Abstract
This study investigated the performance of continuous-flow electrocoagulation (CFR-EC) reactor for aged landfill leachate treatment with a novel configuration of iron and aluminum electrodes to enhance the applicability of the process. The effects of the applied current density (ACD), initial pH, and hydraulic retention time (HRT) on the percentage removal of COD, TOC, BOD5, color, turbidity, and heavy metals (HMs) were modeled with Box-Behnken design (BBD). The results demonstrated that the models are significant (R2 0.97—p-value < 0.0029 and R2 0.92—p-value < 0.0001 for Fe and Al electrodes). COD, TOC, BOD5, and NH3-N removal were maximized at HRT 50 min (40.0 mL min−1) and pH 11 reaching 59, 64, 55, and 27%, respectively, by applying the ACD of 1.1 mA cm−2 in the CFR-ECFe reactor. The CFR-ECFe reactor presented a higher color (59%) and turbidity reduction (86%) than the CFR-ECAl reactor. At optimum condition, the removal percentages of HMs: Cr6+, Pb2+, As3+, Mg2+, B3+, Mn3+, Ni2+, and Ba2+ were 50, 70, 80, 99, 81, 99, 20, and 65%, respectively. The total process cost for landfill leachate treatment was 0.21 $/m3. The CFR-ECFe was an effective and affordable reactor for pollutant removal from landfill leachate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Landfilling is the most frequently used method for solid waste management worldwide (Aslam et al. 2022). One of the environmental threats associated with this waste management procedure is landfill leachate generation. The fluid percolating via the landfill from the liquids present in the waste, rainwater, and outside pool leads to the generation of leachate (Yuan et al. 2021; Kucharska et al. 2022). Leachate is described by a high chemical oxygen demand (COD), biochemical oxygen demand (BOD5), ammoniacal nitrogen (NH3N), total organic carbon (TOC), suspended solids (SS), and heavy metals (Umamaheswari et al. 2020, Paula et al. 2022). Leachate also contains various molecular masses of organics, such as humic substances (HS), which are hazardous and resistant to biological treatment (Argun et al. 2020). The discharge of untreated leachate into the environment can pollute the soil and groundwater resources and is also hazardous to living organisms (Gautam et al. 2022). Therefore, the development of efficient methods for the treatment of landfill leachate has been actively studied. Over time the chemical composition of leachate changes due to the degradation of organic matter (Xu et al. 2018). Aged landfill leachate often contains higher recalcitrant organic matter than the young one, and biological treatment is not appropriate for decreasing the organic pollutants of aged leachate (Chen et al. 2020; Wu and Li 2021).
A combination of various treatment approaches such as chemical, biological, and physicochemical treatments is widely used to treat leachate. Recently, electrochemical processes have attracted widespread attention to treat landfill leachate (Ghimire et al. 2019, Guo et al. 2022). The electrocoagulation process is known as a promising technology because it has advantages such as high performance, operation at ambient pressure and temperature, and the ability to adjust to variations in flow rate and influent compositions (Niza et al. 2021). The results of a study by Huda et al. showed that the electrocoagulation process using iron electrodes reduced BOD, COD, and chromium content (Huda et al. 2017). Bagastyo et al. (2022) investigated the effectiveness of the electrocoagulation (EC) process based on the reduction of organic and nitrogenous contaminants in landfill leachate. Compared to the conventional coagulation process, this process also induces a small volume of sludge that could be proposed as an environmentally friendly process (Mehralian et al. 2021). In the electrocoagulation process, an electrolytic cell with an anode and a cathode involves the generation of coagulant ions by dissolving electrodes electrically. In this method, the metal ions and hydrogen gas generation occur at the anode and the cathode, respectively. Destabilizing of contaminants takes place by different reactions (Eqs. 1–2) occurring on the electrode's surface (Galvão et al. 2020).
The formation of hydroxides/oxyhydroxides provides active sites to adsorb contaminants. Simultaneously, the hydrogen and oxygen gas bubbles released at the cathode and anode surfaces move the contaminant to the top of the solution (Eqs. 3–4), where it can be more easily agglomerated and removed (Huda et al. 2017, Galvão, de Souza et al. 2020).
The application of the electrocoagulation process to remove different contaminants has been presented frequently; however, most of these investigations were focused on development at the batch mode studies. Gautam et al. (2022) studied the treatability of landfill leachate by electrocoagulation process for COD reduction. Results indicated that the galvanized iron electrode could remove 90% of COD and other parameters (Gautam et al. 2022). Niza et al. (2021) investigated the efficiency of batch-mode electrocoagulation using plate electrodes in an old leachate treatment. Electrocoagulation with plate electrodes was able to achieve a 50% reduction of NH3-N (Niza et al. 2021). Galvão et al. (2020) studied the electrocoagulation process to treat landfill leachate utilizing a 2-L reactor with aluminum electrodes. The best removal efficiency was obtained 40% for COD at a current density of 128 A m−2 (Galvão et al. 2020).
The response surface methodology (RSM) is a practical approach for analyzing the relationships between several input and response parameters. The RSM technique was applied to improve the optimized response that is affected by various independent parameters. This method will use to reduce the requirement for performing repeated experiments for tests with several factors.
Based on our knowledge, no study has been performed to convert a landfill leachate treatment system developed from a batch experiment into a continuous-flow landfill leachate treatment system that can be used in industrial-scale systems. Accordingly, this study aims to develop an electrocoagulation continuous-flow reactor for treating landfill leachate in terms of COD, BOD5, TOC, NH3-N, color, turbidity, and heavy metals removal. In addition, the optimization of the continuous-flow electrocoagulation process was carried out using response surface methodology. The performance of electrocoagulation continuous-flow reactors (CFR) was also compared by using both aluminum and iron electrodes. The effect of various parameters on the performance of CFR-EC reactor as well as electrical energy consumption was also studied.
Materials and methods
Analysis and characterization of landfill leachate
The landfill leachate was collected from a detention pond at the sanitary landfill treatment plant in Yazd (Iran). The landfill leachate samples were kept at 4 ◦C for all experiments. The characteristics of leachate are depicted in Table 1. The landfill leachate was analyzed based on standard methods in terms of physicochemical parameters such as chemical oxygen demand (COD-5220), total organic carbon (TOC-5310), biochemical oxygen demand (BOD5-5210), total suspended solids (TSS-2540 D), NH3-N (4500), pH (AZ Instrument Corp, 86,502 AZ), turbidity, and color (APHA 2005). The COD concentration was measured using COD reagent vials at the wavelength of 600 nm (Lovibond COD Tube Tests, Germany). For the BOD5 analysis, a dissolved oxygen meter was utilized to measure the dissolved oxygen values at 5 days of incubation (YSI 5000, USA). The color was determined using a UV–Vis spectrophotometer at the wavelength of 720 nm (DR6000, HACH, USA). The concentrations of heavy metals (Cr6+, Pb2+, As3+, Mg2+, B3+, Mn3+, Ni2+, and Ba2+) were measured by atomic absorption spectrometry (PinAAcle 900F, PerkinElmer, USA). All chemicals utilized were purchased from Merck Company.
Set-up of CFR-EC reactor
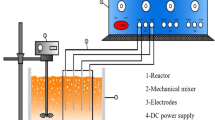
Figure 1a, b depicts the schematic of the CFR-EC reactors with the new configuration of the anode and cathode. Based on Fig. 1, the two cylindrical electrocoagulation reactor of 2-L internal volume was equipped with twelve parallel tubes (20 cm × 0.6 cm; gap e = 4 cm), utilized as anode and cathode, respectively. In both reactors, the anode with a smaller diameter (19 cm × 16.83 cm) was placed into the anode with a larger diameter (19 cm × 21.91 cm) to achieve more surface area. The gap between the anode and cathode was fixed at 1.27 cm to decrease energy failures. Either aluminum or iron electrodes could be used to compare the effect of electrode metal. The two CFR-EC reactors were connected to a digital DC power supply (DAZHENG PS-305D, China) supplying a current density ranging from 0.14 to 1.1 mA cm−2. The efficiency of the CFR-EC reactor for the removal of contaminants was calculated using Eq. (5).
where Ci (mg L−1) and Cf (mg L−1) are related to the initial and final contaminant concentrations of raw and treated leachate, respectively.
A Schematic of the CFR-EC reactor: (1) input, (2) peristaltic pump, (3) flow control valve, (4) CFR-EC reactor, (5) anodes, (6) cathodes, (7) output valve, (8) treated leachate, (9) effluent, (10) sludge disposal. B Cross-sectional view of CFR-EC reactor surface: a anode [diameter: 20.32 cm], b anode [diameter: 15.24 cm], c cathodes, d flow direction
Leachate treatment in the CFR-EC reactor
First, 2 L of landfill leachate was mixed on the magnetic stirrer at 150 rpm to achieve a homogenized solution. Then, landfill leachate through a pump was transferred to the CFR-EC reactor after pH adjustment (3.0–11.0). After a hydraulic retention time (HRT: 10.0–90.0 min, Q: 200 − 22.2 mL min−1), the treated leachate was discharged into the effluent tank through the outlet valve. The effluent was collected in a 50-ml beaker. Three applied current densities (ACD) of 0.14, 0.64, and 1.1 mA cm−2 were chosen to evaluate the influence of current density on the removal efficiency. The generated sludge in the reactor was then permitted to settle for 30 min. All experiments were conducted at room temperature (25 ± 0.5 °C).
Optimization of the CFR-EC reactor
The Box-Behnken model was performed to optimize the influences of ACD, pH, and HRT on the removal rate using the Design Expert software (Ver. 13).
Cost-effectiveness analysis
Analysis of operating costs including consumptions of electrodes, energy, and disposal of sludge.
From an economic view, the operating cost of the new CFR-EC reactor was evaluated based on the amount of electrode and electrical power consumed (Eq. 6).
where α1 and α2 are the unit cost of electricity and electrode materials, respectively. CPower (kWh m−3) and CElectrode (kg m−3) are associated with power consumed and electrodes, respectively. According to the Iran market 2022, unit costs were 0.002 $/kWh of electricity and 0.66 $/kg of iron, respectively. Based on Faraday’s second law (Eq. 7), the rate of electrodes consumed can be determined by the consumed weight of electrodes (Abdulhadi et al. 2021).
where j, HRT, MW, and V are the applied current density (amp), retention time (sec), molecular weight (g mol−1), and volume of solution (m3), respectively. Energy consumption was also calculated based on Eq. (8).
where V is the actual cell voltage (volt).
Results and discussion
Interaction of variables on CFR-EC reactor performance
Different electrode materials affect the performance of the CFR-EC process. The most widely applied electrodes are aluminum and iron since they are effective, cheap, and available. The applicability of the CFR-EC process and the interaction between the COD removal with different variables are displayed in Fig. 2 and Table 2. Based on Table 2, the CFR-EC reactor with an iron electrode had a better removal rate than the aluminum electrode concerning COD removal. The maximum removal rate of COD (59%) with CFR-ECFe reactor was obtained at an ACD of 1.1 mA cm−2, pH of 11.0, and HRT of 50 min (40 mL min−1), while the least amount of COD reduction (9.5%) occurred when the pH of leachate was neutral (7.0), ACD at its lowest (0.14 mA cm−2), and HRT value of 10.0 min. As exhibited in Fig. 2a, c, an increase in ACD enhanced the COD removal efficiency using both electrodes. With the increase in ACD, the extent of anodic dissolution of aluminum and iron leads to the generation of a greater amount of hydroxide flocs to remove contaminants. Also, the bubble-generation rate with rising ACD increases, the bubble size declines, and the amount of metal oxidized boosts producing more efficient and swifter elimination (Abbasi et al. 2020). In this condition, the electrocoagulation process improves and further gas bubbles will be distributed at the cathode, inducing the flotation of pollutants (Betancor-Abreu et al. 2019; Igwegbe et al. 2021). Similarly, raising the HRT shows an increase in the generation of Fe (OH)3, Al (OH)3, and H2 gas bubbles, which also positively correspond to the removal of COD. The highest COD removal was achieved in the first 50.0 min at 1.1 mA cm−2 using both electrodes. Within 90.0 min applying ACD of 0.64 mA cm−2, the removal rate of COD was relatively reduced and reached 39% and 48% using Fe and Al electrodes, respectively (Fig. 2b–d). The shorter HRT required to reach more satisfactory performance of EC process (i.e., HRT: 50.0 min) could be expressed by the presence of coagulant species [(Fe(OH)3(s) and Al(OH)3 (s)] that were produced in the first times of the process. In this state, the capture of colloidal particles and soluble organic compounds by agglomeration and adsorption processes causes faster contaminant removal in the solution (Hakizimana et al. 2017). Generally, the degradation of COD by the electrocoagulation process occurs through coagulant species [(Fe(OH)3(s)], where the colloidal particles and soluble organic compounds are captured by agglomeration and adsorption processes that lead to faster pollutant removal in the solution. Zampeta et al. (2022) reported a similar result using aluminum electrodes in a continuous electrocoagulation reactor. When treating printing ink wastewater with COD 20,000 mg L−1, the maximum removal efficiency was obtained after 65-min treatment at 83 mA cm−2 of ACD, while the HRT raised to 90 and 110 min at the lower ACD (42 and 21 mA cm−2) (Zampeta et al. 2022). The removal efficiency of contaminants is directly influenced by the concentration of metal ions and hydroxyl generated on the electrodes that are proved by Faraday’s law (Safari et al. 2016; Igwegbe et al. 2021). According to Faraday's law, coagulant amounts theoretically have a direct relationship with the ACD and HRT in an electrocoagulation reactor. Thus, when the HRT of the process is increased, the amount of metal ions as coagulant agents increases; consequently, the percentage of COD removal is improved. Figure 2 also presents the removal of COD as a function of the pH, which was varied from pH 3.0 to pH 11.0 for both the iron and aluminum electrodes. From the results, it is evident that the removal of COD was higher in the alkaline conditions compared to the acidic condition. The removal of COD was found to be more effective at the initial pH of 11.0. This finding was in accordance with other results that investigated the removal efficiency of pollutants (Manikam et al. 2019). Indeed, the acidic condition is undesirable for COD removal due to the reduction in the amount of OH− species generated during the electrocoagulation process and the possibility of corrosion occurring within the aluminum electrodes, which leads to lower removal efficiency. With the increase in pH during the electrocoagulation process due to the hydrogen gas evolution on the cathode and the hydroxyl ions enhancement in the solution, high percentage removal was achieved. Generally, the iron electrode was more effective for landfill leachate treatment than aluminum which may be due to the high adsorption capacity of hydrous ferric oxides.
Optimization of the parameters
The BBD was employed to develop a regression model that investigates the influence of parameters on COD removal using both electrodes. Equations (9, 10) are related to COD removal using iron and aluminum electrodes, respectively. Coefficients in Eqs. (9 and 10) show the effect of all influential parameters on COD removal.
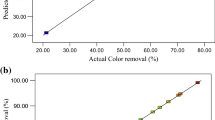
Also, Fig. 3 presents data related to the relationship between the experimental and predicted values. The results of ANOVA for evaluation of the accuracy and significance of the quadratic model are shown in Table 3. Three indexes of the ANOVA, R2, P-value, and lack of fit, have been utilized to evaluate the accuracy of the model (Pormazar and Dalvand 2022). From Table 3, it can be concluded that the selected model has high R2 and adj R-squared. Two models have a very low P-value (< 0.0029 and < 0.0001 for Fe and Al electrodes), demonstrating the model is significant (Zarei et al. 2017). The lack of fit index with 0.0771 and 0.0831values indicates the excellent significance of the models concerning COD removal. As shown in Table 3, all parameters are significant in COD removal. The high F-values of 36.45 and 126.76 with Fe and Al electrodes are related to the effect of pH demonstrating that pH is the most effective variable for COD removal. After that, the parameters of ACD and HRT have a great effect on the electrocoagulation process to remove COD, respectively.
To assess the compressive performance of the CFR-EC in the treatment of landfill leachate, the treatment was performed at optimized conditions. Figure 4 presents the percentage removal of COD, BOD5, TOC, NH3-N, color, turbidity, and heavy metals of the landfill leachate after treatment using the iron electrode. The findings illustrated that the removal efficiency of COD, TOC, BOD5, and NH3-N reached 59, 55, 64, and 27%, respectively, at optimum conditions. In this condition, the removal rate of color and turbidity were 59 and 86%, respectively. Also, 50 to 99% of heavy metals were successfully eliminated during the EC process. Figure 5 shows the photographs of real landfill leachate before and after treatment using the CFR-EC process. As shown in Fig. 5, the CFR-EC process can successfully treat and eliminate the pollutants from the real landfill leachate.
Mechanism of COD degradation by CFR-EC process
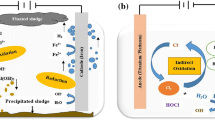
Figure 6 displays the degradation pathway of COD using the CFR-EC process. As shown in Fig. 6, COD degradation usually involves the following steps: 1. sacrificing the anode under the effect of a current density to generate of metal cations; 2. the production of OH− ions on the cathode; 3. formation of MOH(s) with high adsorption capacity to adsorb COD molecules; 4. oxidation of COD molecules to less toxic products; 5. neutralization of COD molecules with metal ions; 6. aggregation of neutralized pollutants and their adsorption; 7. flotation using the gases produced in the CFR-EC reactor. Generally, degradation of contaminants takes place through neutralization of negatively charged colloidal particles via cation hydrolysis products and removal of pollutants in the form of amorphous hydroxides.
Operating cost of the CFR-EC process
The cost–benefit analysis demonstrates that the energy consumption in the CFR-ECFe reactor was 0.013 kWh m−3 in the optimum condition. The electrode consumption (0.33 Kg m−3) cost at the optimum condition estimated based on a price of 0.66 $ kg−1 was 1.6 × 10−4 $. Then, the total operating cost for the CFR-EC process was 0.21 $/m3 of landfill leachate treatment. It can be concluded that the CFR-EC process is a cost-efficient and viable technique for treating landfill leachate with high COD concentration.
Comparison of the CFR-EC reactor with other studies
Different types of electrocoagulation reactor have been developed to treat leachate. Table 4 shows the comparative efficiency of different electrocoagulation reactor used for landfill leachate treatment. As is depicted, the CFR-EC reactor with novel configuration indicates a suitable performance to treat landfill leachate.
Conclusions
Successful application of the CFR-EC reactor was reported to treat aged landfill leachate with high COD values (approximately 75,000 mg L−1) by choosing three basic operational variables (ACD, pH, and HRT) and providing information about the cost–benefit analysis of the process. The CFR-EC reactor with an iron electrode had better removal efficiency than the aluminum electrode. The results illustrated that the treatment of leachate using CFR-ECFe reactor leads to 59, 64, 55, and 27% COD, TOC, BOD5, and NH3-N reduction, while the removal percentage of color and turbidity was obtained 59 and 86%, respectively. Removal percentages of heavy metals such as Cr6+, Pb2+, As3+, Mg2+, B3+, Mn3+, Ni2+, and Ba2+ were obtained 50, 70, 80, 99, 81, 99, 20, and 65% at optimized conditions (ACD: 1.1 mA cm−2, pH: 11.0, and HRT: 50.0 min [40.0 mL min−1]). Total operating costs were calculated as 0.21 $/m3 with the iron electrode. In summary, the application of a CFR-EC reactor to treat aged landfill leachate with high COD concentrations is efficient and cost-effective.
Data availability
The authors do not have permission to share data.
References
Abbasi S, Mirghorayshi M, Zinadini S, Zinatizadeh AA (2020) A novel single continuous electrocoagulation process for treatment of licorice processing wastewater: optimization of operating factors using RSM. Process Saf Environ Prot 134:323–332
Abdulhadi B, Kot P, Hashim K, Shaw A, Muradov M, Al-Khaddar R (2021) Continuous-flow electrocoagulation (EC) process for iron removal from water: Experimental, statistical and economic study. Sci Total Environ 760:143417
APHA (2005). "Standard methods for the examination of water and wastewater." 21.
Argun ME, Akkuş M, Ateş H (2020) Investigation of micropollutants removal from landfill leachate in a full-scale advanced treatment plant in Istanbul city, Turkey. Sci Total Environ 748:141423
Aslam B, Maqsoom A, Tahir MD, Ullah F, Rehman MSU, Albattah M (2022) Identifying and ranking landfill sites for municipal solid waste management: an integrated remote sensing and GIS approach. Buildings 12(5):605
Bagastyo AY, Sidik F, Anggrainy AD, Lin JL, Nurhayati E (2022) The performance of electrocoagulation process in removing organic and nitrogenous compounds from landfill leachate in a three-compartment reactor. J Ecol Eng 23(2):235–245
Betancor-Abreu A, Mena VF, González S, Delgado S, Souto RM, Santana JJ (2019) Design and optimization of an electrocoagulation reactor for fluoride remediation in underground water sources for human consumption. J Water Process Eng 31:100865
Chen W, He C, Gu Z, Wang F, Li Q (2020) Molecular-level insights into the transformation mechanism for refractory organics in landfill leachate when using a combined semi-aerobic aged refuse biofilter and chemical oxidation process. Sci Total Environ 741:140502
Galvão N, de Souza JB, Vidal CMDS (2020) Landfill leachate treatment by electrocoagulation: effects of current density and electrolysis time. J Environ Chem Eng 8(5):104368
Gautam P, Kumar S, Vishwakarma S, Gautam A (2022) Synergistic optimization of electrocoagulation process parameters using response surface methodology for treatment of hazardous waste landfill leachate. Chemosphere 290:133255
Ghimire U, Jang M, Jung SP, Park D, Park SJ, Yu H, Oh SE (2019) Electrochemical removal of ammonium nitrogen and COD of domestic wastewater using platinum coated titanium as an anode electrode. Energies 12(5):883
Guo Z, Zhang Y, Jia H, Guo J, Meng X, Wang J (2022) Electrochemical methods for landfill leachate treatment: a review on electrocoagulation and electrooxidation. Sci Total Environ 806:150529
Hakizimana JN, Gourich B, Chafi M, Stiriba Y, Vial C, Drogui P, Naja J (2017) Electrocoagulation process in water treatment: a review of electrocoagulation modeling approaches. Desalination 404:1–21
Huda N, Raman AAA, Bello MM, Ramesh S (2017) Electrocoagulation treatment of raw landfill leachate using iron-based electrodes: Effects of process parameters and optimization. J Environ Manage 204:75–81
Igwegbe CA, Onukwuli OD, Ighalo JO, Umembamalu CJ, Adeniyi AG (2021) Comparative analysis on the electrochemical reduction of colour, COD and turbidity from municipal solid waste leachate using aluminium, iron and hybrid electrodes. Sustain Water Resour Manag 7(3):39
Kucharska MA, Mirehbar S, Ładyńska JA (2022) Novel combined IME-O3/OH−/H2O2 process in application for mature landfill leachate treatment. J Water Process Eng 45(2022):102441
Manikam MK, Halim AA, Hanafiah MM, Krishnamoorthy RRJDWT (2019) Removal of ammonia nitrogen, nitrate, phosphorus and COD from sewage wastewater using palm oil boiler ash composite adsorbent. Desalin Water Treat 149(2019):23–30
Mehralian M, Khashij M, Dalvand A (2021) Treatment of cardboard factory wastewater using ozone-assisted electrocoagulation process: optimization through response surface methodology. Environ Sci Pollut Res 28(33):45041–45049
Niza NM, Yusoff MS, Zainuri MAAM, Emmanuel MI, Shadi AMH, Hanif MHM, Kamaruddin MA (2021) Removal of ammoniacal nitrogen from old leachate using batch electrocoagulation with vibration-induced electrode plate. J Environ Chem Eng 9(2):105064
Pormazar SM, Dalvand A (2022) Adsorption of Reactive Black 5 azo dye from aqueous solution by using amine-functioned Fe3O4 nanoparticles with L-arginine: process optimisation using RSM. Int J Environ Anal Chem 102(8):1764–1783
Safari S, Azadi Aghdam M, Kariminia HR (2016) Electrocoagulation for COD and diesel removal from oily wastewater. Int J Environ Sci Technol 13(1):231–242
Santos APF, Gozzi F, de Carvalho AE, de Oliveira KRF, Caires ARL, Cavalcante RP, Junior AM (2022) Leachate degradation using solar photo-fenton like process: Influence of coagulation-flocculation as a pre-treatment step. Separat Purific Technol 289:120712
Umamaheswari J, Bharathkumar T, Shanthakumar S, Gothandam KM (2020) A feasibility study on optimization of combined advanced oxidation processes for municipal solid waste leachate treatment. Process Saf Environ Prot 143:212–221
Wu C, Li Q (2021) Characteristics of organic matter removed from highly saline mature landfill leachate by an emergency disk tube-reverse osmosis treatment system. Chemosphere 263:128347
Xu Q, Siracusa G, Di Gregorio S, Yuan Q (2018) COD removal from biologically stabilized landfill leachate using advanced oxidation processes (AOPs). Process Saf Environ Prot 120:278–285
Yuan Y, Liu J, Gao B, Hao J (2021) Ozone direct oxidation pretreatment and catalytic oxidation post-treatment coupled with ABMBR for landfill leachate treatment. Sci Total Environ 794(10):148557
Zampeta C, Mastrantonaki M, Katsaouni N, Frontistis Z, Koutsoukos PG, Vayenas DV (2022) Treatment of printing ink wastewater using a continuous flow electrocoagulation reactor. J Environ Manage 314:115033
Zarei H, Nasseri S, Nabizadeh R, Shemirani F, Dalvand A, Mahvi AH (2017) Modeling of arsenic removal from aqueous solution by means of MWCNT/alumina nanocomposite. Desalin Water Treat 67:196–205
Acknowledgements
The authors thank the Shahid Sadoughi University of Medical Sciences for the financial support of the study (Grant No: 8505).
Funding
The authors declare that no funds, grants, or other supports were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This research was approved by Iran National Committee for Ethics in Biomedical Research (code: IR.SSU.SPH.REC.1399.188).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mehralian, M., Ehrampoush, M.H., Ebrahimi, A.A. et al. Development of electrocoagulation-based continuous-flow reactor for leachate treatment: Performance evaluation, energy consumption, modeling, and optimization. Appl Water Sci 13, 162 (2023). https://doi.org/10.1007/s13201-023-01966-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01966-7