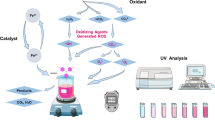
Abstract
MPs are widely found in various environments. PS is the second most common microplastic in sediments, freshwater, soil, and coastal ecosystems. S. cerevisiae was studied as a biocoagulant due to its advantages such as ease of use, non-toxicity, large-scale cultivability and low cost. The aim of this study was to evaluate the efficiency of S. cerevisiae in removing PS from aqueous solutions. BBD was used to determine the optimal removal conditions. The MPs were washed, dried, crushed, sieved, and kept in a closed container to avoid exposure to light and moisture. PS removal was measured under various parameters such as the dose of S. cerevisiae (100–300 mg/L), the concentration of PS (200–900 mg/L), and the pH (4–10). The suspension of PS and S. cerevisiae was stirred and subjected to variable speeds to disperse yeast cells and contact with PS particles. The formed clots were settled under static conditions, and the suspended MPs in the aqueous solution were measured by filtering through Whatman filter paper and recording its weight after drying. The maximum PS removal efficiency was 98.81% under optimized conditions, i.e., the PS concentration of 550 mg/L, the yeast dose of 200 mg/L, and the pH of 7. With regard to the mentioned results, it can be said that S. cerevisiae can be used as a natural and environmentally friendly biocoagulant to remove PS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plastics used throughout life are made of organic and inorganic raw materials such as silicon, nitrogen, hydrogen, carbon, oxygen and chloride. Raw materials for making plastic are extracted from natural gas, coal and oil (Shah et al. 2008). According to the reports, the global production of plastic follows an increasing trend, so that their amount is expected to reach 445.25 million tons in 2025 from 1.5 million tons in 1950 (Rout et al. 2022). Plastics provide durable, lightweight, cheap and strong products, which is why around 300 million tons of plastic products are produced worldwide. Due to low recycling, 90% of plastics are discarded in aquatic environment (Du et al. 2022). Plastics in the environment can break down into smaller particles due to a process called photodegradation, which is caused by exposure to sunlight. This process can cause the plastic to become brittle and crack, leading to the formation of microplastics. Temperature can also play a role in the breakdown of plastics, as higher temperatures can accelerate the rate of degradation. Additionally, other factors such as wind, water, and mechanical stress can also contribute to the fragmentation of plastics into microplastic particles (Bonyadi et al. 2022a, b, c). Microplastic particles were first detected in the Sargasso Sea, an area in the North Atlantic Ocean. The term “microplastic” was first published in the scientific literature by Bayo et al. (2020) (Bayo et al. 2020). As an emerging pollutant, MPs may be more harmful than large plastic (Zhou et al. 2021). These emerging pollutants absorb other pollutants such as pharmaceuticals and personal care products (Esmaili et al. 2023), nanoparticles (Bonyadi et al. 2022a, b, c; Bonyadi et al. 2023), pesticides and herbicides (Pirsaheb et al. 2013), and heavy metals (Davodi et al. 2019). MPs enter the environment in two primary and secondary forms, which enter the environment directly from raw materials, and secondary, which are caused by the decomposition of large plastics by environmental factors such as sunlight, wind, and water (Napper et al. 2015). Due to their small size and sharp ends, MPs cause damage and inflammation in the organs of marine organisms. Also, due to their similarity to natural prey, MPs are ingested by organisms and cause malnutrition and reproductive disorders. In a study, it was demonstrated that MP can be transported through the gut into the circulatory system of aquatic species (Sun et al. 2019). These emerging pollutants accumulate in the body of marine organisms and enter the human body through the food chain (Okoye et al. 2022). MPs in various types, including polystyrene, polyethylene, polyvinyl chloride, polypropylene, and others, are released into seawater, fresh water, coastal waters, sediments, soil, foods, and even the human body [7, 8]. Polystyrene is the second most common microplastic in sediments, freshwater, soil, and coastal ecosystems, widely used in containers, protective packaging, lids, and bottles (Sun et al. 2019; Yilimulati et al. 2021). The mitigation of environmental pollution through the removal of organic pollutants from wastewater has emerged as a promising approach. Over the past few years, several studies have been conducted to explore the degradation of various pollutants through different techniques (Toolabi et al. 2017, 2018). So far, the removal of MPs has been done by different techniques such as membrane ultrafiltration (Gonzalez-Camejo et al. 2023), membrane bioreactor (Bayo et al. 2020), filtration (Bitter et al. 2022), rapid sand filtration (Bitter et al. 2022), sedimentation and disinfection. Among these, methods based on coagulation and flocculation mechanisms are more common due to the formation of large flocs of MPs that are removed from water by sedimentation. In a study, it has been proven that the coagulation process was effective in removing polyethylene MPs (Bayarkhuu et al. 2022). Using chemical coagulants to remove these pollutants causes environmental and health problems. The problems caused by the use of chemical coagulants in water treatment include the production of large amounts of sludge, the indestructibility of chemical compounds, having residues in water, biological accumulation in the body of organisms, and negative effects on human health such as the occurrence of Alzheimer’s disease and dementia (Mazloomi et al. 2018). Therefore, these problems have caused the use of biological coagulants to be considered as a suitable alternative to chemical coagulants. Microorganisms can remove emerging pollutants from aqueous solutions through mechanisms such as biological decomposition, biosorption, and biocoagulation. Biological decomposition breaks down pollutants into simpler compounds, while biosorption involves adsorbing pollutants onto the surface of microorganisms (Nasoudari et al. 2021) The advantage of using biocoagulants includes positive features such as compatibility with the environment, cost-effectiveness, and biodegradability (Amran et al. 2021; Bonyadi et al. 2022a, b, c). Yeast, belonging to the family of unicellular fungi, is widely present in nature (Yang et al. 2014). S. cerevisiae, which is known as baker’s yeast, in the processes of removing pollutants from the environment due to its advantages such as ease of use, non-toxicity, ability to be cultivated on a large scale and low cost (Hadiani et al. 2018). Skaf et al. (2020) investigated the removal of microplastic particles from simulated drinking water through alum (Skaf et al. 2020). Zhang et al. (2020) showed that enzymes extracted from Aspergillus fungus minimize polyethylene microplastic particles through biodegradation (Zhang et al. 2020). Sánchez (2020) investigated the potential of fungi to degrade petroleum polymers. According to its results, fungi can use MPs as a source of carbon and energy. Also, due to having an enzyme system with the ability to detoxify pollutants and the ability of their cells to penetrate three-dimensional layers, it can be said that fungal species have a high potential in reducing microplastic pollution (Sánchez 2020). Cunha et al. (2020) used microalgae based on biopolymers to remove nano and MPs (Cunha et al. 2020). S. cerevisiae is an effective tool for removing pollutants and microplastics from the environment due to its ability to adhere to microplastic particles and remove them through a process called biocoagulation. This yeast has been used in various biotechnological applications (Ramavandi et al. 2019; Sadeghi et al. 2019; Mazloomi et al. 2021), making it a promising and environmentally friendly solution for pollutant removal.
Therefore, the removal of polystyrene MPs by S. cerevisiae from aqueous solutions is important. Further, the characteristics of S. cerevisiae and polystyrene were investigated by FESEM, EDX and FTIR tests.
Materials and methods
Chemicals and reagents
Commercial grade plastic materials were prepared from Pishgaman Plastic Company. S. cerevisiae (ATCC 9763) was obtained from Iran Science and Technology Research Organization. The chemicals were obtained from Merck, Germany.
Preparation of PS MPs
First, the plastic granules were washed with concentrated hydrochloric acid. Then, they were dried in the oven at a temperature of 60 °C for 24 h. Then, the plastic granules were crushed into small particles and sieved in sizes less than 100 µm. The MPs were kept in a closed glass container in a dark environment to avoid exposure to light and moisture.
Design of experiments
In this study, the main parameters, such as pH (4–10), S. cerevisiae dose (100–300 mg/L), and initial PS concentration (200–900 mg/L), were tested (Table 1). The size of microplastic in all samples was less than 100 µm. All experiments were done in a 300-ml flux containing 200 ml of reaction mixture.
All tests were done at room temperature. At first, based on the parameters defined in Table 1, 200 ml of reaction mixture containing the different amounts of PS and S. cerevisiae was prepared. Then, the prepared suspension was subjected to variable speed of 400 rpm for 1 min, to disperse yeast cells and contact with PS particles. Subsequently, in order to promote the formation of sizable clots, the suspension was agitated at 100 rpm for a duration of 15 min. Upon completion of this stage, the mixture was transferred to an Imhof funnel and allowed to settle undisturbed for a period of 30 min, facilitating the formation of clots. The supernatant was filtered using Whatman paper to measure the suspended MPs in the aqueous solution, and then filter paper. It was dried in an oven at 60 °C for 24 h and the weight of the paper was recorded (Zhou et al. 2021). PS removal efficiency was calculated by the following formula:
where M1 and M2 are PS weight before and after the removal process, respectively.
Modeling PS removal
This study was designed applying the response level method, BBD, to optimize the removal efficiency of microplastic by S. cerevisiae. The quadratic model, suggested by BBD, was expressed as the following formula:
In this formula, Y, βi, βij, βii, β0, and xj or xi, respectively, express predicted response, regression coefficients for linear impacts, interaction coefficients, quadratic coefficients, the constant coefficients, and coded values of factors (Anbarani et al. 2023).
Results and discussion
Characterization
FT-IR
Figure 1a illustrates the FT-IR spectra for PS before removal process. The peak at 807.87 cm−1 was associated with the presence of a benzene ring in PS, which disappeared after coagulation (Zhou et al. 2021). The peak at 1656.29 cm−1 indicated the presence of C=O in PS (Yao et al. 2023). The band appeared at 2926.04 cm−1 is related to C–H asymmetric stretching (Lin et al. 2020). The peak at 3377.56 cm−1 was attributed to the O–H stretching vibration (Du et al. 2022). The peak at 1402.20 cm−1 introduces the PS carbon chain. The peak appeared at 1241.07 cm−1, which corresponds to the ester groups in PS (Sun et al. 2021).
Figure 1b represents the FT-IR spectra after the removal process. The C=O peak for the flocs shifted from 1656.29 to 1657.08 cm−1 (Lu et al. 2021). After the elimination process, the O–H peak changed to 3404.22 cm−1 (Chen et al. 2016). The peak at 1152.62 cm−1 indicates ether and ester groups. In fact, O–H and ester provide an active site for the attachment of coagulant particles (Wang et al. 2023; Onukwuli et al. 2021). A new peak appeared at 1601.48 cm−1, which confirms the presence of (C=O–) group. Based on previous studies, (O–H) and (C=O–) groups offer evidence of microplastic removal by yeasts (Zhang et al. 2020). The change in the peak from 1402.20 to 1402.06 cm−1 was caused by a decrease in carbon content in the sludge. The peak at 2923.21 cm−1 represents the C-H group, which did not change significantly compared to before removal.
FESEM
The microscopic morphology of S. cerevisiae and PS after elimination is represented in Fig. 2. According to Fig. 2, PS particles are accumulated on the surface of S. cerevisiae fact, the pores of S. cerevisiae are a suitable space for keeping PS particles (Machado et al. 2008). Hydrophobins are proteins produced by yeasts. Negatively charged MPs can bind to the hydrophobin surface. As a result, large spherical clots are produced. Therefore, it can be said that the surface charge neutralization process has occurred. Usually, due to being hydrophobic, yeasts have unique properties such as high adhesion, firm structure, and suitable surface activity that enable attachment to plastic surfaces. The effect of yeast on plastic includes changes in molecular weight, elasticity, crystallinity and fragmentation of functional groups on the plastic surface (Sánchez 2020). This evidence proves that S. cerevisiae acts as a coagulant.
EDX
The technique of energy-dispersive X-ray spectroscopic patterns (EDX) was used to analyze the elements of PS particles. Figure 3a–b shows the EDX of PS particles before and after removal process. In this analysis, the percentages of carbon, nitrogen, oxygen and phosphorus elements are mentioned. As shown in Fig. 3a, the percentage of carbon in PS particles before the coagulation process was 88.09%, and other elements accounted for small percentages. It can also be seen carefully in Fig. 3b that the percentage of carbon in the mixture of PS and S. cerevisiae has decreased and other elements have increased. So that the amounts of carbon, oxygen, nitrogen and phosphorus in the studied samples were 45.88%, 37.83%, 14.95%, and 1.34%, respectively. The high content of carbon and oxygen facilitates the formation of hydrogen bonds with the coagulant and improves the ability of PS to bridge between clots or absorb them (Yao et al. 2023).
Response model
In this test, the effect of S. cerevisiae on PS removal from aqueous media was investigated. Table 2 indicates PS removal by S. cerevisiae.
Based on Table 2, the lowest and highest PS removal efficiency was 27.77% and 98.81%, respectively. The experimental findings for 2FI, linear, cubic and quadratic models were statistically estimated to determine the best model. Table 3 exhibits the decision of the statistical adequacy of the models.
Table 4 demonstrates the quadratic model coefficients of PS elimination by S. cerevisiae particles. Table 4 shows the fit of the quadratic model (QM) to the experimental data.
From Table 4, the QM of PS removal pursuant to the coded factors is presented in Eq. (3):
Accordingly, each model consists of two variable and fixed components. With regard to the various laboratory parameters, the elimination rate was predicted to be 91.72%. The coded factors of A, B, and C had the coefficients of − 4.31, + 9.44, and + 3.83, respectively. The dose of S. cerevisiae (code B) had the greatest effect on the removal efficiency with a coefficient of 9.44. AB had the highest interaction effect with a coefficient of 11.1 and B2 had the highest square effect with a coefficient of 27.6. Table 5 demonstrates the analysis of variance (ANOVA) for the QM of PS elimination by S. cerevisiae particles.
The values of R2, predicted R2, adjusted R2, and adequacy precision were determined 0.97, 0.80, 0.94 and 18.87, respectively. Based on Table 5, the p value for all the coded factors, including S. cerevisiae dose, PS concentration, and pH, was obtained to be less than 0.05. Therefore, all three variables are significant. In this model, the difference between R2 and predicted R2 was less than 0.2. Therefore, this model is correct. Figure 4 illustrates actual versus predicted removal, showing the adequacy of the model to provide a good prediction for PS removal.
The effect of main parameters on removal efficiency
Figure 5 indicates the impact of important parameters, containing S. cerevisiae dose, PS concentration, and pH and on the removal rate. It is necessary to note that to express the effect of one parameter on a response; other factors are fixed at the zero level. For example, when the pH variable increases from level 1 to + 1, other variables such as S. cerevisiae dose and PS concentration are constant at zero level.
Adsorbent dose effect
Determining the appropriate dose for S. cerevisiae is important. According to Fig. 5a, the highest PS removal efficiency (98.81%) was discovered at the dose of 200 mg/L S. cerevisiae (p value < 0.05). Generally, with the increase in S. cerevisiae dosage, the removal rate of PS enhances. The reason for the low removal efficiency can be explained as small, fragile and unstable flocs are formed in low dosages of yeast. Therefore, the removal of microplates is reduced due to the suspension of flocs and their weak sedimentation. By increasing the dose of S. cerevisiae, yeast cells linked to MPs stick together and form larger flocs, which leads to their rapid sedimentation and high efficiency (Ma et al. 2019). Due to its hydrophobin properties, S. cerevisiae can adhere to hydrophobin substrates through cell penetration into the layers of MPs (Sánchez 2020). Frantz et al. (2020) performed coagulation and flocculation using shrimp waste, and the results showed that the percentage of turbidity removal at a dose of 200 mg/L of coagulant was approximately 100%, but at coagulant doses higher than 200 mg/L, the removal decreased (Frantz et al. 2020). Machado et al. (2008) investigated the removal of heavy metals by S. cerevisiae. The results showed that at the dose of 0.25 g/L, sedimentation was weak, but at the dose of 0.5 g/L, complete sedimentation occurred. This evidence is consistent with the concept of “critical cell density” proposed by Miki et al. 1982 (Miki et al. 1982). “Critical cell density” means that the presence of a low dose of S.cerevisiae cells limits the possibility of attachment and the rate of coagulation is practically zero (Machado et al. 2008). In a study, yeast enzymes could shorten the length of microplastic polyethylene chains and eliminate or reduce MPs (Restrepo-Flórez et al. 2014). In another study, fungi can remove polyurethane and polyethylene better than bacteria (Muhonja et al. 2018; Sánchez 2020).
Effect of initial PS concentration
PS concentration was one of the important factors in PS removal. Figure 5a indicated that the PS removal efficiency was increased by 8.12% from 200 to 500 mg PS concentration and then decreased by 16.73% from 500 to 900 mg/L concentration (p value < 0.05). Considering that PS particles are mostly negatively charged. Therefore, at high concentrations of PS, repulsive forces prevail between particles, which lead to their floating in aqueous solutions. At low concentrations of PS, van der Waals forces between PS particles are dominant due to the bonding with a balanced ratio of yeasts, which results in the production of large and strong flocs. Yao et al. (2023) investigated the microplastic characteristics of PS in the coagulation process. The results showed that when the PS concentration increased from 0.25 to 1 g/L, the removal efficiency was high. While the concentration of PS increased from 1 to 5 g/L, the percentage of PS removal decreased (Yao et al. 2023).
pH effect
The solution pH can affect the process of removing MPs by affecting the balance of negative and positive charges in PS particles and yeast cells, respectively (Zhang et al. 2021). Figure 5b shows the removal efficiency of PS MPs at different pH. From Fig. 5b, PS removal enhances with increasing pH (p value < 0.05). According to the findings, maximum and minimum removal of PS occurred at pH 10 and 4, respectively. The reason can be interpreted as that in alkaline pH, there is more negative charge on the surface of PS. As a result, it increases the probability of yeast cells with positive surface charge to bind to PS particles and also increases coagulation (Sillanpää et al. 2018). On the other hand, the size of the flocs in alkaline pH is larger than in acidic pH, provides optimal conditions for flocs deposition and thus increases the PS removal efficiency (Zhou et al. 2021).
Optimum operational conditions
In this study, we utilized BBD to analyze the results and determine the optimal conditions for dye removal. Based on the quadratic model, we found that the highest removal rate (97.4%) was achieved at a pH of 10, a yeast dose of 219.96 mg/L, and PS level of 462.97 mg/L.
Conclusion
In this study, the use of S. cerevisiae yeast as a natural coagulant to remove polystyrene MPs was evaluated. To achieve the optimal conditions of PS removal using Box–Behnken model, the effect of different parameters such as yeast dose, PS concentration, and pH was investigated. In this study, FT-IR, FESEM and EDX analyses were performed. FT-IR was performed to determine the functional groups active in the process. FESEM also investigated the effect of surface morphology, which showed the accumulation of S. cerevisiae yeast on the surface of PS particles. EDX analysis was also performed to determine the percentage and name of the elements in polystyrene before and after the coagulation process. According to the findings, the relationship between the removal efficiency with yeast dose and pH was direct, while it was inverse with PS concentration. Moreover, alkaline conditions were favorable for PS removal. The maximum PS removal efficiency was 98.81% under optimized conditions, i.e., the PS concentration of 550 mg/L, the yeast dose of 200 mg/L, and the pH of 7. With regard to the mentioned results, it can be said that S. cerevisiae can be used as a natural and environmentally friendly coagulant to remove PS.
Data availability
All necessary data are included in the document.
Abbreviations
- MPs:
-
MPs
- PS:
-
Polystyrene
- S. cerevisiae :
-
Saccharomyces cerevisiae
- BBD:
-
Box–Behnken design
- FT-IR:
-
Fourier-transform infrared spectroscopy
- EDX:
-
Energy-dispersive X-ray
- FESEM:
-
Field emission scanning electron microscopy
- ANOVA:
-
Analysis of variance
References
Amran AH, Zaidi NS, Syafiuddin A, Zhan LZ, Bahrodin MB, Mehmood MA, Boopathy R (2021) Potential of Carica papaya seed-derived bio-coagulant to remove turbidity from polluted water assessed through experimental and modeling-based study. Appl Sci 11(12):5715
Anbarani MZ, Ramavandi B, Bonyadi Z (2023) Modification of chlorella vulgaris carbon with Fe3O4 nanoparticles for tetracycline elimination from aqueous media. Heliyon 9(3):e14356
Bayarkhuu B, Byun J (2022) Optimization of coagulation and sedimentation conditions by turbidity measurement for nano-and microplastic removal. Chemosphere 306:135572
Bayo J, López-Castellanos J, Olmos S (2020) Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar Pollut Bull 156:111211
Bitter H, Krause L, Kirchen F, Fundneider T, Lackner S (2022) Semi-crystalline microplastics in wastewater plant effluents and removal efficiencies of post-treatment filtration systems. Water Res. X 17:100156
Bonyadi Z, Khatibi FS, Alipour F (2022a) Ultrasonic-assisted synthesis of Fe3O4 nanoparticles-loaded sawdust carbon for malachite green removal from aquatic solutions. Appl Water Sci 12(9):221
Bonyadi Z, Maghsodian Z, Zahmatkesh M, Nasiriara J, Ramavandi B (2022b) Investigation of microplastic pollution in Torghabeh River sediments, northeast of Iran. J Contam Hydrol 250:104064
Bonyadi Z, Nasoudari E, Ameri M, Ghavami V, Shams M, Sillanpää M (2022c) Biosorption of malachite green dye over Spirulina platensis mass: process modeling, factors optimization, kinetic, and isotherm studies. Appl Water Sci 12(7):167
Bonyadi Z, Fouladi Z, Robatjazi A, Zahmatkesh Anbarani M (2023) Reactive red-141 removal from synthetic solutions by γ-Al2O3 nanoparticles: process modeling, kinetic, and isotherm studies. Appl Water Sci 13(2):52
Chen C, Wang J (2016) Uranium removal by novel graphene oxide-immobilized Saccharomyces cerevisiae gel beads. J Environ Radioact 162:134–145
Cunha C, Silva L, Paulo J, Faria M, Nogueira N, Cordeiro N (2020) Microalgal-based biopolymer for nano-and microplastic removal: a possible biosolution for wastewater treatment. Environ Pollut 263:114385
Davodi M, Alidadi H, Ramezani A, Jamali-Behnam F, Bonyadi Z (2019) Study of the removal efficiency of arsenic from aqueous solutions using Melia azedarach sawdust modified with FeO: isotherm and kinetic studies. Desalination Water Treat 137:292–299
Du H, Zhang Y, Jiang H, Wang H (2022) Adsorption of rhodamine B on polyvinyl chloride, polystyrene, and polyethylene terephthalate microplastics in aqueous environments. Environ Technol Innov 27:102495
Esmaili Z, Barikbin B, Shams M, Alidadi H, Al-Musawi TJ, Bonyadi Z (2023) Biosorption of metronidazole using Spirulina platensis microalgae: process modeling, kinetic, thermodynamic, and isotherm studies. Appl Water Sci 13(2):63
Frantz TS, de Farias BS, Leite VRM, Kessler F, Cadaval Jr TRSA, de Almeida Pinto LA (2020) Preparation of new biocoagulants by shrimp waste and its application in coagulation-flocculation processes. J Clean Prod 269:122397
Gonzalez-Camejo J, Morales A, Peña-Lamas J, Lafita C, Enguídanos S, Seco A, Martí N (2023) Feasibility of rapid gravity filtration and membrane ultrafiltration for the removal of microplastics and microlitter in sewage and wastewater from plastic industry. J Water Process Eng 51:103452
Hadiani MR, Khosravi-Darani K, Rahimifard N, Younesi H (2018) Assessment of mercury biosorption by Saccharomyces cerevisiae: response surface methodology for optimization of low Hg (II) concentrations. J Environ Chem Eng 6(4):4980–4987
Lin L, Tang S, Wang X, Sun X, Yu A (2020) Adsorption of malachite green from aqueous solution by nylon microplastics: reaction mechanism and the optimum conditions by response surface methodology. Process Saf Environ Prot 140:339–347
Lu S, Liu L, Yang Q, Demissie H, Jiao R, An G, Wang D (2021) Removal characteristics and mechanism of microplastics and tetracycline composite pollutants by coagulation process. Sci Total Environ 786:147508
Ma B, Xue W, Hu C, Liu H, Qu J, Li L (2019) Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem Eng J 359:159–167
Machado MD, Santos MS, Gouveia C, Soares HM, Soares EV (2008) Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: the flocculation as a separation process. Bioresour Technol 99(7):2107–2115
Mazloomi S, Ghodsei S, Amraei P, Bonyadi Z (2018) Data on the removal of turbidity from aqueous solutions using polyaluminum chloride. Data Brief 20:371–374
Mazloomi S, Bonyadi Z, Haghighat GA, Nourmoradi H, Soori MM, Eslami F (2021) Removal of methylene blue by Saccharomyces cerevisiae: process modelling and optimization. Desalination Water Treat 236:318–325
Miki B, Poon N, Seligy V (1982) Repression and induction of flocculation interactions in Saccharomyces cerevisiae. J Bacteriol 150(2):890–899
Muhonja CN, Makonde H, Magoma G, Imbuga M (2018) Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 13(7):e0198446
Napper IE, Bakir A, Rowland SJ, Thompson RC (2015) Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar Pollut Bull 99(1–2):178–185
Nasoudari E, Ameri M, Shams M, Ghavami V, Bonyadi Z (2021) The biosorption of Alizarin Red S by Spirulina platensis; process modelling, optimisation, kinetic and isotherm studies. Int J Environ Anal Chem 103:1–15
Okoye CO, Addey CI, Oderinde O, Okoro JO, Uwamungu JY, Ikechukwu CK, Okeke ES, Ejeromedoghene O, Odii EC (2022) Toxic chemicals and persistent organic pollutants associated with micro-and nanoplastics pollution. Chem Eng J Adv 11:100310
Onukwuli O, Nnaji P, Menkiti M, Anadebe V, Oke E, Ude C, Ude C, Okafor N (2021) Dual-purpose optimization of dye-polluted wastewater decontamination using bio-coagulants from multiple processing techniques via neural intelligence algorithm and response surface methodology. J Taiwan Inst Chem Eng 125:372–386
Pirsaheb M, Khodadadi T, Bonyadi Z, Sharafi K, Khosravi T (2013) Evaluation of pesticide residues 2, 4-D, Atrazine and Alachlor concentration in drinking water well of Mahidasht district-Kermanshah, Iran, 2010–2011. World Appl Sci J 23(11):1530–1537
Ramavandi B, Najafpoor AA, Alidadi H, Bonyadi Z (2019) Alizarin red-S removal from aqueous solutions using Saccharomyces cerevisiae: kinetic and equilibrium study. Desalination Water Treat 144:286–291
Restrepo-Flórez J-M, Bassi A, Thompson MR (2014) Microbial degradation and deterioration of polyethylene–a review. Int Biodeterior Biodegradation 88:83–90
Rout PR, Mohanty A, Sharma A, Miglani M, Liu D, Varjani S (2022) Micro-and nanoplastics removal mechanisms in wastewater treatment plants: a review. J Hazard Mater Adv 6:100070
Sadeghi A, Ehrampoush MH, Ghaneian MT, Najafpoor AA, Fallahzadeh H, Bonyadi Z (2019) The effect of diazinon on the removal of carmoisine by Saccharomyces cerevisiae. Desalination Water Treat 137:273–278
Sánchez C (2020) Fungal potential for the degradation of petroleum-based polymers: an overview of macro-and microplastics biodegradation. Biotechnol Adv 40:107501
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26(3):246–265
Sillanpää M, Ncibi MC, Matilainen A, Vepsäläinen M (2018) Removal of natural organic matter in drinking water treatment by coagulation: a comprehensive review. Chemosphere 190:54–71
Skaf DW, Punzi VL, Rolle JT, Kleinberg KA (2020) Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem Eng J 386:123807
Sun J, Dai X, Wang Q, Van Loosdrecht MC, Ni B-J (2019) Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res 152:21–37
Sun Y, Wang X, Xia S, Zhao J (2021) New insights into oxytetracycline (OTC) adsorption behavior on polylactic acid microplastics undergoing microbial adhesion and degradation. Chem Eng J 416:129085
Toolabi A, Malakootian M, Ghaneian MT, Esrafili A, Ehrampoush MH, Tabatabaei M, AskarShahi M (2017) Optimization of photochemical decomposition acetamiprid pesticide from aqueous solutions and effluent toxicity assessment by Pseudomonas aeruginosa BCRC using response surface methodology. AMB Express 7:1–12
Toolabi A, Malakootian M, Ghaneian MT, Esrafili A, Ehrampoush MH, AskarShahi M, Tabatabaei M (2018) Modeling photocatalytic degradation of diazinon from aqueous solutions and effluent toxicity risk assessment using Escherichia coli LMG 15862. AMB Express 8(1):1–13
Wang W, Yang M, Ma H, Liu Z, Gai L, Zheng Z, Ma H (2023) Removal behaviors and mechanism of polystyrene microplastics by coagulation/ultrafiltration process: co-effects of humic acid. Ultrafiltration process: co-effects of humic acid. Sci Total Environ 881:163408
Yang M, Zheng S (2014) Pollutant removal-oriented yeast biomass production from high-organic-strength industrial wastewater: a review. Biomass Bioenergy 64:356–362
Yao J, Peng Z, Chen W, Lin Q, Cheng M, Li H, Yang Y, Yang HY (2023) Surface characteristics of polystyrene microplastics mainly determine their coagulation performances. Mar Pollut Bull 186:114347
Yilimulati M, Wang L, Ma X, Yang C, Habibul N (2021) Adsorption of ciprofloxacin to functionalized nano-sized polystyrene plastic: kinetics, thermochemistry and toxicity. Sci Total Environ 750:142370
Zhang J, Gao D, Li Q, Zhao Y, Li L, Lin H, Bi Q, Zhao Y (2020) Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci Total Environ 704:135931
Zhang Y, Zhou G, Yue J, Xing X, Yang Z, Wang X, Wang Q, Zhang J (2021) Enhanced removal of polyethylene terephthalate microplastics through polyaluminum chloride coagulation with three typical coagulant aids. Sci Total Environ 800:149589
Zhou G, Wang Q, Li J, Li Q, Xu H, Ye Q, Wang Y, Shu S, Zhang J (2021) Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: performance and mechanism. Sci Total Environ 752:141837
Acknowledgements
Not applicable.
Funding
The authors would like to thank the financial support provided by the Student Research Committee of Mashhad University of Medical Science (Iran) through the Grant Number of 4010688.
Author information
Authors and Affiliations
Contributions
MZ performed the experiments and wrote the paper; AE designed the experiments and analyzed data; ZB wrote and edited the paper and conceived and designed the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zahmatkesh Anbarani, M., Esmaeili Nasrabadi, A. & Bonyadi, Z. Use of Saccharomyces cerevisiae as new technique to remove polystyrene from aqueous medium: modeling, optimization, and performance. Appl Water Sci 13, 166 (2023). https://doi.org/10.1007/s13201-023-01970-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01970-x