Abstract
This study aimed to create activated carbon thin film (ACTF) as a hybrid nanocarbon via a simple and efficient method through a single-step mixing process using thermal functionalization techniques. TEM, BET, BJH, FTIR, XRD, and TGA analyses were used to investigate the prepared ACTF. The results exhibited that ACTF has a porous structure with a high surface area of 318 m2/g and important functional groups, which are considered significant adsorption sites. The adsorption performance of ACTF for phenol and p-nitrophenol (PNP) removal from aqueous solutions using batch adsorption mode was studied. Evaluations were conducted on experimental factors influencing the adsorption process, such as pH, initial phenol and PNP concentrations, adsorbent dose, contact time, and temperature. Under the optimized conditions, the phenol and PNP were removed with a maximum efficiency of 89.98% and 92.5%, respectively. The results of linear and nonlinear isotherms and kinetic models of phenol and PNP showed that both pollutants were well fitted with the Freundlich model (R2 = 0.99, χ2 = 0.13, RMSE = 1.6), (R2 = 0.99, χ2 = 0.42, RMSE = 2.8), and the pseudo-second-order model (R2 = 0.999, χ2 = 0.03, RMSE = 0.31), (R2 = 0.99, χ2 = 0.01, RMSE = 0.24), for phenol and PNP, respectively. According to the calculated thermodynamic results, the adsorption of phenol and p-nitrophenol onto the ACTF surface was a spontaneous and exothermic reaction. The regeneration experiments showed that the spent ACTF could be reused up to the fifth cycle while maintaining noteworthy removal efficiency.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In recent years, water pollution has become one of the major issues endangering the ecological environment, human existence, and growth. Among all pollutants, water contamination with phenolic compounds was seen as a serious problem and a major source of concern owing to their toxicity and harm to living organisms even at low concentrations (Chowdhary et al. 2020). Phenol and PNP have been classified as priority toxic phenolic compounds (Sam et al. 2021). Phenol and PNP are released from various industries, such as petrochemical, petroleum refineries, pharmaceutical, paint, coal conversion industries, and others, in addition to domestic and agricultural activities (Ahmaruzzaman 2008). These pollutants have serious negative consequences for people, including liver, pancreatic, and kidney damage, degradation of proteins and tissues, and paralysis of the central nervous system. They can also result in headaches, elevated blood pressure, and severe eye discomfort (Saravanakumar and Kumar 2013). Therefore, the United States Environmental Protection Agency (EPA) regards them as priority pollutants (Ramos et al. 2021). So, it is necessary to remove these compounds from the effluents before discharging them into the stream of water.
Several traditional methods such as precipitation, coagulation, chemical oxidation, filtration, photocatalytic degradation, membrane separation, electrochemical degradation, and adsorption have been employed for the purpose of removing phenols (El-Maghrabi et al. 2019; Said et al. 2021; Dehmani et al. 2022). However, it is worth noting that adsorption is the most extensively utilized and effective approach for eliminating environmental pollutants due to its ease of operation and implementation, high removal efficiency, nonpoisonous by-products, low cost, and regenerative ability (Hosny et al. 2016, 2023).
A variety of adsorbents are utilized to remove phenolic compounds from wastewater, which include carbonaceous materials, metal oxides, polymers, biosorbents, and clay (Ahmaruzzaman 2008; Jaspal and Malviya 2020). Among these materials, carbonaceous materials such as graphene, activated carbon (AC), and carbon nanotubes (CNS) have received a lot of attention as effective adsorbents for phenolic compounds due to their high surface area, abundant active sites, chemical stability, and strong π–π interactions with the aromatic rings of phenolic compounds (Haydari et al. 2023).
Biomass-derived activated carbon is a particularly popular adsorbent for phenolic compounds due to its high adsorption capacity, low cost, and regeneration ability. A low cost activated carbon derived from black wattle bark waste showed high adsorption performance for phenol removal from aqueous solutions (Lütke et al. 2019). The mechanisms of adsorption by ACs were also stated in many studies through hydrogen bonding, π–π interactions, and van der Waals forces. Carbon nanotubes (CNTs) have also demonstrated promising results for the removal of phenolic compounds due to their distinctive tubular structure, which provides a high surface area and an abundance of active sites. For example, the hybrid material (CNT/PEG) used as an adsorbent for the removal of phenol exhibited an effective removal mechanism mainly through π–π interactions and hydrogen bonding (Bin-Dahman and Saleh 2020). Graphene and its derivatives, such as graphene oxide and reduced graphene oxide, have also shown promising results for the adsorption of phenolic compounds due to their large surface area, sp2 hybridized carbon substrates with conjugated ring structure, and abundant active sites. Zhang et al. demonstrated the ability of nanographite oxide to have a higher adsorption capacity for p-nitrophenol through hydrogen bonding, electron donor–acceptor interactions, and dispersive interactions as the main mechanisms affecting the adsorption removal (Zhang et al. 2015).
Based on the above, this research focuses on improving the adsorption mechanism of phenolic compounds by presenting an innovative adsorbent with all the properties suitable for the nature of these compounds. Activated carbon thin film (ACTF) is a novel type of carbonaceous material that is made up of thin graphene sheets with an uneven surface that link both internally and externally with amorphous porous carbon. This networked configuration provides several benefits to ACTF, such as a carbon-rich solid with high aromaticity, π electronic domains, a porous structure with a large surface area, and numerous and varied functional groups on its surface. All these properties make ACTF a promising adsorbent for wastewater treatment. Activated carbon thin films (ACTF) were first created using the batch acid spray technique using cobalt silicate as a catalyst at low temperatures by Fathy et al. (2017).
The first challenge in this work is to prepare ACTF from low-cost natural resources, using a new technique different from the technique used previously by Hosny et al. (2019). The ACTF was fabricated through a simple one-pot synthesis reaction using a thermofabrication approach to facilitate the incorporation of the AC layers into and out of the graphene layers to create a connected porous carbon network structure that aids in the diffusion of contaminants inside it. The second challenge of this research is to apply linear and nonlinear kinetic and isotherm models, as well as adsorption thermodynamics, to the experimental data that will be obtained from batch studies. These models, and their assumptions, are essential in understanding the adsorption mechanism, surface properties, and affinity of adsorbents. Error analysis methods, such as correlation coefficient (R2), root mean square error (RMSE), and chi-square (χ2), were used to determine the most suitable linear and nonlinear kinetic and isotherm equations, as reported by Karri et al. (2017) and Bopda et al. (2019).
The current study aimed to study the adsorption of phenol and p-nitrophenol onto ACTF from aqueous solutions. Adsorption parameters of phenol and PNP on ACTF were studied, such as pH, adsorbent dose, initial concentration of phenolic pollutants, contact time, and temperature. The linear and nonlinear kinetic and isotherm models, as well as the error parameters and adsorption thermodynamics, were studied. Finally, the capability of ACTF to be reused was evaluated through NaOH regeneration.
Materials and methods
Material and reagents
The utilized raw herb sample was collected from agricultural land in Cairo, Egypt. The collected sample was ground and sieved with particle sizes of 0.5–2 mm, washed, and dried at 105 °C overnight. As well as waste industrial graphite from used batteries, it was grinded by a planetary ball milling apparatus and sieved to obtain particle sizes of 60 mesh. All of the chemicals utilized in this study, including hydrochloric acid (HCl, 37%), Sodium hydroxide (NaOH, 98%), sulfuric acid (H2SO4, 95–98%), NaNO3 (99.5%), Potassium permanganate (KMnO4, 99.5%), Hydrogen peroxide (H2O2, 30%), and o-Phosphoric acid (H3PO4, 85%), were supplied by Merck India, Mumbai. Phenol (99%) and PNP (99%) were obtained from JHD Guangdong Guanghna Sci-Tech. Ltd., Guangdong, China. All the reagents and materials used in this study were of analytical grade and used directly without additional purification.
Synthesis of graphene oxide (Go) by a modified hummer’s method
The GO sample was synthesized following the modified Hummers method, as 1 g of the resultant graphite powder was mixed with 46 ml of H2SO4 under stirring in an ice bath, followed by the addition of 0.5 g of sodium nitrate (NaNO3) and 3 g of KMnO4 with stirring at a temperature less than 5 °C for 2 h. The mixture was then transferred to a water bath at 35 °C with stirring for 1 h, and then 150 ml of deionized water was slowly added and heated to 95 °C for 30 min until the solution turned dark green. To eliminate residual oxidants, 5 ml of H2O2 was slowly added, resulting in a brilliant yellow color and stirred for 10 min. The mixture was then centrifuged at 5000 rpm for 10 min to separate the precipitate from the liquid, and the precipitate was washed several times with deionized water and a 5% HCl solution. Finally, the obtained precipitate was dried in an oven at 60 °C for 24 h to produce a brown GO powder (Majumder et al. 2018).
Synthesis of activated carbon (AC)
In this experiment, the crushed herb was washed multiple times with distilled water to remove any dust or contaminants and then dried in an oven at 70 °C for 12 h. Next, the material was chemically activated by impregnating 10 g of the powdered material with H3PO4 in a 1:1 (w/w) ratio and leaving the mixture to keep for 24 h. The resulting mixture was dried in a ceramic crucible and placed in a closed ceramic crucible in the muffle furnace at 500 °C for 3 h (Mohammadi et al. 2020; Strelkov et al. 2021). The synthesized activated carbon (AC) was then washed several times with hot distilled water, followed by 0.1 M of HCl, and finally with distilled water until it reached a neutral pH. The sample was then dried overnight in an oven at 80 °C and ground in a glass mortar to a fine powder.
Synthesis of ACTF through thermal functionalization
The as-prepared GO and AC particles were combined at a mass ratio of 1:1. At first, a certain mass of activated carbon (AC) was added to 100 ml of distilled water and stirred for 1 h in a magnetic stirrer. The prepared GO was sonicated in a 250-ml glass beaker with the same volume of distilled water for 2 h at room temperature. The described GO was then added to the AC solution and stirred at 70 °C for 1 h to allow the AC, to act as a spacer between the GO layers. The mixture was then subjected to thermal functionalization in a preheated muffle furnace for 300 s at 350 °C, which successfully incorporated AC between the expanded GO layers. Carefully the crucible was removed from the furnace to prevent material loss due to pressure difference. Finally, the resulting amorphous carbon thin film was washed with distilled water until the pH was neutral, and then dried in a vacuum oven at 60 °C for 12 h.
Characterization methods
The analysis of XRD was performed by a D8 Focus X-ray diffractometer (Bruker, Germany) with an acceleration voltage of 40 kV and a current of 30 mA. The intensity is in the scan range of 4°–80°, with a rate 6° per minute. The FTIR spectra of the prepared samples were investigated on a Perkin-Elmer IR spectrophotometer in the range of 4000–400 cm−1. The surface morphological image of the resultant sample was picked up using a high-resolution transmission electron microscope (HRTEM, model JEOL JEM 2200FS TEM with a field emission gun operating at 200 kV). The BET approach was used to assess the specific surface area and pore properties (BET model BELSORP, BEL Master—Ver 7.1.0.0, MicrotracBEL, Corp.). The BJH method was used to estimate the size distribution. Thermogravimetric analyzer model: (Shimadzu thermal gravimetric analysis (TGA)—50, Japan, was used in a nitrogen atmosphere to determine the thermal stability of the resultant sample. The thermal decomposition was performed with a heating rate of 10 °C/min and a temperature range of 30–1000 °C.
Adsorption studies
Batch adsorption experiments were applied to evaluate the adsorption performance of ACTF for phenol and PNP removal. For each experiment, 100 ml stopper conical flasks were filled with 50 ml of the desired concentration of phenol and PNP solution mixed with a definite amount of adsorbent dose, and the samples were placed in a shaker at 150 rpm. The initial pH of phenol and PNP solutions was adjusted at different pH values in the range from 4 to 10 using 0.1 M HCl and NaOH to determine the optimum pH. The other adsorption parameters, such as initial concentration of phenolic compounds, temperature, contact time, and adsorbent dosage, varied from 50 to 200 mg/L, 25 to 55 °C, 0 to 180 min, and 0.0125 to 0.075 g, respectively. After the adsorption, centrifugation was immediately used to determine the amount of phenol and PNP in the supernatant using the JASCO V-750 Spectrophotometer at wavelengths of 270 and 317 nm, respectively (Chaudhary et al. 2022).
The adsorption capacity of phenol and PNP adsorbed (qe, mg/g) was determined from the following equation:
Co and Ce are the initial and equilibrium concentration (mg/L) of phenol and PNP in the solution, respectively. V (L) is the total volume of the solution, m (g) is the mass of the ACTF.
The following equation determined the removal efficiency of phenol and PNP.
Adsorption isotherm and kinetic models
Linear and nonlinear kinetic and isotherm models (Hidayat et al. 2021), were fitted to the data obtained from experimental batch adsorption, to explain the mechanism of the adsorption phenomena of phenol and PNP onto ACTF adsorbent. The kinetics models applied to the adsorption of phenol and PNP, namely the Pseudo-first-order model (PFO), Pseudo-second-order model (PSO), and intra-particle diffusion model (IPD), whose equations are presented in Table 1, The Pseudo-first-order model (PFO), is a commonly used assumption to describe the rate at which a solute adsorbs onto a solid surface and mostly depends on the concentration of the adsorbate in the bulk solution (López-Luna et al. 2019a, b). The Pseudo-second-order model (PSO), assumes that the rate-controlling step in the adsorption process is chemisorption. It further predicts the adsorption behavior across the entire range of adsorption. And its advantage is that the rate of adsorption is influenced by the adsorption capacity rather than the concentration of the adsorbate (Khandaker et al. 2020). The intra-particle diffusion model (IPD), is the most commonly used model to describe the mechanism of adsorption, the reaction pathway, and the rate-determining step. This model is commonly employed in three different forms, as follows: (Pezoti et al. 2016):
-
1.
The first form involves plotting a straight line that is forced to pass through the origin, and in this form, IPD is only the rate-controlling step.
-
2.
The second form includes a multi-linearity plot with two or three distinct steps, encompassing the entire adsorption process. The initial step corresponds to external surface adsorption, or instantaneous adsorption, followed by a gradual adsorption step where intraparticle diffusion is the controlling factor. The final step represents the equilibrium stage, where the solute's movement from larger pores to micropores leads to a slower adsorption rate.
-
3.
The third form results in a straight line, but it does not necessarily pass through the origin, indicating the presence of an intercept. The magnitude of the intercept is proportional to the thickness of the boundary layer, with a larger intercept indicating a greater boundary layer effect.
As the sorption kinetics reached equilibrium, linear and nonlinear Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (D − R) isotherm models were used, the equations for which are also shown in Table 1 (Batool et al. 2018; López-Luna et al. 2019a, b). The Langmuir isotherm assumes the surface of the adsorbent is homogeneous with sites of similar energies of adsorption, while the Freundlich isotherm assumes that adsorption occurs on a heterogeneous surface with an uneven distribution of adsorption energy (Moubark and Hosny 2021). Also, the Temkin isotherm assumes that the heat of the adsorption (ΔH) process of all molecules decreases linearly rather than logarithmically with coverage, and it is more suitable for moderate concentrations of pollutants, while D–R is typically used to identify adsorption mechanisms by calculating the mean free energy (E) value as given from the equation in Table 1, which can indicate either physical or chemical adsorption depending on whether E is more than or equal to 8 kJ/mol. This model successfully fits a range of adsorbate concentrations between moderate and high values, with the adsorption process taking place through a pore-filling mechanism on the heterogeneous surface of the adsorbent (Chaudhary et al. 2022).
Data evaluation and error functions
The linear or nonlinear regression analysis of kinetic and isotherm models was applied to verify the compatibility of theoretical adsorption models with experimental adsorption data. Linear regression analysis is made by fitting a linear model to the experimental data to obtain a direct linear relationship between dependent and independent variables, and from slop and intercept, calculated adsorption capacity (qe,cal) and other theoretical parameters will be measured, while nonlinear regression analysis will be fitted by using the solver add-in functions of Microsoft Excel software(Lin and Wang 2009). Error functions such as coefficient of determination (R2), Chi-square (χ2), and the Root Mean Square Error (RMSE), whose equations are shown below in equations from 3 to 5, respectively, The coefficient of determination, R2, is a measure of the correlation between the experimental data and the model equations(Chan et al. 2012). On the other hand, χ2 and RMSE are indicators of how closely the experimental values of the amount of phenol adsorbed at equilibrium (qe,exp) match the calculated values obtained from the model equations (qe,cal). The best-fitting model of linear and nonlinear models based on the value of error functions is that R2 has the highest value closer to unity, with minimum values of χ2 and RMSE.
qe,exp, qe,cal and qe,mean are the experimental, calculated adsorption capacities and the mean of the qe,exp values, n is the number of measurement.
Thermodynamic studies
Thermodynamic parameter studies help to know better about the mechanism of the adsorption process, whether exothermic, endothermic, or spontaneous(Romero-Gonzalez et al. 2005). Parameters such as entropy change (ΔS°: kJ/mol), enthalpy change (ΔH°: kJ/mol) and Gibbs free energy change (ΔG°: kJ/mol) were determined according to the following equations:.
T (k) is the absolute temperature, kd is the distribution coefficient t (L/g), and R is the universal gas constant (8.314 J/mol K). The values of thermodynamic parameters (ΔS, ΔH) were calculated from the intercept and slope that were obtained from the graph of the Van’t Hof plot (ln K against T −1) (Jain et al. 2022).
Reusability
Reusability studies of the adsorbent in any adsorption process are of utmost importance because they affect the purification cost of industrial waste water (Ighalo et al. 2022). To assess if the ACTF (an adsorbent material) can be regenerated and reused, the material was subjected to a desorption process after being used to absorb phenol and PNP. This involved soaking the material in 25 mL of 0.1 M NaOH while being stirred magnetically, to remove the absorbed phenol and PNP from its surface (Fseha et al. 2023). The regenerated adsorbent was then washed with distilled water and reused in the next adsorption cycle. This cycle of adsorption and desorption was repeated five times to evaluate the regeneration potential of the ACTF.
Results and discussion
Characterization of ACTF
X-ray diffraction analysis (XRD)
X-ray diffraction (XRD) analysis confirms the crystallographic properties of the AC, GO and ACTF nanostructure as displayed in Fig. 1. In this regard, the crystal structure of AC is confirmed by the existence of two main peaks at 2θ of 23.9° and 42° for (002) and (101) planes (Qiu et al. 2019). The X-ray diffraction patterns of GO show a single, sharp diffraction peak at 2θ of 10.39° for (001) plane, with an interlayer space of 0.81 nm, indicating the presence of oxygen-based cracks on the surface of GO. This indicates successful exfoliation and the formation of highly structured GO graphite oxide crystals (Fathy et al. 2019). The XRD analysis of the ACTF shows a significant decrease in intensity and a shift in the peak positions when compared to the original samples, demonstrating the ability of the alternating AC to interact with the graphene layers within the composite ACTF. This result confirms the efficient thermal functionalization of ACTF through GO and AC.
The high-resolution transmission electron microscope (HR-TEM) and selected area electron diffraction (SAED) analysis
The surface morphology of the porous ACTF nanostructure was investigated in Fig. 2 by HR-TEM analysis. Figure 2a reveals the presence of clustered flakes and sheet-like structures, and Fig. 2b and c reveal a well-defined, distinct flattened morphology, characterized by the presence of two-dimensional graphene sheets and dark-colored activated carbon particles. The images further demonstrate that the ACTF material consists of numerous rippling and frizzing interconnected layers of graphene sheets, to which the dispersed black-colored activated carbon contributes to the overall nanostructure. The SAED pattern in Fig. 2d was examined alongside the XRD patterns, demonstrating a consistent match between them. Consequently, the HR-TEM studies provide clear evidence that this one-step synthetic approach enables the easy and spontaneous self-assembly of AC on and in between the graphene nanosheets. This assembly is facilitated by π-π stacking and electrostatic interactions, leading to a strong integration of the ACTF nanostructure.
FTIR analysis
The FTIR analysis was performed to examine the chemical composition of GO, AC, and ACTF and the resulting spectra are shown in Fig. 3. In the FTIR spectrum of AC (Fig. 3), distinct bands are observed at 3422 cm−1, 1617 cm−1, and 1041 cm−1. These bands correspond to the stretching vibrations of O–H functional groups, C = C groups, and C–O groups in the sample (Iwanow et al. 2020). In the IR spectrum of GO (Fig. 3), specific signals corresponding to various functional groups were identified. These include the O–H stretching vibration at 3445 cm−1, the H–C–sp3 stretching vibration at 2977 cm−1, the C = O stretching vibration at 1705 cm−1, which indicates the presence of carboxylic groups at the edges of GO, and the epoxy stretching vibration C–O at 1054 cm−1.The presence of enlarged bands associated with oxygen-containing functional groups in the produced GO indicates the successful oxidation of graphite (Surekha et al. 2020). In the case of ACTF, the FTIR spectrum in Fig. 3 exhibits peaks in the range of 3738–3441 cm−1, which correspond to the overlap of multiple O–H groups. Additionally, there are other main peaks observed at 1622 cm−1, 1384 cm−1, and 1042 cm−1. Notably, these bands show significant changes in peak position, intensity, and the absence of certain peaks after thermal treatment. These observations suggest a successful interconnection between GO and AC in the prepared material.
Surface area and pore size analysis
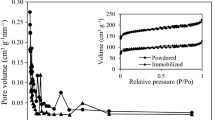
The nitrogen adsorption isotherms and pore size distribution of the ACTF nanostructure are demonstrated in Fig. 4a and b. In this regard, the N2 isotherm of the ACTF displayed in Fig. 4a exhibits a Type IV isotherm pattern (Mojoudi et al. 2019). This characteristic is associated with the presence of numerous mesopores within the material. The specific surface area was measured to be 318 m2/g using the BET method, as shown in Table 2. The pore size distribution, as determined by the BJH plot, exhibited a single prominent peak with an average pore diameter of 4.09 nm. This demonstrated that the ACTF had a mesoporous structure matching what was depicted in the adsorption–desorption isotherm graph. Because of its large specific surface area and porous structure, ACTF provides excellent adsorption capabilities and improves pollutant removal efficacy.
Thermal stability analysis
The thermal stability of AC, GO, and ACTF was investigated using thermogravimetric analysis (TGA) at 850 °C under nitrogen atmosphere. As shown in Fig. 5, ACTF showed greater thermal stability compared to AC and GO at 430 °C, with its weight decreasing by 3–4% due to the evaporation of intercalated adsorbent water and the elimination of oxygen functional groups. Moreover, ACTF showed a moderate weight loss of approximately 31% in the temperature range between 430 and 800 °C due to unstable carbon decomposition, generating carbon dioxide and carbon monoxide. The rate of weight loss remained constant at 800 °C, indicating no further dissociation or degradation. Overall, ACTF demonstrates favorable synergistic effects and significantly higher thermal stability compared to AC and GO feedstocks.
Determination of point zero charge (pHzpc)
The point of zero charge (pHzpc) is the pH at which an adsorbent's net surface charge equals zero. It is crucial for understanding adsorbent surface charge characteristics, as they significantly affect contaminant removal efficiency. The relationship between the pH of the solution and the adsorbent surface charge depends on pHzpc. When the pH is greater than pHzpc, the adsorption surface is negative, while when it is less than pHzpc, the adsorbent surface is positive. The salt addition method was used to determine the pHzpc of ACTF (Yeganeh et al. 2022). The experimental results revealed that the prepared ACTF has a pHzpc value of 6 (Fig. 6). This suggests that water donates more H+ ions, than OH− ions to the functional groups present on the ACTF surface.
Effect of pH
The pH of the solution is a crucial factor that affects the dissociative behavior (ionization degree) of adsorbates (phenol and p-nitrophenol) and the surface charge of the adsorbent (ACTF). For this reason, the influence of pH values on the adsorption of phenol and PNP onto the ACTF surface, was investigated at various pH values ranging from 4 to 10, as shown in Fig. 7a. The obtained results showed that the phenol removal efficiency reaches its maximum percentage (~ 90%) at pH = 4, with a minor decline (from 89.9 to 79.7%) with increasing pH values from 4 to 10. But in the case of p-nitrophenol, it is notable that high removal efficiency was preserved at a pH of 4–7 and quickly declined to ~ 61% at pH 8–10. This is due to the pKa values of phenol (9.89) and p-nitrophenol (7.15), where phenol has a higher pKa value than p-nitrophenol, which is the reason for the higher stability of the pH curve of phenol than p-nitrophenol. To further, understand the adsorption mechanism, it is necessary to determine the surface charge of the absorbent by point zero charge (pHzpc). The pHzpc of the prepared adsorbent is 6, as was illustrated in Fig. 6.
At maximum phenol and PNP adsorption disclosed at pH = 4, this meant pH < pHzpc, according to which the surface of ACTF will have a positive charge and phenol and PNP present in their molecular forms. As a result, the electrostatic interaction between the positive charge of the ACTF surface and phenol and PNP can be attribute to electron-donar acceptor complex between aromatic ring of phenolic compounds (electron acceptor) and oxygenated functional groups on the surface of ACTF(electron donar), resulting in the formation of a partly covalent bond. Additionally, π–π dispersion interaction and hydrogen bond formation may be the most plausible mechanisms for higher phenol removal efficiency on the ACTF surface at acidic pH. The obtained results in this study are similar to many earlier studies (Dehmani et al. 2020; Bibi et al. 2022).
While at pH ˃ pKa and pHzpc, the molecules of phenol and p-nitrophenol were transformed to an anionic form bearing a negative charge, which led to more electrostatic repulsion with the negative charge of the ACTF surface, resulting in a reduction in the removal efficiency. However, since PNP is more polar than phenol, it can form additional interactions such as hydrogen bonds, as well as PNP have a nitro group, electron withdrawing group, that decrease π electrons in the ring which improve electron donar acceptor complex interaction between PNP and ACTF, further increasing the removal efficiency of PNP over phenol. As a result, hydrogen bonding, π–π dispersion, and Electron donor–acceptor complex formation at the carbon surface may be the major adsorption mechanisms at low pH levels. As a result of this, pH = 4 was chosen as the ideal pH value for more adsorption experiments.
Effect of adsorbent dose
The removal efficiency of phenols and PNPs on the ACTF surface is influenced by the surface area and active sites on the adsorbent. The effect of adsorbent dose on removal efficiency was studied using varying ACTF doses of 0.0125, 0.025, 0.05, and 0.1 dissolved in 50 ml of phenol or PNP solutions (which correspond to 0. 25, 0. 5, 1, and 2 g/L, respectively). The other adsorption parameters were maintained constant at 50 mg/L phenol or PNP concentration, a pH of 4, a temperature of 30 °C, and an equilibrium time of 3 h. Results are shown in Fig. 7b.
The maximum phenol removal efficiency was achieved at 1 g/L (0.05 g of ACTF dissolved in 50 ml of phenol solutions); after that, efficiency decreases significantly due to an agglomerate of ACTF molecules and a decline in the total adsorption surface area. Leading to a decrease in its performance and decreasing the phenols adsorption rate on their surface (Papaoikonomou et al. 2021). Therefore, 0.05 g was chosen as the best adsorbent dosage for further adsorption experiments.
Whereas, PNP removal efficiency reached 92.4% at 1 g/L (0.05 g of ACTF dissolved in 50 ml of PNP solutions), followed by a gradual increase to 97.5% at 2 g/L (0.1 g of ACTF dissolved in 50 ml of PNP solutions). Higher ACTF adsorbent doses may offer more adsorption sites or porous sites, indicating PNP's higher affinity for ACTF promotes more removal efficiency than phenol.
Effect of initial phenol and PNP concentration
The effect of initial phenol and PNP concentration on the removal efficiency are shown in Fig. 7c. The variation of initial phenol concentration is from 50 to 200 mg/L at constant pH (4), constant adsorbent dose (1 g/L), constant time (3 h), and at room temperature. It can be observed in Fig. 7c, that the removal efficiency decreased with an increase of initial concentration of phenol and PNP by ACTF; Maximum phenol (89.87%) and PNP (92.4%) adsorption was found when using 50 mg/L of initial phenol and PNP concentration and a minimum amount of adsorption was observed at an initial concentration of 200 mg/L. The rise in R (%) was induced at lower phenol and PNP concentration due to availability of more number of vacant active sites, but these are gradually filled by adsorbates, resulting in an adsorption equilibrium (Qiao et al. 2018). The increase in the concentration of phenol and PNP after reaching the saturation state on the surface of the adsorbent results in the electrostatically repulsion interaction between the phenolic compounds adsorbed on the surface of the adsorbent and the free phenolic molecules, thus causing decrease in the removal efficiency of phenol and PNP. A Similar result was stated in previous published research (Ganguly et al. 2020).
Influence of contact time
The removal efficiency of phenol and PNP was studied using contact time between 10–180 min Fig. 7d. The initial stage of adsorption showed a sharp increase of 75%, with adsorption time increasing to 30 min for phenol and 83% for PNP. After a specific time, equilibrium was achieved around 150 min for phenol and 120 min for PNP. This increase is due to the collision between phenol, PNP, and adsorbent, leading to mass transfer of adsorbate onto active sites. As time progresses, more internal sites become available, resulting in a gradual increase in removal efficiency. Adsorption equilibrium occurs when phenolic compounds fully occupy the adsorption sites due to strong interactions between oxygenated functional groups on the surface and hydroxyl groups of phenol compounds via hydrogen bonding and π–π interactions between aromatic rings. A similar result was stated in previous published research (Wadhawan et al. 2020; Dutta et al. 2021).
Effect of temperature and thermodynamic study
Another important factor that influenced on adsorption directly was temperature, because it influences on the solid interface and movement of pollutants during adsorption. As illustrated in Fig. 8a, adsorption effectiveness of phenol and PNP reduces as temperature rises from 25 to 55 °C, and the ideal temperature has been recorded at 25 °C. This likely denotes a weak chemical interaction between the surface oxygenated functional groups of ACTF and adsorbates.
The reason for the decrease in the removal efficiency with the increase in temperature is that with the increase in temperature the heat energy of the system increases, leading to causes the adsorbate molecules to have more kinetic energy and become more mobile, limiting their ability to adhere to the adsorbent surface, resulting in a lower interaction between the adsorbate and surface functionality of ACTF adsorbent causing decrease in removal efficiency (Priyadarshini et al. 2021). In addition, higher temperatures may produce structural changes in the adsorbent or desorption of earlier adsorbed molecules, lowering its efficacy even further. So, the maximum removal of phenol and PNP was attained at 25 °C, which is an exothermic process.
The correlation between temperature and adsorption can be better understood by evaluating thermodynamic parameters like Gibbs free energy (ΔG°), enthalpy (ΔH°), and entropy (ΔS°). The values of ΔH° and ΔS° were calculated from the slopes and intercepts of the Van’t Hoff plots as shown in Fig. 8b and Table 3. The values of ΔG° and ΔHo, for phenol and PNP, indicate spontaneous adsorption, an exothermic nature, and a decrease in degree of freedom at the solid-solution interface. Adsorption mechanisms can be categorized into chemisorption and physisorption, with ΔG values typically ranging between − 20 and 0 kJ mol−1. The range of − 20 to − 80 kJ mol−1 indicates a combination of physisorption and chemisorption, while values between − 80 and − 400 kJ mol−1 suggest chemisorption. In the present study, all the calculated ΔG values fall within the range of − 20 and 0 kJ mol−1, indicating that the adsorption mechanism is physisorption. This suggests that the interaction between phenol, PNP, and the surface of ACTF most likely involves π–π interactions and hydrogen bonds.
Linear and nonlinear kinetic models
Pseudo-first-order model (PFO)
As presented in Fig. 9a and b and Table 4, the experimental data did not show a linear trend across the whole range of phenolic compound concentrations, despite having adequate linear regression coefficients (R2). In this regard, it appears that the PFO model, at least in its linear form, was unable to represent the kinetic behavior of phenol and PNP adsorption onto ACTF. Additionally, logarithmic indeterminate values in the present work impeded the improved fitting of the PFO linear model. As a result, the PFO adsorption rate constants (k1) for both phenol and PNP were negative and of a tiny order of magnitude, possibly indicating a lack of affinity or repulsion between phenolic solutions and the ACTF adsorbent. This model has the significant drawback of making it difficult to forecast how an adsorption process would behave when it is slow, necessitating a theoretical assessment of adsorption capacity (qe). As it can be observed from Table 4, the calculated qe,cal was significantly different from the experimental data; hence, higher error functions (χ2 and RMSE) were obtained.
As for the PFO nonlinear model, it allows for the direct calculation of the equilibrium absorption capacity (qe) based on the adsorption path over time; this capability offers a significant advantage compared to the linear model. Hence, it was observed that the calculated adsorption capacity (qe,cal) of phenol and PNP that was obtained from nonlinear PFO is in good agreement with the experimental adsorption capacity (qe,exp), as indicated in Table 4. Moreover, the values of χ2 and RMSE have been recorded at 0.73 and 1.4 for phenol and 0.51 and 1.93 for PNP (Table 4), which means that nonlinear PFO has a low error function. Therefore, it is possible that the PFO nonlinear model provides a dependable description of the kinetics of phenol and PNP adsorption onto ACTF. It is crucial to thoroughly investigate the nonlinear form of the pseudo-first-order model before dismissing it based on its inability to accurately fit the data using linear adjustments.
Pseudo-second-order model (PSO)
The PSO model has been widely employed to explain chemisorption processes that involve valence forces, such as the sharing electrons between the adsorbent and adsorbate, representing covalent forces (López-Luna et al. 2019a, b). Unlike the pseudo-first-order kinetic model, this particular model predicts adsorption behavior throughout the entire adsorption range. As a result, reaching true equilibrium becomes more feasible, and the adsorption capacity (qe) can be accurately determined.as shown from Fig. 10a and b and Table 4. the adsorption of phenol and PNP onto ACTF exhibited excellent conformity with both the linear and nonlinear types of the pseudo-second-order kinetic model. The high value of coefficient determination (R2 ~ 1) with low minimum error values and close agreement between (qe,exp) and qe,cal indicated a stronger fit with the nonlinear model compared to the linear model for phenol and PNP. Thus, the kinetics of adsorption of phenolic compounds onto ACTF nanostructures was better accuracy described by PSO than the PFO. And the mechanism of the adsorption according to the hypnosis of the model is the π–π electron donar acceptor complex between π acceptor of benzene rings in phenolic compounds and π donar of oxygenated functional groups in ACTF surface.
Intra-particle diffusion model (IPD)
Nevertheless, the conventional kinetic models mentioned above are not able to accurately describe the complex mechanism of adsorption due to the involvement of multiple interconnected regulatory mechanisms and certain assumptions (Matera et al. 2019). Therefore, the use of an intraparticle diffusion model (IPD) can be employed to effectively describe the adsorption mechanism, reaction pathway, and steps that determine the rate of the process. Figure 11 shows the IPD fit for phenol and PNP adsorption onto the ACTF nanostructure. In both of the two plots, there were three straight lines that intercepted the origin but not for the whole time, which indicated that IPD was not only the rate-determining step. The first portion of the curve showed a straight-line relationship between 0 and 15 min of the contact time between phenolic solutions and nanostructures and refers to rapid mass transfer from the phenolic solution to the ACTF's surface external layer. The rate of change (kid1) of concentration with contact time was rapid in this initial stage due to the availability of free active sites as well as the high phenolic bulk solution concentration. The second portion of the curve showed a straight line from 20 to 60 min. The rate of change (kid2) of concentration with contact time was progressively decreasing in this second stage as a result of the gradual diffusion of phenolic compounds into the ACTF inner pores and surface active sites. The third portion of the curve showed a straight line from 90 to 180 min. The rate of change (kid3) of concentration with contact time was relatively slower in this third stage due to the saturation of ACTF active sites. The disparity in the rate of mass transfer during the three different stages of adsorption is responsible for the deflection of straight lines away from the origin point. It was observed from Table 4, that the values of boundary layer thickness (Ci) increase with increasing phenol and PNP concentrations, indicating that the larger the boundary layer thickness, the slower the external mass transfer and the longer the diffusion pathway, implying a slower adsorption process (Santhi et al. 2010). The R2 values provided in Table 4, which are close to unity, suggest that this model has been successfully applied. This observation potentially verifies that the intraparticle diffusion process is the rate-determining step. The linear relationship observed in the plots further supports the significant involvement of intraparticle diffusion in the adsorbate uptake by the adsorbent. To summarize and align with our findings, the adsorption mechanism can be described as a sequence of three consecutive steps. Firstly, there is the external or film mass transfer, where solute molecules move from the bulk of the solution to the surface of the sorbent particle. This step is followed by diffusion within the internal structure of the particle, leading to the transportation of molecules toward the sorption sites. Finally, at these sorption sites, an exceptionally fast uptake of molecules takes place, which is challenging to measure. Therefore, both film mass transfer and intraparticle diffusion are identified as the rate-determining steps in this process.
Linear and nonlinear equilibrium isotherm
Langmuir isotherm
The linear and nonlinear forms of the Langmuir model are used to examine the process of monolayer adsorption onto a homogenous surface where there is no diffusion of the sorbate into the surface plane. Linear and nonlinear regression of isotherm models of adsorption of phenol and PNP on ACTF were depicted in Fig. 12a and b, and the plot parameters and values of χ2 and RMSE are represented in Table 5. Based on the values of the correlation coefficients (R2), χ2, and RMSE, the Langmuir model best matches the experimental procedures in a nonlinear rather than linear model. But the theoretical adsorption capacity (qe,cal) obtained from both models did not match the experimental adsorption capacity (qe,exp), indicating that the adsorption of phenol and PNP onto ACTF was not best fitted with the assumptions of the Langmuir model.
Freundlich isotherm
The linear and nonlinear forms of the Freundlich model are used to investigate whether the adsorption of phenol and PNP on ACTF nanostructure occurs on a heterogeneous surface with interactions between surface-bonded and unbound molecules, leading to the multi-layered formation of sorbate molecules or not. As observed from Fig. 13a, b and Table 5, the correlation coefficients (R2) of phenol and PNP in both models are nearly at unity, with the lowest values of χ2 and RMSE, indicating the Freundlich model is the preferred choice for evaluating phenol and PNP adsorption data due to its optimality as compared to the Langmuir model. The Freundlich isotherm demonstrates a higher adsorption capacity value (Kf = 14) for phenol and (Kf = 22) for PNP, indicating a robust connection between phenol and PNP and the sorption sites of ACTF. Moreover, the Freundlich constant n was found to be higher than 1, which indicated that the adsorption process is favorable. Hence, the experimental data exhibits a greater resemblance to the Freundlich model as compared to the Langmuir model.
Temkin isotherm
This model explores the interaction between the sorbent and sorbate, and it is built upon the assumption that the heat of adsorption is not constant. Instead, the heat of adsorption decreases as a result of the interaction between the sorbent and sorbate during the adsorption process. Linear and nonlinear regression of isotherm models of adsorption of phenol and PNP on ACTF were depicted in Fig. 14a and b, and the plot parameters and values of χ2 and RMSE are represented in Table 5. Based on the values of the correlation coefficients (R2), χ2, and RMSE, the Temkin best matches the experimental procedures. The values of heat of adsorption (bt) for phenol and PNP were 39.5 and 43.04 kJ/mol, respectively. The positive value of bt is an indicator of the exothermic process of the adsorption of phenol and PNP on the ACTF nanostructure. As a result, the Temkin adsorption isotherm fits well with the experimental data.
Dubinin–Radushkevich Isotherm (D–R) Model
The Dubinin–Radushkevich isotherm (D–R) model has proven to be very useful for determining the type of sorption process, whether it is physical adsorption or chemical adsorption, through the determination of the mean free energy (E) value, which could be either chemical adsorption in the case of E < 8 kJ/mol or physical adsorption in the case of E < 8 kJ/mol (Puccia and Avena 2021). Linear and nonlinear forms of the D–R model are given in Fig. 15a, b and Table 5. Since both calculated E in the linear forms for phenol and PNP (296 and 383 kJ/mol) and in the nonlinear form (228 and 334 kJ/mol), it can be concluded that the adsorption of phenol and PNP ACTF is chemical. Moreover, the values of the correlation coefficients (R2) are low compared with other isotherm models with high values of χ2 and RMSE, indicating that the D–R model doesn’t fit the experimental procedures. Table 5 provides a summary of the isotherm parameters for the four isotherm models that were previously mentioned. The findings in Table 5 provide clear evidence that the Freundlich isotherm model is the most appropriate for explaining the equilibrium adsorption of both phenol and PNP onto ACTF in both linear and nonlinear regression. This is supported by the model's lowest χ2 and RMSE value and highest R2 values. These results suggest that the adsorption process of phenol and PNP onto the produced ACTF is characterized by heterogeneity.
Regeneration studies
The recyclability test (Fig. 16) clearly demonstrates the remarkable recyclability of the ACTF nanostructure. Even after the 5th cycle, the adsorbent maintained a high adsorption performance toward phenol and PNP, with removal efficiency (R%) values of 76% and 79%, respectively. It was observed that the removal efficiency decreased slightly from the first to the fifth cycle, indicating that the morphological properties of the adsorbent surface have remained largely intact as the sites on the surface retained their functionality. The adsorption of phenol and PNP on ACTF exhibits reversibility. As a result, the used adsorbent can be effectively regenerated and reused by subjecting it to treatment with 0.1 M NaOH, followed by thorough washing and subsequent drying at 105 °C. This regeneration process is facilitated by the reaction of phenol and PNP with sodium hydroxide, resulting in the formation of easily desorbable sodium phenolates from the ACTF.
Comparison with other adsorbents
The adsorption capacities of the ACTF nanostructure for phenol and PNP were compared to those of other carbon-related adsorbents. Table 6 provides a comparison of carbon materials used for the adsorption of phenol and PNP from aqueous solutions. According to the table, ACTF has the maximum adsorption capacity compared to most of the materials used in previous research to remove phenol and PNP. This indicates that the ACTF is highly effective for treating wastewater contaminated with phenol and PNP.
Conclusion
In this work, Activated carbon thin film (ACTF) has been synthesized through a thermal functionalization step in which activated carbon was incorporated as a spacer material between layers of graphene oxide (GO) through the intercalation step between the expanded layers during the thermal process. The adsorption performance of ACTF toward phenol and PNP was studied. ACTF exhibits a high specific surface area, porous structure, abundant functional groups, and structural stability, which enhance the uptake of phenol and PNP onto ACTF. The optimized adsorption parameters were pH 2, initial phenol concentration of 50 mg/L, ACTF dose of 1 g/L for phenol pollutant, and pH 2, initial PNP concentration of 50 ppm, and ACTF dose of 2 g/L for PNP pollutant. The adsorption process reached equilibrium in 150 min for phenol and 120 min for PNP, showing a maximum removal efficiency of 89.5% and 92.5% for phenol and PNP, respectively, at 25 °C. The removal efficiency of ACTF was more effective in adsorbing PNP than phenol, indicating the relevance of the nitro groups on the adsorbate. The experimental data showed that the linear and nonlinear Freundlich models provided the best fit for phenol and PNP adsorption, showing that it occurs in multiple layers and that the surface of the ACTF adsorbent is heterogeneous. Additionally, the linear and nonlinear pseudo-second-order kinetics model was found to be the best fit for the data, suggesting that chemisorption is the key mechanism for the adsorption of phenol and PNP onto ACTF. According to the thermodynamic study, the adsorption process was exothermic, favorable, and spontaneous, as evidenced by the negative values of ΔHo and ΔGo. According to kinetics, isotherms, and thermodynamics, it was deduced that the adsorption mechanism of phenol and PNP onto ACTF was primarily physical (π–π dispersion interaction and hydrogen bond), and partly chemical (electron donor–acceptor complex). The utilized ACTF was effectively recovered from the phenol-loaded solid using a 0.1 M NaOH solution and showed promising adsorption efficiencies of (76–79%) for phenol and PNP, respectively, after five successive adsorption–desorption cycles.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACTF:
-
Activated carbon thin film
- PNP:
-
P-nitrophenol
- χ 2 :
-
Chi-square
- RMSE:
-
Root mean square error
- R 2 :
-
Coefficient of determination
- PSO:
-
Pseudo-first-order model
- PSO:
-
Pseudo-second-order model
- IPD:
-
Intra-particle diffusion model
- q e :
-
Equilibrium adsorption capacity mg/g
- C i :
-
Concentration of the influent mg/L
- Ce:
-
Concentration of the effluent mg/L
- V :
-
Sample's volume L
- ΔG°:
-
Gibbs free energy (kJ/mol)
- ΔH o :
-
Enthalpy (kJ/mol)
- ΔS o :
-
Entropy (kJ/mol)
- K 1 :
-
Pseudo-first-order rate constant rate constant (min−1)
- K 2 :
-
Pseudo-second-order rate constant (g/mg min)
- K id :
-
Intra-particle diffusion rate constant (mg/g min−1/2)
- C :
-
Boundary layer thickness constant
- K L :
-
Langmuir constant (L mg−1)
- q m :
-
Maximum adsorption capacity (mg g−1)
- b T :
-
Temkin isotherm constant
- K T :
-
Temkin isotherm equilibrium binding constant (L g−1)
- R :
-
Universal gas constant (8.314 J mol − 1 K−1)
- K ad (mol2 kJ−2):
-
Dubinin–Radushkevich isotherm constant (mol2 kJ−2)
- Ε :
-
Dubinin–Radushkevich isotherm constant
- E :
-
Mean free energy (kJ mol−1)
- T :
-
Absolute temperature (K)
- R%:
-
Removal efficiency
References
Ahmaruzzaman M (2008) Adsorption of phenolic compounds on low-cost adsorbents: a review. Adv Colloid Interface Sci 143(1–2):48–67
Batool F, Akbar J, Iqbal S, Noreen S, Bukhari SNA (2018) Study of isothermal, kinetic, and thermodynamic parameters for adsorption of cadmium: an overview of linear and nonlinear approach and error analysis. Bioinorgan Chem Appl 2018:1–11
Bibi A, Bibi S, Abu-Dieyeh M, Al-Ghouti MA (2022) New material of polyacrylic acid-modified graphene oxide composite for phenol remediation from synthetic and real wastewater. Environ Technol Innov 27:102795
Bin-Dahman OA, Saleh TA (2020) Synthesis of carbon nanotubes grafted with PEG and its efficiency for the removal of phenol from industrial wastewater. Environ Nanotechnol Monitor Manag 13:100286
Bopda A, Tchuifon DRT, Ndifor-Angwafor NG, Doungmo G, Gabche AS (2019) Non-linear equilibrium and kinetic study of the adsorption of 2, 4-dinitrophenol from aqueous solution using activated carbon derived from olives stones and cotton cake. Afr J Environ Sci Technol 13(9):365–380
Chan L, Cheung W, Allen S, McKay G (2012) Error analysis of adsorption isotherm models for acid dyes onto bamboo derived activated carbon. Chin J Chem Eng 20(3):535–542
Chaudhary M, Suhas S, Kushwaha S, Chaudhary I, Tyagi MH, Dehghani BS, Inbaraj JG, Sharma M (2022) Studies on the removal of phenol and nitrophenols from water by activated carbon developed from demineralized Kraft Lignin. Agronomy 12(10):2564
Chowdhary P, Bharagava RN, Mishra S, Khan N (2020) Role of industries in water scarcity and its adverse effects on environment and human health. Environ Concerns Sustain Dev Air Water Energy Resour 1:235–256
Dehmani Y, Alrashdi AA, Lgaz H, Lamhasni T, Abouarnadasse S, Chung I-M (2020) Removal of phenol from aqueous solution by adsorption onto hematite (α-Fe2O3): mechanism exploration from both experimental and theoretical studies. Arab J Chem 13(5):5474–5486
Dehmani Y, Dridi D, Lamhasni T, Abouarnadasse S, Chtourou R, Lima EC (2022) Review of phenol adsorption on transition metal oxides and other adsorbents. J Water Process Eng 49:102965
Dutta S, Gupta B, Srivastava SK, Gupta AK (2021) Recent advances on the removal of dyes from wastewater using various adsorbents: a critical review. Mater Adv 2(14):4497–4531
El-Maghrabi HH, Hosny R, Ramzi M, Zayed MA, Fathy M (2019) Preparation and characterization of novel magnetic ssZNFe2O4–hydroxyapatite core-shell nanocomposite and its use as fixed bed column system for removal of oil residue in oily wastewater samples. Egypt J Pet 28(2):137–144
Fathy M, Moghny TA, Mousa MA, El-Bellihi A-HA, Awadallah AE (2017) Synthesis of transparent amorphous carbon thin films from cellulose powder in rice straw. Arab J Sci Eng 42:225–233
Fathy M, Hosny R, Keshawy M, Gaffer A (2019) Green synthesis of graphene oxide from oil palm leaves as novel adsorbent for removal of Cu(II) ions from synthetic wastewater. Graphene Technol 4(1):33–40
Fseha YH, Shaheen J, Sizirici B (2023) Phenol contaminated municipal wastewater treatment using date palm frond biochar: optimization using response surface methodology. Emerg Contamin 9(1):100202
Ganguly P, Sarkhel R, Das P (2020) Synthesis of pyrolyzed biochar and its application for dye removal: Batch, kinetic and isotherm with linear and non-linear mathematical analysis. Surfaces and Interfaces 20:100616
Haydari I, Aziz K, Kaya S, Daştan T, Ouazzani N, Mandi L, Aziz F (2023) Green synthesis of reduced graphene oxide and their use on column adsorption of phenol from olive mill wastewater. Process Saf Environ Protect 170:1079–1091
Hidayat ARP, Sulistiono DO, Murwani IK, Endrawati BF, Fansuri H, Zulfa LL, Ediati R (2021) Linear and nonlinear isotherm, kinetic and thermodynamic behavior of methyl orange adsorption using modulated Al2O3@UiO-66 via acetic acid. J Environ Chem Eng 9(6):106675
Hosny R, Fathy M, Ramzi M, Abdel Moghny T, Desouky SEM, Shama SA (2016) Treatment of the oily produced water (OPW) using coagulant mixtures. Egypt J Pet 25(3):391–396
Hosny R, Moubark M, Abdelraheem OH, Zayed M (2019) Utilization of cross-linked chitosan/ACTF biocomposite for softening hard water: optimization by adsorption modeling. Egypt J Chem
Hosny R, Mohamed AMG, Abdelraheem O, Hussein MF, Mubarak MF, Ahmed HA (2023) Synthesis, characterization, and adsorption study of magnetic superhydrophobic monolithic core-shell polystyrene composite for the removal of ethyl naphthalene from produced water using fixed column bed. Egypt J Chem
Ighalo JO, Omoarukhe FO, Ojukwu VE, Iwuozor KO, Igwegbe CA (2022) Cost of adsorbent preparation and usage in wastewater treatment: a review. Clean Chem Eng 3:100042
Ismail AI (2015) Thermodynamic and kinetic properties of the adsorption of 4-nitrophenol on graphene from aqueous solution. Canad J Chem 93(10):1083–1087
Iwanow M, Gärtner T, Sieber V, König B (2020) Activated carbon as catalyst support: precursors, preparation, modification and characterization. Beilstein J Organ Chem 16(1):1188–1202
Jain M, Khan SA, Sahoo A, Dubey P, Pant KK, Ziora ZM, Blaskovich MAT (2022) Statistical evaluation of cow-dung derived activated biochar for phenol adsorption: adsorption isotherms, kinetics, and thermodynamic studies. Bioresour Technol 352:127030
Jaspal D, Malviya A (2020) Composites for wastewater purification: a review. Chemosphere 246:125788
Karri RR, Sahu J, Jayakumar N (2017) Optimal isotherm parameters for phenol adsorption from aqueous solutions onto coconut shell based activated carbon: error analysis of linear and non-linear methods. J Taiwan Inst Chem Eng 80:472–487
Khandaker S, Toyohara Y, Saha GC, Awual MR, Kuba T (2020) Development of synthetic zeolites from bio-slag for cesium adsorption: kinetic, isotherm and thermodynamic studies. J Water Process Eng 33:101055
Lin J, Wang L (2009) Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front Environ Sci Eng China 3:320–324
López-Luna J, Ramírez-Montes LE, Martinez-Vargas S, Martínez AI, Mijangos-Ricardez OF, González-Chávez MDCA, Carrillo-González R, Solís-Domínguez FA, Cuevas-Díaz MDC, Vázquez-Hipólito V (2019a) Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl Sci 1:1–19
López-Luna J, Ramírez-Montes LE, Martinez-Vargas S, Martínez AI, Mijangos-Ricardez OF, González-Chávez MDCA, Carrillo-González R, Solís-Domínguez FA, Cuevas-Díaz MDC, Vázquez-Hipólito V (2019b) Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl Sci 1(8):950
Lütke SF, Igansi AV, Pegoraro L, Dotto GL, Pinto LAA, Cadaval TRS (2019) Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J Environ Chem Eng 7(5):103396
Majumder S, Sardar M, Satpati B, Kumar S, Banerjee S (2018) Magnetization enhancement of Fe3O4 by attaching onto graphene oxide: an interfacial effect. J Phys Chem C 122(37):21356–21365
Matera S, Schneider WF, Heyden A, Savara A (2019) Progress in accurate chemical kinetic modeling, simulations, and parameter estimation for heterogeneous catalysis. Acs Catal 9(8):6624–6647
Mohammadi SZ, Darijani Z, Karimi MA (2020) Fast and efficient removal of phenol by magnetic activated carbon-cobalt nanoparticles. J Alloys Compd 832:154942
Mojoudi N, Mirghaffari N, Soleimani M, Shariatmadari H, Belver C, Bedia J (2019) Phenol adsorption on high microporous activated carbons prepared from oily sludge: equilibrium, kinetic and thermodynamic studies. Sci Rep 9(1):19352
Moubark M, Hosny R (2021) Comparative analysis on adsorption properties and mechanisms of nitrate and phosphate ions by a Zn Fe3O4/SiO2 MCM-48 magnetic composite: kinetic and isotherm studies, pp 247–261
Nirmala G, Murugesan T, Rambabu K, Sathiyanarayanan K, Show PL (2021) Adsorptive removal of phenol using banyan root activated carbon. Chem Eng Commun 208(6):831–842
Papaoikonomou L, Labanaris K, Kaderides K, Goula AM (2021) Adsorption–desorption of phenolic compounds from olive mill wastewater using a novel low-cost biosorbent. Environ Sci Pollut Res 28:24230–24244
Pezoti O, Cazetta AL, Bedin KC, Souza LS, Martins AC, Silva TL, Júnior OOS, Visentainer JV, Almeida VC (2016) NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem Eng J 288:778–788
Priyadarshini B, Patra T, Sahoo TR (2021) An efficient and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: kinetics, thermodynamics and isotherm studies. J Magnes Alloys 9(2):478–488
Puccia V, Avena MJ (2021) On the use of the Dubinin–Radushkevich equation to distinguish between physical and chemical adsorption at the solid-water interface. Colloid Interface Sci Commun 41:100376
Qiao K, Tian W, Bai J, Dong J, Zhao J, Gong X, Liu S (2018) Preparation of biochar from Enteromorpha prolifera and its use for the removal of polycyclic aromatic hydrocarbons (PAHs) from aqueous solution. Ecotoxicol Environ Saf 149:80–87
Qiu Y, Ali S, Lan G, Tong H, Fan J, Liu H, Li B, Han W, Tang H, Liu H (2019) Defect-rich activated carbons as active and stable metal-free catalyst for acetylene hydrochlorination. Carbon 146:406–412
Ramos RL, Lebron YAR, Moreira VR, de Souza Santos LV, Amaral MCS (2021) Phenolic compounds in surface water: methodology and occurrence in Doce River, Brazil. Environ Monitor Assessm 193:1–16
Romero-Gonzalez J, Peralta-Videa J, Rodrıguez E, Ramirez S, Gardea-Torresdey J (2005) Determination of thermodynamic parameters of Cr (VI) adsorption from aqueous solution onto Agave lechuguilla biomass. J Chem Thermodyn 37(4):343–347
Said KAM, Ismail AF, Karim ZA, Abdullah MS, Hafeez A (2021) A review of technologies for the phenolic compounds recovery and phenol removal from wastewater. Process Saf Environ Protect 151:257–289
Sam SP, Tan HT, Sudesh K, Adnan R, Ting ASY, Ng SL (2021) Phenol and p-nitrophenol biodegradations by acclimated activated sludge: influence of operational conditions on biodegradation kinetics and responding microbial communities. J Environ Chem Eng 9(4):105420
Saravanakumar K, Kumar A (2013) Removal of phenol from aqueous solution by adsorption using zeolite. Afr J Agric Res 8(23):2965–2969
Sarker N, Fakhruddin A (2017) Removal of phenol from aqueous solution using rice straw as adsorbent. Appl Water Sci 7:1459–1465
Strelkov V, Shirkunov A, Ryabov V, Chuchalina A (2021) Granulated activated carbon production based on petroleum coke. In: IOP conference series: earth and environmental science. IOP Publishing
Surekha G, Krishnaiah KV , N. Ravi and R. P. Suvarna (2020). FTIR, Raman and XRD analysis of graphene oxide films prepared by modified Hummers method. Journal of Physics: Conference Series, IOP Publishing.
Wadhawan S, Jain A, Nayyar J, Mehta SK (2020) Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: a review. J Water Process Engi 33:101038
Wang X, Hu Y, Min J, Li S, Deng X, Yuan S, Zuo X (2018) Adsorption characteristics of phenolic compounds on graphene oxide and reduced graphene oxide: a batch experiment combined theory calculation. Appl Sci 8(10):1950
Yadav N, Narayan Maddheshiaya D, Rawat S, Singh J (2020) Adsorption and equilibrium studies of phenol and para-nitrophenol by magnetic activated carbon synthesised from cauliflower waste. Environ Eng Res 25(5):742–752
Yeganeh M, Farzadkia M, Jafari AJ, Sobhi HR, Esrafili A, Gholami M (2022) Application of a magnetic solid-phase extraction method using a novel magnetic metal organic framework nanocomposite for extraction of malathion and diazinon pesticides from environmental water samples. Microchem J 183:108082
Zhang B, Li F, Wu T, Sun D, Li Y (2015) Adsorption of p-nitrophenol from aqueous solutions using nanographite oxide. Colloids Surf A Physicochem Eng Aspects 464:78–88
Acknowledgements
The authors Acknowledgements the Academy of Scientific Research and Technology (ASRT), for financial support provided through the Scientists for Next Generation (SNG) scholarship, and the Egyptian Petroleum Research Institute (EPRI) for continued support and assistance.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The funding provided by the Academy of Scientific Research & Technology (ASRT), as represented by the Scientists for Next Generation scholarship (ASRT-SNG). This work was financially supported by the Egyptian Petroleum Research Institute (EPRI), Egypt.
Author information
Authors and Affiliations
Contributions
The student, SSG, prepared the compounds and confirmed the description of the prepared materials using different techniques, and studied the factors that affected the efficiency of the prepared compounds, to remove phenol and PNP from industrial wastewater. Each of, Dr. MFM, Dr. MAl-K, Prof. TA-M and Prof. ITEl-S, have developed the research plan, supervised its implementation methodology, interpreted the results, and wrote and reviewed the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to publish and consent to participate
Not applicable.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gabr, S.S., Mubarak, M.F., Keshawy, M. et al. Linear and nonlinear regression analysis of phenol and P-nitrophenol adsorption on a hybrid nanocarbon of ACTF: kinetics, isotherm, and thermodynamic modeling. Appl Water Sci 13, 230 (2023). https://doi.org/10.1007/s13201-023-02018-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-02018-w