the amount of pollutant removal, the number of oxidizing radicals and, as a result, the number of electron–hole pairs should be increased. Increasing the irradiation time significantly increases the electron–hole pairs as well as the removal efficiency of methyl orange. Investigating the effect of pH on pollutant removal efficiency shows the absence of a specific sequence. As the pH increases from 3 to 11, the removal efficiency decreases at first and then increases. This process of changes is observed using both synthesized photocatalysts. As can be seen, the highest amount of methyl orange removal occurs in acidic conditions (pH = 3) and the lowest amount of decomposition occurs in neutral environment (pH = 7). As mentioned earlier, the formation of active oxidizing radicals such as hydroxyl and oxygen are effective in the rate of decomposition of organic pollutants. Therefore, with the increase in the number of such radicals, the photocatalytic degradation also increases. Due to the fact that the amount of free hydrogen ions (\({H}^{+})\) in acidic suspension is much higher than in neutral and alkaline suspensions, the possibility of absorbing dissolved oxygen by hydrogen ions and forming hydroxyl radicals (\({OH}^{\bullet }\)) in acidic environment increases significantly (Abbasi 2021a, b). Therefore, with the increase in radical species in acidic suspension, the rate of photocatalytic decomposition also increases significantly compared to neutral and alkaline environment. The greater efficiency of photocatalytic removal of methyl orange in alkaline suspension compared to neutral suspension can be due to the presence of hydroxide ions (\({OH}^{-}\)) because such free ions in the suspension have the ability to transform into hydroxyl radicals and pollutant degradation.
Figure 5 shows the comparison of methyl orange removal efficiency using Fe3O4@TiO2 and MGO@TiO2 at different pHs. It is quite clear that in all time intervals and all suspensions, nanocomposite has more photocatalytic activity than nanohybrid. The greater removal efficiency of methyl orange using nanocomposite can be attributed to the effect of the presence of GO in the nanocomposite structure. Due to the presence of functional groups containing oxygen on the surface of graphene oxide sheets, the active sites for the absorption of iron ions (\({\mathrm{Fe}}^{+3}\)) resulting from the ionization of Fe(acac)3 increase, and this prevents the agglomeration of Fe3O4 nanoparticles and also increases the active contact surface. Therefore, TiO2 nanoparticles are also synthesized with proper dispersion on the surface of Fe3O4 nanoparticles. Another reason for the higher photocatalytic activity of the nanocomposite compared to the nanohybrid is the possibility of organic pollutants being absorbed into the functional groups of graphene oxide through ionic interactions. Therefore, by increasing the binding of methyl orange to the surface of the photocatalyst, the rate of decomposition also increases (Kim et al. 2015). Considering the structure of graphene oxide and the ability to transfer electric charges, the amount of electron–hole separation produced in the nanocomposite is much higher than that of the nanohybrid, and this reduces the recombination of electron–hole pairs (Abbasi et al. 2020) and increases the photocatalytic activity of the nanocomposite compared to the nanohybrid. The photocatalytic degradation of methyl orange using MGO@TiO2 is schematically depicted in Fig. 6.
Investigating the statistical models
Tables 1 and 2 show the results of statistical modeling of experimental data for the removal efficiency of methyl orange using Fe3O4@TiO2 and MGO@TiO2, respectively. The applied statistical models are linear, 2FI, quadratic and cubic. The most appropriate model that has the ability to predict the changes in methyl orange removal efficiency with irradiation time and pH is selected based on statistical parameters. The most important parameter for determining the best statistical model is the P-value. Therefore, statistical models that have P-value less than 0.5 are selected as meaningful models. According to the results of Tables 1 and 2, it can be seen that both linear and quadratic models have P-value less than 0.05. Therefore, these two models are the most adequate to estimate the response. Another statistical parameter that is used to select the most adequate statistical model is lack of fit. This parameter includes factors that do not have a significant effect on the response. Therefore, the statistical models that are proposed as adequate models should have non-significant lack of fit. In other words, the P-value of lack of fit should be greater than 0.05 (Madadi et al. 2012). According to the P-value of the proposed models (linear and quadratic) presented in Tables 1 and 2, it can be seen that only the P-value of the quadratic model is greater than 0.05, which indicates that it is not significant. Therefore, the quadratic model is the most efficient model for fitting the changes in methyl orange removal efficiency with irradiation time and pH.
The statistical parameters of the quadratic model for Fe3O4@TiO2 and MGO@TiO2 as photocatalysts are listed in Table 3. The most important statistical parameter that determines the adequacy of the model is the R-squared (R2). As can be seen, the value of this parameter for Fe3O4@TiO2 and MGO@TiO2 is 0.9902 and 0.9927, respectively. Therefore, it can be claimed with 99% confidence that the quadratic model has the ability to predict the changes in the MO removal efficiency using both photocatalysts. The comparison between the values of R-squared and adjusted R2 (R2adj) shows that there is no significant difference between these two parameters. Therefore, it can be concluded that the quadratic model, which is selected as the best model, only includes significant variables because the inclusion of non-significant variables in the model causes a significant decrease in the value of R2adj compared to R2 (Namvar-Mahboub and Pakizeh 2014).
The estimated coefficients for the quadratic model according to the coded factors are listed in Table 4. According to the coefficients of factor A (irradiation time) and factor B (pH), it can be seen that the coefficient of factor A has a higher value in both quadratic models proposed using Fe3O4@TiO2 and MGO@TiO2. Therefore, the greater effect of irradiation time on the removal efficiency of methyl orange can be inferred. Also, the results of Table 4 show that the individual factors (A and B) and the interaction between them (A-B) have a nonzero coefficient, so they are effective in estimating the removal efficiency.
In addition to the statistical parameters obtained in the analysis of variance, which leads to the selection of the best model to fit the experimental data, graphical statistical methods are also used to confirm the proposed models. The most common method is to compare the experimental data with the predicted data by the selected model. If the laboratory data are compatible with the values estimated by the model, the adequacy of the model can be confirmed. Figures 7 and 8 show the comparison between the experimental data and the estimated values of MO removal efficiency using Fe3O4@TiO2 and MGO@TiO2, respectively. As can be seen, the quadratic model, which is chosen as the best statistical model for both photocatalysts, has high accuracy in estimating the MO removal efficiency. Therefore, the results of the graphical analysis are completely consistent with the analysis of variance.
Figures 9 and 10 show the effect of independent variables such as irradiation time and pH on the MO removal efficiency using Fe3O4@TiO2 and MGO@TiO2, respectively. Figures 9a and 10a show the response surface curve depicted in three dimensions, while Figs. 9b and 10b show the effect of independent variables in the form of counter lines and in two dimensions. It is clear that by increasing the irradiation time from 5 to 40 min, the MO removal efficiency also increases obviously due to the increase in the number of excited electrons and electron–hole pairs, while increasing the pH from 3 to 11 causes a double behavior in the MO removal efficiency. As shown, the lowest removal efficiency occurs at pH = 7. This may be due to the low electric charges in the neutral suspension, while at pH = 3, which has a large amount of free hydrogen ions and positive electric charge, the removal efficiency is at the highest value (Abbasi and Hasanpour 2017; Abbasi et al. 2017b).
Conclusions
In this study, Fe3O4 nanoparticles are successfully attached on the surface of graphene oxide and then TiO2 nanoparticles are synthesized on the surface of them. The results showed that increasing the irradiation time from 5 to 40 min increases the amount of photocatalytic activity. Also, the effect of pH on the removal efficiency of methyl orange showed that the lowest and highest levels of pollutant removal occur in neutral (pH = 7) and acidic (pH = 3) conditions, respectively. Considering the positive effect of graphene oxide in the separation of electric charges and preventing the recombination of electron–hole pairs, the photocatalytic activity of the nanocomposite containing graphene oxide is much higher than the sample without graphene oxide. The quadratic model proposed using the response surface method is acceptable enough to predict the changes in the removal efficiency of methyl orange.
Data availability
Not applicable.
References
Abareshi M et al (2010) Fabrication, characterization and measurement of thermal conductivity of Fe3O4 nanofluids. J Magn Magn Mater 322:3895–3901
Abbasi S (2018) Investigation of the enhancement and optimization of the photocatalytic activity of modified TiO2 nanoparticles with SnO2 nanoparticles using statistical method. Mater Res Expr 5(6):066302
Abbasi S (2019) Photocatalytic activity study of coated anatase-rutile titania nanoparticles with nanocrystalline tin dioxide based on the statistical analysis. Environ Monit Assess 191(4):206
Abbasi S (2020) Adsorption of dye organic pollutant using magnetic ZnO embedded on the surface of graphene oxide. J Inorg Organomet Polym Mater 30:1924–1934
Abbasi S (2021a) Improvement of photocatalytic decomposition of methyl orange by modified MWCNTs, prediction of degradation rate using statistical models. J Mater Sci: Mater Electron 32(11):14137–14148
Abbasi S (2021b) Response surface methodology for photo degradation of methyl orange using magnetic nanocomposites containing zinc oxide. J Cluster Sci 32(4):805–812
Abbasi S (2022a) The degradation rate study of methyl orange using MWCNTs@ TiO2 as photocatalyst, application of statistical analysis based on Fisher’s F distribution. J Cluster Sci 33(2):593–602
Abbasi S (2023a) Studying the destruction of pollutant in the presence of photocatalysts based on MWCNTs with controlled values of TiO2 nanoparticles. Appl Water Sci 4(13):100
Abbasi S (2023b) Magnetic photocatalysts based on graphene oxide: synthesis, characterization, application in advanced oxidation processes and response surface analysis. Appl Water Sci 13(6):1–11
Abbasi S, Hasanpour M (2017) The effect of pH on the photocatalytic degradation of methyl orange using decorated ZnO nanoparticles with SnO2 nanoparticles. J Mater Sci: Mater Electron 28:1307–1314
Abbasi S et al (2015) Synthesis of TiO2 nanoparticles and decorated multiwalled carbon nanotubes with various content of rutile titania. Synth React Inorg, Met-Org, Nano-Met Chem 45(10):1539–1548
Abbasi S, Ekrami-Kakhki M-S, Tahari M (2017a) Modeling and predicting the photodecomposition of methylene blue via ZnO–SnO2 hybrids using design of experiments (DOE). J Mater Sci: Mater Electron 28:15306–15312
Abbasi S, Hasanpour M, Ekrami-Kakhki M-S (2017b) Removal efficiency optimization of organic pollutant (methylene blue) with modified multi-walled carbon nanotubes using design of experiments (DOE). J Mater Sci: Mater Electron 28:9900–9910
Abbasi S et al (2020) Synthesis of magnetic Fe3O4@ ZnO@ graphene oxide nanocomposite for photodegradation of organic dye pollutant. Int J Environ Anal Chem 100(2):225–240
Abbasi S et al (2021) Application of the statistical analysis methodology for photodegradation of methyl orange using a new nanocomposite containing modified TiO2 semiconductor with SnO2. Int J Environ Anal Chem 101(2):208–224
Abbasi S et al (2022) Evaluation of the dependence of methyl orange organic pollutant removal rate on the amount of titanium dioxide nanoparticles in MWCNTs-TiO2 photocatalyst using statistical methods and Duncan’s multiple range test. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2060085
Ananpattarachai J, Kajitvichyanukul P, Seraphin S (2009) Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J Hazard Mater 168(1):253–261
Ghaderi A, Abbasi S, Farahbod F (2018a) Synthesis, characterization and photocatalytic performance of modified ZnO nanoparticles with SnO2 nanoparticles. Mater Res Expr 5(6):065908
Ghaderi A, Abbasi S, Farahbod F (2018b) Synthesis of ZnO-SnO2-Ag nanocomposite and investigation of the photocatalytic decomposition of methyl orange using synthesized nanocomposite. J Environ Health Eng 5(4):337–344
Huang BS, Wey MY (2011) Properties and H2 production ability of Pt photodeposited on the anatase phase transition of nitrogen-doped titanium dioxide. Int J Hydrogen Energy 36(16):9479–9486
Ismail AA et al (2013) TiO2 decoration of graphene layers for highly efficient photocatalyst: impact of calcination at different gas atmosphere on photocatalytic efficiency. Appl Catal B 129(17):62–70
Kim SP, Choi MY, Choi HC (2015) Characterization and photocatalytic performance of SnO2–CNT nanocomposites. Appl Surf Sci 357:302–308
Kumar KM et al (2016) Green synthesis of S-doped rod shaped anatase TiO2 microstructures. Mater Lett 183:211–214
Lin Y et al (2012) Ternary graphene–TiO2–Fe3O4 nanocomposite as a recollectable photocatalyst with enhanced durability. Eur J Inorgan Chem 28:4439–4444
Liu Y et al (2012) A magnetically separable photocatalyst based on nest-like γ-Fe2O3/ZnO double-shelled hollow structures with enhanced photocatalytic activity. Nanoscale 4:183–187
Ma P et al (2013) Synthesis and photocatalytic property of Fe3O4@TiO2 core/shell nanoparticles supported by reduced graphene oxide sheets. J Alloy Compds 578:501–506
Madadi F, Ashrafizadeh F, Shamanian M (2012) Optimization of pulsed TIG cladding process of stellite alloy on carbon steel using RSM. J Alloy Compd 510(1):71–77
Namvar-Mahboub M, Pakizeh M (2014) Optimization of preparation conditions of polyamide thin film composite membrane for organic solvent nanofiltration. Korean J Chem Eng 31(2):327–337
Park JH, Kim S, Bard AJ (2006) Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett 6(1):24–28
Roozban N, Abbasi S, Ghazizadeh M (2017a) Statistical analysis of the photocatalytic activity of decorated multi-walled carbon nanotubes with ZnO nanoparticles. J Mater Sci: Mater Electron 28:6047–6055
Roozban N, Abbasi S, Ghazizadeh M (2017b) The experimental and statistical investigation of the photo degradation of methyl orange using modified MWCNTs with different amount of ZnO nanoparticles. J Mater Sci: Mater Electron 28:7343–7352
Tao L et al (2023) Metal-decorated InN monolayer senses N2 against CO2. ACS Appl Mater Interfaces 15(9):12534–12544
Wang X et al (2022) Fe3O4@m-ZrO2@Ag ternary magnetic nanocomposites for sensitive SERS sensing and photocatalytic removal of Cr(VI) and organic dyes. Compos B Eng 239:109959
Acknowledgements
The authors of this article are grateful for the assistance of the honorable director of the Central Research Laboratory of Esfarayen University of Technology to conduct laboratory tests.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SA analyzed the results, conducted the laboratory tests, and wrote the manuscript; MT analyzed the results; M. I conducted the laboratory tests.
Corresponding author
Ethics declarations
Conflict of interest
All the authors of this article undertake that they do not have any financial relationship with any person or institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbasi, S., Tahari, M. & Imani, M. Prediction of pollutant removal from aqueous solutions using magnetic photocatalysts. Appl Water Sci 13, 219 (2023). https://doi.org/10.1007/s13201-023-02027-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-02027-9