Abstract
CuCoFe2O4@Activated Carbon (AC) was synthesized by a fast, simple, and green microwave-assisted coprecipitation method, and then used as a new heterogeneous magnetic nanocatalyst in Fenton-like reaction for ciprofloxacin (CIP) degradation from aqueous media. CuCoFe2O4@AC was characterized by Field emission scanning electron microscopy (FE-SEM), Energy-Dispersive Spectroscopy (EDS), Mapping, Line scan, Fourier-transform infrared spectroscopy (FT-IR), Thermal gravimetric analysis (TGA), X-Ray diffraction analysis (XRD), vibrating-sample magnetometer (VSM), and Brunauer–Emmett–Teller (BET) techniques. The characterization results showed that the CuCoFe2O4@AC nanocomposite was in the ferrite phase with a mesoporous, uniform, quasi-spherical surface and a particle size of about 25 nm. The total volume of single-point adsorption pores was equal to 0.22 cm3 g−1 and the specific surface area was determined to be 199.54 m2 g−1. This nanocomposite had good thermal stability with high magnetic strength. In the presence of H2O2, the synthesized nanocomposite provided a Fenton-like reaction for CIP removal from aqueous solutions. The investigation of this process showed that neutral pH, 1 g L−1 of the nanocomposite, and 73.5 mM of H2O2 were the optimal conditions for CIP removal with an initial CIP concentration of 20 mg L−1. The maximum removal efficiency of 95.77% was attained after 120 min of contact time under the optimum conditions. The CIP degradation during this Fenton-like process followed a pseudo-first-order kinetic model with rate constants (Kapp) of 0.01 min−1. Finally, the CIP removal efficiency after 5 cycles of recovery and regeneration of CuFe2O4@AC was 87.65%. The excellent performance and high catalytic activity of CuCoFe2O4@AC in Fenton-like reaction for CIP removal make it have potential application foreground in the treatment of pharmaceutical wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pharmaceutical compounds are administrated to humans and animals and mainly include micro-pollutants that pass from the body through urine or feces without any changes. A similar process occurs to these compounds alongside other organic and inorganic chemicals in the sewage. Evidence shows that antibiotics and similar medications with low concentrations (mg L−1 to ng L−1) are present in wastewater, sediments, soil, and water sources (surface and groundwater, drinking water, and ocean water). Available data suggests that eliminating these contaminants from water and wastewater through current treatment techniques does not tend to be helpful (Fadário-Frade et al. 2014). According to the current body of research, aquatic reservoirs contain persistent pharmaceuticals (Mohammed et al. 2018). In addition, Sewage sludge can absorb Fluoroquinolone drugs, such as Ciprofloxacin, Norfloxacin, and Ofloxacin. Furthermore, according to a recent study, Fluoroquinolones commonly exist in plant effluents used in wastewater trials (Vieno et al. 2006). Owing to its widespread bactericidal activity, the fluoroquinolone antibiotic, namely, Ciprofloxacin (CIP), is frequently used in hospitals to deal with bacterial infections. Additionally, due to its high-water solubility, only 25% of this antibiotic is absorbed by the body, with the remainder being flushed into the sewage. Moreover, it contaminates surface and subsurface waters due to its great stability, endurance, and low environmental degradation (Nasiri et al. 2022). Many investigations have shown varying levels of CIP, including 249–405 ng L−1 in Swiss home sewage, an average concentration of 0.02 g L−1 in US surface streams, 0.7–124.5 g L−1 in hospital wastewater in Switzerland, and 45–568 ng L−1 in sewage and Swiss wastewater treatment plants. In wastewater treatment plants, this antibiotic is removed with an efficiency of 79–87% (Mohammed et al. 2018). However, the environmental behavior of quinolone antibiotics has turned to a global problem. By fostering the growth of bacteria resistant to antibiotics, negative consequences can be observed in the ecosystem and human health (Peng et al. 2017). Fluoroquinolones are efficacious against a variety of harmful bacterial species as they selectively limit the synthesis of bacterial DNA (Fadário-Frade et al. 2014). Due to their high adsorption in minerals and organic matter, these chemicals quickly move from water bodies to the soil and sediments. In addition to being antibiotic-resistant, Fluoroquinolones have adverse eco-toxicity effects and may be responsible for a sizable amount of detected bacterial genotoxicity in hospital effluents (Fadário-Frade et al. 2014). Furthermore, fluoroquinolones have the capacity to develop drug resistance by changing the drug target through mutation (Mohammed et al. 2018). Effective treatment techniques are warranted to remove low quantities of this antibiotic from contaminated wastewater before its spread to the environment (Pandey et al. 2022).
Numerous purification techniques have been used to remove antibiotics from wastewater. Special attention has been devoted to adsorption as an appealing and advantageous removal method due to its ease of use, a broad range of applications, and straightforward regeneration. For example, activated carbon has been considered a dominant absorbent in removing antibiotics from wastewater due to certain features, such as excellent selectivity, usage simplicity, an abundance of developed internal pore structure, and large specific surface area (Peng et al. 2017) CIP and other antibiotics have been removed from wastewater using a variety of physical, chemical, and biological techniques, such as membrane separation (Calero-díaz et al. 2017), ion exchange resin (Am et al. 2015), nano-filtration (Doğan 2016), oxidation (Nekouei 2018; Malakootian et al. 2019; Khani 2020; Revathi 2019), and adsorption processes (Peng et al. 2017; Sbardella et al. 2018). Due to the lack of complete pollutant degradation, limited efficiency, unreasonable investment and management costs, and inadequate maintenance, the majority of these approaches are often expensive (Malakootian et al. 2020; Nasiri et al. 2022). Advanced Oxidation Processes (AOPs) have been extensively tested and employed in removing persistent organic pollutants (POPs) from contaminated water (Pandey et al. 2022; Thabet et al. 2023; Thabet et al. 2020; Tony 2022, Tony and SM. 2019;).
As an AOP, the Fenton-based oxidation system has drawn a lot of attention due to its capacity to eliminate POPs from aqueous solutions (Fadário-Frade et al. 2014). H2O2 and Fe (II) salt are combined in this process, and they exhibit potent oxidizing characteristics for most organic molecules under specific circumstances (Fenton 1894). The Fenton procedures are preferable to other processes due to their POP degradation, application at room temperature and atmospheric pressure, and accessible reagents. Moreover, the Fenton process can be combined with various water and wastewater treatment processes. For example, it can be integrated with coagulation, filtration and biological oxidation processes. In addition, it has the fastest reaction time among advanced oxidation processes. Nonetheless, the low pH requirement of the reaction mixture is one of the drawbacks of the traditional Fenton reaction (≈ 3) (Ziembowicz and Kida 2022).
This issue can be resolved using metal ferrites and accelerating the reaction rate (Fadário-Frade et al. 2014; Mohammed et al. 2018). Due to their limited solubility and high oxidative characteristics, ferrites constituting transition metals can be utilized to oxidase contaminants (Torre et al. 2018). Ferrites made from transition metals are frequently employed as magnetic materials, semiconductors, pigments, and catalysts (Torre et al. 2018). These structures are classified into hexagonal, garnet and spinel types according to the crystallization form they take. Examples that can be cited are MFe12O19, M3Fe5O12, and MFe2O4, respectively. With the explanation that the letter M refers to one or more divalent intermediate metals (Mn, Fe, Co, Ni, Cu and Zn) (Pandey et al. 2022). The transition metals contribute to ferrite structure and distribution and shape their catalytic capabilities. Consequently, ferrites play effective roles in phenol hydroxylation, crude hydrodesulfurization, compound oxidation, and alkylation processes. Besides, they encompass magnetic qualities, making it easy to recover them with a magnet. The spinel structure of copper and cobalt ferrites typically has a mixed distribution of Cu2+ and CO2+ ions, which are dispersed in both the tetrahedral (A) and octahedral (B) orientations. At normal temperature, the Fe3+ ions are evenly distributed in places A and B, which is the mean of the inverse structure, while the Cu2+ or CO2+ ions occupy position B. This distribution is affected by temperature. The Cu2+ or CO2+ ions occupy position A at temperatures above 360 °C, and the spinel has a regular shape that enhances the ferrite's magnetic characteristics (Torre et al. 2018).
To deal with wastewater, a number of composites consisting of biomass or active carbon have been used recently. The ability of these composites to separate these products or reagents without the processes like filtration, centrifugation, or decantation is of paramount significance. These composites can be thermally revived if they lose their activity. The most recent developments in green chemistry, clean technology, and sustainability led to heterogeneous catalysis, which resolved such issues to a great extent (Torre et al. 2018). Graphene oxide (GO), ordered mesoporous carbon (OMC), fullerene (C60), carbon nanotubes (CNTs), and activated carbon (AC) are the main types of carbon materials. Advantageous characteristics of carbon materials include wide specific surface area, good conductivity, and chemical stability (Zhao et al. 2017; Navalon et al. 2011; Patnaik et al. 2016). This toxically harmful reagent might be converted into more environmentally friendly compounds using activated carbon. Moreover, activated carbon can notably contribute to creating catalysts that can operate as oxidizing agents (24). Several magnetic nanocatalysts have been created thus far and utilized in the AOPs as heterogeneous nanocatalysts, including CuFe2O4/GO (Noroozi et al. 2020), CoFe2O4/AC (Ma et al. 2020), Fe3O4@AC (Liu et al. 2020), Fe3O4/GO (Pervez et al. 2020), Fe3O4@Alg-Fe (Ahmadi H 2016), Carbon dots/Fe3O4@Carbon sphere (Chang et al. 2020), Fe3O4@His@Ag (Amir et al. 2015), Urea/TiO2/ZnFe2O4/Clinoptilolite (Aram et al. 2020), Cu2S/Ag2S/BiVO4 (Fakhravar et al. 2020), Fe3O4@SiO2@TiO2 (Sobhi et al. 2020), Fe3O4@Al2O3-PMO (Ammar et al. 2020), MnFe2O4/BiVO4 (Cam et al. 2021), ZnFe2O4@MC (Tamaddon et al. 2020a), CoFe2O4@MC (Nasiri et al. 2022; Malakootian et al. 2019a), CuFe2O4@MC (Nasiri et al. 2022; Tamaddon et al. 2020b), ZnFe2O4@CMC (Malakootian et al. 2019b, 2019c).
As previous research suggests, efficient technologies should be designed and implemented to remove micro-pollutants from the aquatic environment. In order to treat wastewater containing POPs, additional research studies should design nanocatalysts with sufficient foundations and create industrial-grade materials. According to the literature review, CuCoFe2O4@AC has not been synthesized nor used as the heterogeneous magnetic nanocatalyst to CIP degradation from aqueous environments by the Fenton-like advanced oxidation process. In this study, to create a new magnetic heterogeneous nanocatalyst, CuCoFe2O4@AC magnetic nanocomposite was created and probed its structural and stability features. This nanocatalyst was implemented in the Fenton-like process to remove CIP from aqueous media.
Method and materials
Chemicals
Chemical materials including Cobalt (II), Chloride hexahydrate (CoCl2.6H2O), Iron (III), Chloride hexahydrate (FeCl3.H2O), Copper (II), Chloride dihydrate (CuCl2.2H2O), Sodium hydroxide (NaOH), Sulfuric acid (H2SO4), Activated carbon (AC), and H2O2 were purchased from Sigma Aldrich and Merck Companies (USA and Germany). Double distilled water was used to make all the solutions. The solutions' pHs were adjusted by HCl (1N) and (NaOH) (1N), and measured with a digital pH meter (Jenway meter 3510).
Instrumentation
The catalyst was characterized using XRD, BET, FESEM, EDS-Mapping and Line scan, FTIR, VSM, and TGA techniques. XRD (PHILIPS PW1730) to define the crystal structure and phase of the catalyst, FESEM (TESCAN MIRA III) to observe the morphology, composition, and surface characteristics of the catalyst on the nanoscale, EDS, Mapping and Line scan (TESCAN MIRA II, SAMX Detector) to evaluate element weight (%), types, and distribution patterns at the surface of the catalyst, FTIR (AVATAR, Thermo) to evaluate chemical bonding in catalyst and define functional groupings, VSM (LBKFB, Kashan Kavir Magnet Company) to assess the magnetic properties of the catalyst, BET (BELSORP MINI II) to measure surface area and pore size, and TGA (TGA8000-PerkinElmer) to evaluate the thermal resistance of the catalyst at high temperatures. To evaluate CIP concentrations, a UV–vis spectrophotometer (S2100 Series UV/Vis Spectrophotometers) was used (λmax = 275 nm).
Preparation of CuCoFe2O4@AC
The CuCoFe2O4@AC was synthesized according to previous method with some modifications (Torre et al. 2018). In order to synthesize CuCoFe2O4@AC magnetic nanocatalyst, initially Iron (III) chloride hexahydrate (FeCl3.6H2O), Cobalt (II) chloride hexahydrate (CoCl2.6H2O), and Copper (II) chloride dihydrate (CuCl2.2H2O) with a molar ratio of 2:0.5:0.5 was dissolved in 100 mL distilled water (DW). Then 1 g of activated carbon (AC) was added to the reaction vessel. Subsequently, NaOH was added to the resulting suspension for 1 h until the solution pH reached 11. After one hour of stirring, the reaction vessel was transferred to the microwave oven (SAMSUNG, 2450 MHz, 800 W) and irradiated by microwave waves in three periods of 5 min with 450 W power of the microwave. The obtained product was a light and black powder that was washed many times using DW and dried in the oven (70 °C within 24 h). Figure 1 schematically depicts the development process for the heterogeneous magnetic nanocatalyst CuCoFe2O4@AC.
Determination of the pH point of zero charge (pHzpc)
Adhering to a solid addition method, the pHZPC of CuCoFe2O4@AC nanocomposite was determined (Balistrieri and Murray 1981). To this end, conical flasks were filled with 50 mL of a 0.01 M KNO3 solution. By adding 0.1 M HCl or NaOH solutions, the solutions' initial pH was sat between 2 and 9. Then 1 g of CuCoFe2O4@AC nanocomposite was added to each flask and stirred for 24 h, and the final pH of the solutions was obtained. The value of pHZPC can be detected through the curve that crosses the pH0 line in the pH–pH0 plot.
Batch degradation experiments
Process parameters such as pH, CuCoFe2O4@AC nanocatalyst dose, H2O2 concentration, initial concentration of CIP, and contact time were investigated and optimized. The efficiency of each process of nanocomposite, hydrogen peroxide, nanocomposite with hydrogen peroxide was evaluated separately.
After creating a stock solution of CIP at a concentration of 50 mg L−1, the initial solution was diluted to create 40, 30, 20, 10, 5, 3, and 1 mg L−1 concentrations. At pH levels of 3, 5, 7, 9, and 11, various catalyst dosages (0.1, 0.3, 0.6, 0.8, and 1 g L−1) and hydrogen peroxide concentrations (2.94, 5.88, 14.67, 29.4, 44.1, 58.8, and 73.5 mM) were examined. The trials were carried out at contact times of 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, and 120 min and, ultimately, the optimal contact time was detected. The removal efficiency (Eq. 1) was then determined:
where, C0 and Ct (mg L−1) represent the CIP concentration before and after the reaction, respectively (41).
After determining the optimal conditions for synthetic samples, under optimal conditions, the removal efficiency was determined with the Kerman University of Medical Sciences wastewater sample (Kerman, IRAN) as a real sample. Real wastewater samples were obtained before entering the chlorination tank and their physicochemical properties were determined.
Result and discussion
Characterization of CuCoFe2O4@AC magnetic nano-heterogeneous catalyst
Fourier-transform infrared spectroscopy (FTIR)
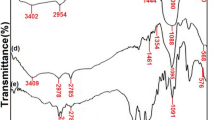
An FTIR analysis was used to discover functional groups and adsorption bonds constituting the chemical structure of the magnetic nanocomposite (Sharifi et al. 2022). According to Fig. 2, the FTIR spectroscopy was carried out to this end with a frequency range of 400–4400 cm−1. As it was found in the FT-IR analysis, the stretching vibrations of the hydroxyl O–H group included a wavelength of 3431 cm−1 (Sharifi et al. 2022; Joshi et al. 2019), the aliphatic C–H group showed a wavelength of 2923 cm−1, and the asymmetric stretching vibrations of the methylene (–CH2) group had a wavelength of 2854 cm−1 (Sharifi et al. 2022; Joshi et al. 2019). The presence of C = C groups is responsible for the adsorption bond at the wavelength of 1627 cm−1 (Sharifi et al. 2022; Parlayıcı and Pehlivan 2017). The spinel metal ferrite structure of the CuCoFe2O4@AC catalyst and its strong and sharp adsorption band at 596 cm−1 and a weak band at 472 cm−1 corresponds to the stretching vibrations of metal oxide in the octahedral and tetrahedral structure of spinel ferrite (Sharifi et al. 2022; Joshi et al. 2019). This finding is consistent with the studies of Sharifi et al., (Sharifi et al. 2022), Rajabi et al., (2022), Joshi et al., (2019), Wang et al., (2021), and Parlayıcı et al., studies (Parlayıcı and Pehlivan 2017).
Field-emission scanning electron microscopy (FESEM)
The FESEM analysis of the CuCoFe2O4@AC nanocatalyst is illustrated in Fig. 3. At a particle size of about 25 nm, it is quite evident that this nanocatalyst was nearly homogeneous, uniform, practically spherical, and with the least amount of agglomeration in terms of particle morphology. Sharifi et al. (Sharifi et al. 2022) reported a similar finding in this respect.
Energy-dispersive X-ray spectroscopy (EDS)
To determine component types and their weight in the synthesized nanocomposite, an EDS analysis was carried out. Figure 4 displays the CuCoFe2O4@AC EDS spectrum. According to the EDS analysis results, there were 87.10% carbon, 8.30% oxygen, 3.80% iron, 0.70% cobalt, and 0.20% copper in the chemical structure of the nanocatalyst, which confirmed the successful synthesis of the nanocatalyst.
Elemental mapping & line scan
In Fig. 5, CuCoFe2O4@AC's elemental mapping pictures (a) and line scan spectra (b) are demonstrated. The components of the CuCoFe2O4@AC magnetic nanocatalyst particles were almost consistently distributed in the structure, as our mapping study confirmed. Based on the results, it could be concluded that the constituent elements on the catalyst's surface were distributed uniformly due to the consistent distribution of copper, iron, cobalt, oxygen, and carbon. A line scan analysis was run across different parts of the CuCoFe2O4@AC magnetic nanocatalyst to confirm EDS and mapping analyses.
X-Ray Diffraction (XRD)
The heterogeneous magnetic nanocomposite CuCoFe2O4@AC's crystal structure and phase were described using the XRD analysis. Figure 6 displays the results that were attained. The structure of activated carbon is amorphous. Activated carbon showed a modest peak at 2θ of 18.32◦ in the XRD pattern (Palanichamy 2013). CuCoFe2O4@AC magnetic nanocomposite's crystal structure in the ferrite phase was substantiated by the strong and distinct peaks at 2θ values of 30.15°, 35.54°, 43.15°, 53.64°, 57.09°, and 66.62° (Joint Committee on Powder Diffraction Standards: JCPDS 96–900-2319). It was further found that the crystal structure of the nanocomposite was maintained after its amalgamation with activated carbon. Using the Scherrer equation (Eq. 2) and the XRD data, it was assessed the average crystal size of the magnetic nanocomposite, which was found to be 16.50 nm.
where, D (nm), β, λ (nm) and θ are the particle diameter, the line broadening at half the maximum intensity X-ray wavelength and Bragg angle.
Vibrating-sample magnetometer (VSM)
VSM analysis of CuCoFe2O4 and CuCoFe2O4@AC was done separately and its results are shown in Fig. 7a, b. The saturation magnetization (Ms), residual magnetism (Mr), and coercivity (Hc) values for CuCoFe2O4@AC were found to be 27.05 emu/g, 8.16 emu/g, and 248 Oe, respectively. The saturation magnetization (Ms) of CuCoFe2O4 (Ms = 53.92 emu/g) and CuCoFe2O4@AC (Ms = 27.05 emu/g) was compared to each other, as it can be seen that by adding activated carbon (AC) to CuCoFe2O4, its magnetic power has decreased, but it still has a high magnetic property to separate from the reaction medium. These results demonstrate that the CuCoFe2O4@AC magnetic nanocomposite possesses strong magnetic properties. Such strong magnetic properties lead to its easy and fast separation from the reaction medium by an external magnet. Therefore, the catalyst could be easily and quickly recovered and regenerated.
Thermogravimetric analysis (TGA)
A TGA analysis was used to examine the weight fluctuations of the CuCoFe2O4@AC heterogeneous magnetic nanocomposite under temperature. The TGA analysis revealed that the magnetic nanocomposite was thermally degraded in two stages. At 20 to 500 °C, the catalyst weight decreased in the initial stage. The catalyst weight declined in the second step at a temperature of 500–600 °C. Overall, the magnetic nanocatalyst's weight was reduced by around 20% up to a temperature of 600 °C. These findings demonstrate adequate thermal stability for the CuCoFe2O4@AC heterogeneous magnetic nanocatalyst (Fig. 8).
Surface area and porosity determinations by Brunauer–Emmett–Tell (BET)
The adsorption/desorption isotherm of CuCoFe2O4@AC nanocomposite are shown in Fig. 9. In the BET study, it was found that the average diameter of absorption pores (4V/A by BET) was 4.37053 nm, the total volume of single point absorption pores (less than 987.808 Å width at p/p = 0.98) was 0.21 cm3/g, and the specific surface area was 199.54 m2 g−1. The International Union of Pure and Applied Chemistry (IUPAC) defines chemistry as follows: Micropores, mesopores, and macropores represent the three types of pores. Mesoporous materials are substances with pores ranging in size from 2 to 50 nm. Materials with pore diameter less than 2 nm and more than 50 nm are called /microporous and macroporous materials, respectively. Thus, as this framework clarifies, CuCoFe2O4@AC magnetic nano-heterogeneous catalyst can be considered in the mesoporous category.
Optimization parameters of CIP degradation
Effect of solution pH on the removal efficiency of CIP
Solid evidence shows that solution pH notably impacts oxidant solubility and activity, reaction kinetics, radical generation, catalyst surface properties, and pollutant levels (Rajabi et al. 2022). In Fig. 10, the impacts of acidic, neutral, and alkaline conditions (pHs 3, 5, 7, 9, and 11) on the effectiveness of the Fenton-like process for CIP removal are shown. In this figure, the initial CIP concentration is 30 mg L−1, the nanocomposite loading is 1 g L−1, and the H2O2 concentration equals 73.5 mM with a 30-min reaction time.
As shown in Fig. 10, the pH of the solution was effective in removing the CIP. As a result, neutral conditions enjoyed prominent removal effectiveness. However, in the traditional Fenton reaction, the reaction mixture had a low pH requirement (about 3) (Ziembowicz and Kida 2022). In this study, it was found that the solution pH influenced the characteristics of the catalyst (pHpzc) and pollutant (pKa). Moreover, the CuCoFe2O4@AC catalyst had a point of zero charge (pHZPC) equal to 8.47. According to pHzpc, the catalyst's surface was positively charged at pH levels lower than 8.47 and negatively charged at higher pH levels. By contrast, CIP had a pka of 6.09, indicating its presence as an anion at pH levels higher than 6.09. CuCoFe2O4@AC and CIP showed a positive charge at the acidic pH (pHs < 6.09), which had a detrimental impact on CIP absorption to the CuCoFe2O4@AC surface. Moreover, CuCoFe2O4@AC and CIP possessed negative charges at the alkaline pH (pHs < 8.47), which had a detrimental impact on CIP absorption to the CuCoFe2O4@AC surface. Nonetheless, CuCoFe2O4@AC and CIP might not create repulsive forces at neutral pH levels. As a result, CIP started to degrade at pH levels other than 6 to 8.5. Given the CIP tendency toward the catalyst surface in this pH range, OH ion production due to the dissociation of water molecules on the catalyst surface is unlikely. As a result, the pH of the solutions increased by 1 unit as the reaction time increased (after 30 min). Due to the repulsive force between CuCoFe2O4@AC and CIP, variations at alkaline and acidic pH values did not significantly affect CIP removal. At pH = 7, non-named loads achieved their maximum value, and electrostatic contact took place, resulting in the highest removal efficiency. It should be observed that the formation of hydroxyl radicals was higher at neutral pH levels than at alkaline or acidic levels. Previous research yielded similar findings in CIP removal by ZnFe2O4@CMC at various solution pH levels (Malakootian et al. 2019b). The production of H2O2 and •OH declines at higher pH levels as an electron cannot form superoxide radical anion (•O2) through oxygen reduction (Eqs. (3) and (4)). As a result, CIP degrades less rapidly.
Effect of the CuCoFe2O4@AC dosage on the removal efficiency of CIP
The Fig. 11 shows the effects of 0.1, 0.3, 0.6, 0.8, and 1 g L−1 CuCoFe2O4@AC with an initial concentration of 30 mg L−1, an H2O2 concentration of 73.5 mM, and a pH of 7 on CIP removal efficiency.
The efficacy of CIP removal increased by raising the concentration of CuCoFe2O4@AC, as shown in Fig. 11. As a result, the highest removal efficiency was found to be 85.37% or 1 g L−1 CuCoFe2O4@AC at a 30-min contact time. Pollutant levels dropped from 30 to ∼4.3 mg L−1 at this stage. Hence, the optimal dosage for the heterogeneous Fenton-like nanocatalyst, namely, CuCoFe2O4@AC, was determined to be 0.1 g in 100 mL of distilled water. The catalyst dosage improved degrading efficiency by offering active sites for the heterogeneous Fenton-like reaction and generating hydroxyl radical. Also, the CIP adsorption was raised with increasing catalyst dosage. The results obtained from investigating the catalyst dose were consistent with Malakootian et al.’s research and confirm these results (Malakootian et al. 2019b).
Effect of the H2O2 concentration
Figure 12 illustrates the effects of 0, 2.94, 5.88, 14.67, 29.4, 44.1, 58.8, and 73.5 mM H2O2 on the CIP removal efficiency at an initial concentration of 30 mg L−1, a CuCoFe2O4@AC loading of 1 g L−1, and a pH of 7 during a 30-min reaction time.
The concentration of hydrogen peroxide was enhanced, which improved the CIP's removal efficiency. At 73.5 mM of hydrogen peroxide, the maximal CIP removal (80.94%) was found. As a result, 73.5 mM of H2O2 was chosen as the ideal dose, which was used for the remaining procedures. Increasing the amount of hydrogen peroxide led to the generation of \({}^{ \bullet }{\text{OH}}\) and \({}^{ \bullet }{\text{HO}}_{{2}}\) free radicals in the reaction medium. This, in turn, increased the effectiveness of pollutant removal, as described the reactions in the preceding section (Ziembowicz and Kida 2022).
Effects of initial concentrations of CIP
Various CIP concentrations (1, 3, 5, 10, 20, 30, 40, and 50) were examined in terms of the removal effectiveness of the Fenton-like process by CuCoFe2O4@AC in the presence of H2O2. Figure 13 presents the results.
The highest removal efficiency (∼86.85%) was detected at the starting concentration of 30 mg L−1 (after 30 min). The Fenton-like process removal efficiency declined as the initial CIP concentrations were lowered. In addition, the pollutant showed the same removal effectiveness at the initial concentration range of 5–50 mg L−1. However, the decrease in the number of accessible sites for contaminant adsorption might contribute to the 6% decline in CIP removal effectiveness at values between 30 and 50 mg L−1 (Malakootian et al. 2018).
Furthermore, it is very likely that the repulsion force in CIP molecules was strong. Moreover, free radicals do not have the capacity to degrade and eliminate the pollutant at greater levels of pollutant concentration. Consequently, the number of hydroxyl radicals in the environment should be increased. Prolonging the reaction time and consuming additional oxidants and catalysts can facilitate this process (Malakootian et al. 2018). Hence, the optimal CIP concentration was considered 30 mg L−1.
Based on Fig. 14, the initial concentration of CIP rose from 1 to 50 mg L−1, and the pseudo-first-order constant degradation for CIP decreased (R2 = 0.8661).
Kinetics models
The rate of contaminant removal improved as reaction time rose. This is due to the fact that CIP degradation in an aqueous solution via the heterogeneous Fenton-like process was associated with reaction time. Figure 15 sketches this outcome.
After 120 min of contact time, the starting CIP concentration of 20 mg L−1 resulted in the greatest removal efficiency (95.774%). In other words, the level of CIP dropped from 20 mg L−1 to 0.845 mg L−1. Moreover, the equilibrium starting point for eliminating different CIP concentrations was discovered to be 20 mg L−1 of the initial concentration, which was 1.286 mg L−1 around 60 min after the reaction began.
It was also probed the kinetic models of the heterogeneous Fenton oxidation process to gain a clear picture of CIP removal procedures. Langmuir–Hinshelwood and pseudo-first-order kinetic models were applied in this respect. Equation (5) was used to calculate the removal coefficient for the pseudo-first-order kinetic model.
where C0, Ct, and Kapp respectively represent initial CIP (mg L−1), CIP at specific reaction times, and the reaction rate constant (min−1) (48).
At various CIP concentrations, the Kapp value was calculated based on the Ln (Ct C0) versus the time curve (Table 1).
In accordance with the rise in CIP concentration up to 50 mg L−1, the Kapp value declines. High correlation coefficient findings (R2 = 0.8461) demonstrate that a pseudo-first-order kinetic model is responsible for oxidizing CIP through heterogeneous Fenton.
The Langmuir–Hinshelwood kinetic model is frequently used to account for heterogeneous catalytic processes. According to this model, pollutant molecules are adsorbed in the catalyst's active areas (Sharifi et al. 2022). Equation (6) represents the Langmuir–Hinshelwood kinetic model for degrading CIP through CuCoFe2O4@AC.
where C0 represents the starting concentration of CIP (mg L−1), Kc shows the rate constant for surface reactions (mg L−1min−1), and KL–H is the equilibrium constant for adsorption in the L–H model (L mg−1). Figure 16 shows the Langmuir–Hinshelwood in the catalytic degradation of CIP (Sharifi et al. 2022).
The initial CIP concentration is plotted against \(K_{obs}^{ - 1}\) in Fig. 17, based on which the Kc and KL–H values were derived.
The surface response rate constant and the Langmuir–Hinshelwood model's adsorption equilibrium constant is represented as KL–H = 0.01 L mg−1 and Kc = 1.80 mg L−1min−1, respectively, in Fig. 16.
The Langmuir–Hinshelwood kinetic model was responsible for catalytically degrading CIP, as the correlation coefficient (R2) of 0.8093 indicates. The pseudo-first-order reaction and Langmuir–Hinshelwood kinetics for the photo-catalytic CIP degradation were described by Malakootian et al. (Malakootian et al. 2019b) and Sharifi et al. (Sharifi et al. 2022).
The synergistic effect of CuCoFe2O4@AC and H2O2
In this study, CuCoFe2O4@AC catalytic capability and the synergistic impacts of the magnetic nanocomposite and H2O2 were assessed. The hydrogen peroxide and the CuCoFe2O4@AC nanocomposite without hydrogen peroxide contributed to removing CIP by 3.4% and 72.91%, respectively, under ideal circumstances, at 30 min reaction time, and with a starting CIP concentration of 30 mg L−1. Moreover, the maximum synergistic impact was to be 86.85%.
Removal of CIP from real wastewater
Real wastewater samples were obtained before entering the chlorination tank, from the Kerman University of Medical Sciences, and their physicochemical properties were determined (Table 2).
In optimal conditions (including the catalyst dose = 1 g L−1, pH = 7, CIP concentration = 20 mg/L, H2O2 = 73.5 mM, and reaction time = 120 min), the efficiency of removing CIP was reported 64.74%. The real wastewater showed a 31% decline in CIP removal effectiveness. Cations and anions such as sulfate, nitrate, and phosphate in real wastewater contributed to this decline in removal efficiency, which plays an inhibitory role in the action of oxidizer radicals and reduces the number of oxidizing radicals in the solution. Also, these cations and anions can occupy the active sites of the catalyst surface and reduce the efficiency of the process (Malakootian et al. 2018). In the study of metronidazole photocatalytic degradation, Tamaddon et al. reported similar results on real wastewater (Tamaddon et al. 2020a).
Proposed mechanisms affecting the processes
Figure 17 depicts the suggested mechanism for degrading CIP through CuCoFe2O4@AC.
Free radicals include the reactants and products of the Fenton reaction as a radical reaction. Due to their high oxidizing potential, \({}^{ \bullet }{\text{OH}}\) radicals produced as a result of the interaction between H2O2 and Fe2+ exhibit great reactivity and strong oxidizing characteristics (Barb et al. 1951; Haber and Weiss 1934). Reaction Eqs. (7) and (15) can be used to elaborate on the Fenton reaction:
However, the Fenton reaction appears to be complicated and involves several reactions represented by Eqs. (7) to (15) (Pandey et al. 2022; Ziembowicz and Kida 2022):
Given that Cu-based materials are known as efficient Fenton-like catalysts in a wide pH range (1, 2, 5), the following reactions may take place when copper is included in the composition of a nanocomposite (Fadário-Frade et al. 2014; Mohammed et al. 2018).
The Fenton process involves the non-selective oxidation of practically all organic molecules by \({}^{ \bullet }{\text{OH}}\) radicals, which produces intermediate, including alcohols, carboxylic acids, aldehydes, water, and carbon dioxide (Ziembowicz and Kida 2022). Based on reactions 18–22 (Ziembowicz and Kida 2022), the oxidation of organic compounds occurs in the Fenton process when generated OH radicals interact with impurity molecules (RH = C17H18FN3O3).
An organic compound molecule loses the hydrogen atom when the radical \({}^{ \bullet }{\text{OH}}\)reacts with it, forming an organic radical (\({}^{ \bullet }{\text{R}}\)). Then, this radical interacts with environmental oxygen, and the radical \({}^{ \bullet }{\text{ROO}}\)is created. Then, the radical converts Fe3+ ions to\({}^{ \bullet }{\text{Fe}}^{{{2} + }}\). The organic substrate molecule and peroxide radical (\({}^{ \bullet }{\text{ROO}}\)) then combine to create another organic radical (\({}^{ \bullet }{\text{R}}\)). The Fenton reaction causes compounds to undergo chemical changes that gradually lower their molecular weight. Equation (24) (Ziembowicz and Kida 2022) presents the breakdown of organic molecules.
Moreover, the Fenton reaction is advantageous since it causes coagulation and oxidation of organic molecules simultaneously. The reaction results in Fe3+ ions as well as \({}^{ \bullet }{\text{OH}}\) radicals, which precipitate as a colloid of Fe (III) hydroxide (Ziembowicz and Kida 2022). Nonetheless, other researchers contend that the ferryl ion (FeO2+) performs the following functions as the oxidizing agent (Ziembowicz and Kida 2022):
Reusability and chemical stability of CuCoFe2O4@AC magnetic heterogeneous nanocatalyst
In AOPs, it is crucial to examine the catalyst's chemical stability and reusability from an economic and environmental standpoint. Figure 18a displays the recovery and redistribution results of the CuCoFe2O4@AC magnetic nanocatalyst after five cycles of use and redistribution. The CIP removal effectiveness dropped from 95.77 to 91.20% in the case of the first catalyst regeneration cycle. The second cycle showed a nearly 4% decrease in removal efficiency, which could be ascribed to the catalyst's active sites being occupied by CIP and its waste during washing. After five cycles of catalyst recovery and regeneration, the CIP removal efficiency dropped to 87.65%. However, it was observed a tolerable efficiency for the catalyst after five stages of recovery and regeneration.
In this study, the chemical stability of CuCoFe2O4@AC magnetic nanocatalyst was examined. To do so, an Atomic Absorption Spectrophotometer (AAS) was used and measured the concentration of copper, iron, and cobalt ions at the wavelengths of 324.8, 240.7, and 248.3 nm, respectively, after their reaction in an aqueous solution in the fifth cycle. The AAS results showed a concentration of 7 ppm for Cu ions, 0.6 ppm for iron ions, and below the device's detection limit for cobalt ions. These results confirm the acceptable chemical stability of the nanocatalyst. FESEM and XRD analyses were run on the CuCoFe2O4@AC magnetic nanocatalyst to verify the chemical stability of the catalyst following five cycles of recovery and regeneration. In Fig. 18b, the findings of these analyses are depicted (c). After five cycles of use and regeneration, the XRD analysis revealed no appreciable changes in the 2θ position and a slight decrease in peak intensity, though the catalyst's crystal structure was still preserved. The results of the FESEM investigation also revealed that, after five cycles of usage and regeneration, the catalyst's morphology did not significantly change. Hence, it can be concluded that this catalyst involves easy recycling and adequate chemical stability.
Comparison of the Fenton-like degradation performance of Ciprofloxacin versus other processes
As shown in Table 3, the present study compared to the mentioned studies has produced a suitable removal efficiency in a short period of time.
Conclusion
This study used an easy and environmentally friendly microwave-assisted coprecipitation approach using iron, cobalt, and copper salts on activated carbon to create a novel CuCoFe2O4@AC heterogeneous magnetic nanocomposite as a magnetically separable catalyst. Techniques such as FESEM, EDS, Mapping, Line Scanning, FT-IR, XRD, VSM, and BET were used for the characterization of the magnetic nanocomposite. The produced nanocatalyst had a pseudo-spherical structure and a particle size of about 25 nm. CuCoFe2O4@AC also has a high specific surface area (199.54 m2/g), good magnetic strength (Ms = 27.05 emu/g), crystalline structure in the ferrite phase, and high thermal stability. In this study, a Fenton-like process was produced by the CuCoFe2O4@AC nanocomposite in the presence of hydrogen peroxide. The interaction of H2O2 with Fe2+, Cu2+, and Co2+ produces \({}^{ \bullet }{\text{OH}}\) and \({\text{HO}}_{{2}}^{ \bullet }\) radicals, which exhibit great reactivity and robust oxidizing properties due to high oxidizing potentials. Finally, removal efficiency under optimal conditions, (including CIP concentration: 20 mg L−1; CuCOFe2O4@AC dose: 1 g L−1; H2O2 concentration: 73.5 mM; pH = 7; reaction time: 120 min), was achieved at 95.77%. In addition, the surface response rate constant (Kc) and adsorption equilibrium constant (KL–H) of the Langmuir–Hinshelwood model were detected at 0.01 L mg−1 and 1.80 mg L−1min−1, respectively. Also, after five recovery cycles, the regenerated catalyst could be effectively employed for CIP removal owing to its strong chemical stability and reusability. Different kinds of carbon structures such as graphene oxide, multi-wall or single-wall nanotube carbons, graphite, or charcoal can be used to modify various spinel metal ferrites to design functional magnetic nanocatalysts. Modified spinel metal ferrites can be used in environmental remediation or catalysis for the removal of various organic and inorganic pollutants from contaminated water and wastewater.
References
Ahmadi M, Rahmani H, Takdastan A, Jaafarzadeh N, Mostoufi A (2016) A novel catalytic process for degradation of bisphenol A from aqueous solutions: a synergistic effect of nano-Fe3O4@ Alg-Fe on O3/H2O2. Process Saf Environ Prot 104:413–421
Amir M, Kurtan U, Baykal A (2015) Rapid color degradation of organic dyes by Fe3O4@ His@ Ag recyclable magnetic nanocatalyst. J Ind Eng Chem 27:347–353
Ammar S, Elaibi A, Mohammed I (2020) Core/shell Fe3O4@ Al2O3-PMo magnetic nanocatalyst for photocatalytic degradation of organic pollutants in an internal loop airlift reactor. J Water Process Eng 37:101240
Aram M, Farhadian M, Nazar A, Tangestaninejad S, Eskandari P, Jeon BH (2020) Metronidazole and cephalexin degradation by using of Urea/TiO2/ZnFe2O4/Clinoptiloite catalyst under visible-light irradiation and ozone injection. J Mol Liq 304:112764
Bai Z, Yang Q, Wang J (2017) Degradation of sulfamethazine antibiotics in Fenton-like system using Fe3O4 magnetic nanoparticles as catalyst. Environ Prog Sustain Energy 36:1743–1753
Balistrieri LS, Murray JW (1981) The surface chemistry of goethite (–FeOOH) in major ion seawater. Am J Sci 281:788–806
Barb WG, Baxendale JH, George P, Hargrave KR (1951) Reactions of ferrous and ferric ions with hydrogen peroxide. Part I Ferrous Ion Reaction Trans Faraday Soc 47:462–500
Calero-Díaz G, Monteoliva-García A, Leyva-Díaz JC, López-López C, Martín-Pascual J, Torres JC, Poyatos JM (2017) Impact of ciprofloxacin, carbamazepine and ibuprofen on a membrane bioreactor system: kinetic study and biodegradation capacity. J Chem Technol Biotechnol 92(12):2944–2951
Cam N, Pham HD, Pham TD, Phuong T, Van HC, Tung M et al (2021) Novel photocatalytic performance of magnetically recoverable MnFe2O4/BiVO4 for polluted antibiotics degradation. Int Ceram 47(2):1686–1692
Chang S, Zhang Q, Lu Y, Wu S, Wang W (2020) High-efficiency and selective adsorption of organic pollutants by magnetic CoFe2O4/graphene oxide adsorbents: Experimental and molecular dynamics simulation study. Sep Purif Technol 238:58–96
de la Torre E, Lozada AB, Adatty M, Gámez S (2018) Activated carbon-spinels composites for waste water treatment. Metals Basel 8(12):58962
Doğan EC (2016) Investigation of ciprofloxacin removal from aqueous solution by nanofiltration process. Glob Nest J 18(2):291–308
Fadário-Frade VM, Dias M, Costa-Teixeira ACS, Alves-Palma MS (2014) Environmental contamination by fluoroquinolones. Braz J Pharm Sci 50(1):41–54
Fakhravar S, Farhadian M, Tangestaninejad S (2020) Excellent performance of a novel dual Z-scheme Cu2S/Ag2S/BiVO4 heterostructure in metronidazole degradation in batch and continuous systems: immobilization of catalytic particles on α-Al2O3 fiber. Appl Surf Sci 505:144599
Fenton HJH (1894) Oxidation of Tartaric Acid in presence of Iron. J Chem Soc Trans 65:899–910
Haber F, Weiss J (1934) The catalytic compensation of hydrogen peroxide by iron salts. Proc R Soc Lond 147:332–351
Hassani A, Karaca M, Karaca S, Khataee A, Açıs O, Yılmaz B (2018) Preparation of magnetite nanoparticles by high-energy planetary ball mill and its application for ciprofloxacin degradation through heterogeneous Fenton process. J Environ Manage 211:53–62
Joshi S, Garg V, Kataria N, Kadirvelu K (2019) Applications of Fe3O4@ AC nanoparticles for dye removal from simulated wastewater. Chemosphere 236:124280
Khani MR, Mahdizadeh H, Kannan K, Kalankesh LR, Kamarehei B, Baneshi MM, Shahamat YD (2020) Olive mill wastewater (OMW) Treatment by hybrid processes of electrocoagulation/catalytic ozonation and biodegradation. Environ Eng Manag J EEMJ 19(8):1401–1410
Liu L, Yang C, Tan W, Wang Y (2020) Degradation of acid red 73 by activated persulfate in a heat/Fe3O4@ AC system with ultrasound intensification. ACS Omega 5(23):13739–13750
Liu H, Liu Y, Li X, Zheng X, Feng X, Yu A (2022) Adsorption and fenton-like degradation of ciprofloxacin using corncob biochar-based magnetic iron-copper bimetallic nanomaterial in aqueous solutions. Nanomaterials 2(4):12
Ma Q, Nengzi LC, Zhang X, Zhao Z, Cheng X (2020) Enhanced activation of persulfate by AC@ CoFe2O4 nanocomposites for effective removal of lomefloxacin. Sep Purif Technol 233:115978
Malakootian M, Nasiri A, Mahdizadeh H (2018) Preparation of CoFe2O4/activated carbon@chitosan as a new magnetic nanobiocomposite for adsorption of ciprofloxacin in aqueous solutions. Water Sci Technol 78(10):2158–2170
Malakootian M, Pournamdari M, Asadipour A, Mahdizadeh H (2019) Degradation and removal of p-nitroaniline from aqueous solutions using a novel semi-fluid Fe/charcoal micro-electrolysis reactor. Korean J Chem Eng Internet 35(3):1–9. https://doi.org/10.1007/s11814-018-0166-x
Malakootian M, Nasiri A, Alibeigi A, Mahdizadeh H, Gharaghani M (2019a) Synthesis and stabilization of ZnO nanoparticles on a glass plate to study the removal efficiency of acid red 18 by hybrid advanced oxidation process (Ultraviolet/ZnO/ultrasonic). Desal Water Treat 170:325–336
Malakootian M, Nasiri A, Asadipour A, Kargar E (2019b) Facile and green synthesis of ZnFe2O4@CMC as a new magnetic nanophotocatalyst for ciprofloxacin degradation from aqueous media. Process Saf Environ Prot 129:138–151
Malakootian M, Nasiri A, Asadipour A, Faraji M, Kargar E (2019c) A facile and green method for synthesis of ZnFe2O4@CMC as a new magnetic nanophotocatalyst for ciprofloxacin removal from aqueous media. MethodsX 6:1575–1580
Malakootian M, Nasiri A, Heidari MR (2020) Removal of phenol from steel plant wastewater in three dimensional electrochemical (TDE) process using CoFe2O4@AC/H2O2. Zeit Phys Chemie 234(10):1661–1679
Mohammed I, Riyami A, Ahmed M, Al A, Choudri BBS (2018) Antibiotics in wastewaters : a review with focus on Oman. Appl Water Sci 8(7):1–10
Nasiri A, Tamaddon F, Mosslemin M, M. (2019) AF microwave assisted method to synthesize nanoCoFe2O4@methyl cellulose as a novel metal-organic framework for antibiotic degradation. MethodsX 6:1557–63
Nasiri A, Tamaddon F, H MM, Gharaghani MA, Asadipour A. (2019) Magnetic nano-biocomposite CuFe2O4@methylcellulose (MC) prepared as a new nano-photocatalyst for degradation of ciprofloxacin from aqueous solution. Environ Health Eng Manag J 6(1):41–51
Nasiri A, Rajabi S, Amiri A, Fattahizade M, Hasani O, Lalehzari A et al (2022) Adsorption of tetracycline using CuCoFe2O4@Chitosan as a new and green magnetic nanohybrid adsorbent from aqueous solutions: isotherm, kinetic and thermodynamic study. Arab J Chem 15(8):545632
Nasiri A, Rajabi S, Hashemi M (2022) CoFe2O4@Methylcellulose/AC as a New, Green, and Eco-friendly Nano-magnetic adsorbent for removal of Reactive Red 198 from aqueous solution. Arab J Chem 15(5):56478
Navalon S, Dhakshinamoorthy A, Alvaro M, Garcia H (2011) Heterogeneous fenton catalysts based on activated carbon and related materials. Chemsuschem 4:1712–1730
Nekouei F, Nekouei S, Noorizadeh H (2018) Enhanced adsorption and catalytic oxidation of ciprofloxacin by an Ag/AgCl@ N-doped activated carbon composite. J Phys Chem Solids 114:36–44
Noroozi R, Gholami M, Farzadkia M (2020) Catalytic potential of CuFe2O4/GO for activation of peroxymonosulfate in metronidazole degradation: study of mechanisms. J Environ Health Sci Eng 18(1):1–14
Palanichamy KAA (2013) Refuse derived energy—tea derived boric acid activated carbon as an electrode material for electrochemical capacitors. Port Electrochim Acta 31:165–174
Pandey NK, Li HB, Chudal L, Bui B, Amador E, Zhang MB et al (2022) Exploration of copper-cysteamine nanoparticles as an efficient heterogeneous Fenton-like catalyst for wastewater treatment. Mater Today Phys 22:2–14
Parlayıcı S, Pehlivan E (2017) Removal of metals by Fe3O4 loaded activated carbon prepared from plum stone (Prunus nigra): kinetics and modelling study. Powder Technol 317:23–30
Patnaik S, Martha S, Acharya S, Parida KM (2016) An overview of the modification of g-C3N4 with high carbon containing materials for photocatalytic applications. Inorg Chem Front 3:336–347
Peng X, Hu F, Zhang T, Qiu F, Dai H (2017) Amine-functionalized magnetic bamboo-based activated carbon adsorptive removal of ciprofloxacin and norfloxacin: a batch and fixed-bed column study. Bioresour Technol 249:924–934
Pervez M, He W, Zarra T, Naddeo V, Zhao Y (2020) New Sustainable approach for the production of Fe3O4/Graphene oxide-activated persulfate system for dye removal in real wastewater. Water Basel 12(3):733
Rajabi S, Nasiri A, Hashemi M (2022) Enhanced activation of persulfate by CuCoFe2O4@MC/AC as a novel nanomagnetic heterogeneous catalyst with ultrasonic for metronidazole degradation. Chemosphere Internet 286(P3):131872. https://doi.org/10.1016/j.chemosphere.2021.131872
Rajesh AM, Bhatt SA, Brahmbhatt H, Anand PS, Popat KM (2015) Taste masking of ciprofloxacin by ion-exchange resin and sustain release at gastric-intestinal through interpenetrating polymer network. Asian J Pharm Sci 10(4):331–340
Revathi V, Karthik K, Mahdizadeh H (2019) Antibacterial activity and physico-chemical properties of metal-organic single crystal: Zinc (Tris) thiourea chloride. Chem Data Collect 24:100279
Sbardella L, Comas J, Fenu A, Rodriguez-roda I, Weemaes M (2018) Advanced biological activated carbon filter for removing pharmaceutically active compounds from treated wastewater. Sci Total Environ 636:519–529
Sharifi N, Nasiri A, Silva Martínez S, Amiri H (2022) Synthesis of Fe3O4@activated carbon to treat metronidazole effluents by adsorption and heterogeneous Fenton with effluent bioassay. J Photochem Photobiol A Chem 427(January):113845
Sobhi H, Badi MY, Esrafili A, Ghambarian M (2020) Evaluation of the efficiency of a photocatalytic process using the magnetic nanocatalyst (Fe3O4@ SiO2@ TiO2) in the removal of ceftriaxone from aqueous solutions. J Environ Health Eng 7(3):229–243
Tamaddon F, Mosslemin M, Asadipour A, Gharaghani M, Nasiri A (2020a) Microwave-assisted preparation of ZnFe2O4@methyl cellulose as a new nano-biomagnetic photocatalyst for photodegradation of metronidazole. Int J Biol Macromol 154:1036–1049
Tamaddon F, Nasiri A, Yazdanpanah G (2020b) Photocatalytic degradation of ciprofloxacin using CuFe2O4@methyl cellulose based magnetic nanobiocomposite. MethodsX 7:74–81
Thabet RH et al (2023) Magnetite-based nanoparticles as an efficient hybrid heterogeneous adsorption/oxidation process for reactive textile dye removal from wastewater matrix. Int J Environ Anal Chem 103(11):2636–2658
Thabet RH, et al. (2020) Catalytic oxidation over nanostructured heterogeneous process as an effective tool for environmental remediation. In: IOP conference series: materials science and engineering. p. 012004.
Tian Y, He X, Zhou H, Tian X, Nie Y, Zhou Z et al (2020) Efficient fenton-like degradation of ofloxacin over bimetallic Fe-Cu@ Sepiolite composite. Chemosphere 257:127209
Tony MA, Ali IA (2022) Mechanistic implications of redox cycles solar reactions of recyclable layered double hydroxides nanoparticles for remazol brilliant abatement. Int J Environ Sci Technol 19(10):9843–60
Tony M, Mansour SA (2019) Microwave-assisted catalytic oxidation of methomyl pesticide by Cu/Cu2O/CuO hybrid nanoparticles as a Fenton-like source. Int J Environ Sci Technol 17:45236
Vieno N, Tuhkanen T, Kronberg L (2006) Analysis of neutral and basic pharmaceuticals in sewage treatment plants and in recipient rivers using solid phase extraction and liquid chromatography–tandem mass spectrometry detection. J Chromatogr 1134(1–2):101–111
Wang H, Li Z, Yahyaoui S, Hanafy H, Seliem MKA, Bonilla-Petriciolet G et al (2021) Effective adsorption of dyes on an activated carbon prepared from carboxymethyl cellulose: experiments, characterization and advanced modelling. Chem Eng J 417:128116
Zhao Q, Mao Q, Zhou Y, Wei J, Liu X, Yang J et al (2017) Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: a review on heterogeneous catalysts and applications. Chemosphere 189:224–238
Ziembowicz S, Kida M (2022) Limitations and future directions of application of the Fenton-like process in micropollutants degradation in water and wastewater treatment: a critical review. Chemosphere 296(January):1–14
Acknowledgements
This research with project number 400000021 and IR.MUBAM.REC.1400.032 ethic approval cod was sponsored by the Vice Chancellor for Research and Technology, Bam University of Medical Sciences, Bam, Iran. The authors wish to thank Research Deputy of Bam University of Medical Sciences for its financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies involving human, animal, or patient participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pourshaban-Mazandarani, M., Ahmadian, M., Nasiri, A. et al. CuCoFe2O4@AC magnetic nanocomposite as a novel heterogeneous Fenton-like nanocatalyst for Ciprofloxacin degradation from aqueous solutions. Appl Water Sci 13, 179 (2023). https://doi.org/10.1007/s13201-023-02002-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-02002-4