Abstract
Wabe River is a vital source of drinking water, irrigation for agriculture, and income for the local residents. However, the river's declining quality has led to an increase in waterborne infections and made it appear muddier. The study's objective was to evaluate the Wabe river water quality using 23 different water quality parameters to calculate the Weighted Arithmetic Water Quality Index (WAWQI), Metal Index (MI), Pollution Index (PI), and Potential Ecological Risk Index (PERI). The origins and hydro-chemical makeup of the river water were also ascertained using Piper diagrams and Gibb's plots. During the wet season, the WAWQI results revealed that the River water is unfit for drinking, which range from 205.4 to 487.8 (> 100) at all sampling stations. During the dry season, the water quality ranged from good to unsuitable (48.7–101.4). Similarly, the results of the Metal Index were greater than 1(MI > 1) indicates ‘Threshold of warning’ for drinking and aquatic life in both seasons. Pollution index results showed ‘seriously’ effect on aquatic life. However, the risk level posed by those metals according to PERI was in the range of 436.9 (Strong) to 1714.1 (Very high) and 288.5 (Moderate) to 704.3 (Very high) during the wet and dry seasons, respectively. The sources of contamination were confirmed by Gibb's plots as rock weathering, evaporation, and anthropogenic activities, and the water type was classified as calcium-magnesium-chloride by the piper diagram. Controlling the sources of pollution is necessary for preserving the Wabe River's sustainability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rivers are the momentous natural resource for drinking, domestic, and irrigation purposes, enhancing the economic status and sustainability of the adjacent communities (Kasa et al. 2022). The surface water highly vulnerable and impair its potential use (Hamid et al. 2020) due to anthropogenic activities such as agricultural runoff water (Ustaoğlu et al. 2021), domestic wastewater disposal (Preisner 2020), industrial (Bougherira et al. 2014; Menberu et al. 2021), open defecation (Okullo et al. 2017) and the improper disposal of solid waste (Menberu et al. 2021), as well as natural activities such as urban sediment inflow (Egbueri 2022), surface runoff (Kasa et al. 2022), the river flows through lithogenic structures (Asnake et al. 2021), crustal material weathering (Sahle et al. 2019) and soil erosion (Reddythota and Timotewos 2022). Rivers, tributaries, and canals transport a huge volume of municipal sewage, industrial wastewater, and agricultural runoff water, resulting in trash and pollutants accumulating in the water bodies (Mustapha et al. 2013). These surface water quality will decline to lead to a negative impact on user communities and aquatic life as a result, more than half a million people, mostly babies and teens, have died from waterborne infections, as well as the extinction of aquatic life (Theodore 2018; Weldeyohanis et al. 2020).
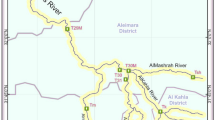
The Wabe River flows 91 km through the Gurage zone of southern Ethiopia, ending up in the Omo-Gibe basin, which is a source of life for the local inhabitants. The river has long been used for drinking, agriculture, industry, and fishing. Soil erosion and sedimentation also have an impact on the quality of Wabe River water, as it contains several pollutants that limit its value (Sahle et al. 2019). Furthermore, contaminants might reach the river from Wolkite Town's municipal dump, which is about 500 m distant from the Wabe River, especially during the rainy season (Kasa et al. 2022; Weldeyohanis et al. 2020). Water quality degradation has rendered some surface water supplies unusable for drinking, industrial, agricultural and other use (Kasa et al. 2022). Diarrhea, which is mostly spread by enteroviruses in the aquatic environment, is the most prevalent illness brought on by water pollution (Lin et al. 2022). The use of hygiene, which is essential to preventing not just diarrheal illnesses but also acute respiratory infections and several neglected tropical diseases, is made easier by the availability of safe and sufficient water (WHO 2014). These pollutants, which are poisonous to aquatic life and frequently shorten an organism's life span, capacity for reproduction, and reduced fish productivity, move up the food chain as predators devour their prey (Reckendorf et al. 2023). As a result, it is vital to understand the water's suitability for drinking and aquatic life.
Water quality assessment of water resources for potential use is indispensable (Edokpayi et al. 2020). As a result, regular water quality testing is necessary to identify contaminants and pollution levels, as well as to categorize water quality for various applications. A water quality index is a tool that represents overall water quality conditions and suitability for the planned use of water resources in a single number (Menberu et al. 2021).Water quality indices result in assists to prepare the mitigation measures to preserve ecosystem health and formulating suitable pollution control strategies to restore the carrying ability of water resources (Vadde et al. 2018). The objective of this study is to use a variety of water quality indices to determine if the water in the Wabe River is appropriate for consumption and aquatic life.
The current study's objectives are to (i) assess the regional and seasonal variations of physicochemical and heavy metals factors impacting the water quality of the Wabe River, (ii) used WAWQI and MI water quality indices to assess whether wabe river water is suitable for drinking during the wet and dry seasons; (iii) assessed MI and PI water quality indices to assess whether wabe river water is suitable for maintaining aquatic life during the wet and dry seasons, and (iv) Identified the Ecosystem of the Wabe River's potential risk from heavy metals, ecological risk components, and potential ecological risk index (v) Gibb's diagrams were utilized to pinpoint the sources of pollution, and Piper diagrams were employed to ascertain the hydrochemistry of the pollutants in the water. Prior to this study, the Wabe River's water quality status for multipurpose usage had not been evaluated. Furthermore, the potential risk posed by trace elements to aquatic ecosystems has not yet been evaluated. Evaluating the river water quality for multipurpose use and determining the danger level of heavy metals in river water on aquatic life is critical for key stakeholders to make pollution control decisions. It can also serve as a baseline for future research.
Methodology
Description of study area
The Wabe River watershed is one of the Omo-Gibe basin's sub-catchments. The river watershed lies between 08°21′30″–08°30′00″ north latitude, and 37°49′00″-38°05′40″ east longitude. The catchment covers 1860 km2 and has elevations ranging from 1014 and 3611masl. The river is 91 km long and has an average annual flow is 30.4 m3/sec (CESI 2009). The Wabe River flows through south-central Ethiopia from west to southwest. It is located near wolkite town and 178 km from Addis Ababa, Ethiopia’s capital city. The Wabe River originates in the Gurage mountain range and flows into the Gibe River.
In the Wabe catchment region, the dry season runs from November to May, and the wet season runs from June to October. The maximum annual rainfall (70–90%) occurs from June to September. According to Ethiopian National Meteorological Agency data, the average annual rainfall in the Wabe catchment ranged from 1111 to 1374 mm, with an average temperature of 18 °C. The river watershed's primary soil types are clay and sandy loam.
Sample site selection and sampling
The Wabe River was divided into ten segments based on the ease and reliability of the access. Sampling sites were purposively selected based on irrigation practices, waste disposal, runoff inlets and domestic activities from the ten segments to determine the spatial variability of the ecological risk of heavy metals. Water samples were collected on a bimonthly basis from ten Sample points (T1, T2, T3, T4, W1, W2, W3, W4, W5, and W6) (Fig. 1) during the wet season (July, August & September) and the dry season (November, December and January). A total of 60 water samples were examined to determine the spatiotemporal quality of river water for drinking and aquatic life. The geographical locations of Sample points were determined using a GPS (Garmin model 60) (Fig. 1).
The time pace composite sampling technique was used to collect samples at a depth of 30 cm from the surface using a manual sampler (Imneisi and Aydin 2016). The polyethene bottles with a capacity of 1.5 L were washed with 2 ml HCl and rinsed with sample water before collection of the sample. The sample bottles were properly labeled and stored in a 4 °C icebox before being delivered to Arba Minch University's water quality laboratory within 24 h for analysis according to the sampling methodology (APHA 2012).
Analysis of water quality parameters
After the equipment was calibrated according to the manufacturer's specifications, a total of 23 water quality parameters were measured. The remaining parameters were examined in the laboratory, whereas the EC, pH, TDS, and DO were determined at the sampling locations using a portable multimeter (HQ40D, USA). The summary of parameters, analytical methods, and instruments used for analysis were presented in Table 4.
Sample preparation for determination of metal ions
A 100 ml of the sample was taken in a beaker. 3 mL concentrated hydrochloric acid and 9 mL nitric acid were added to the sample for digestion, and it was heated for two hours. After cooling, the solution was placed into a standard flask containing Whatmann filter paper after two hours of digestion. The same methodology was used to prepare blank samples for each elemental analysis. Finally, Ca2+, Mg2+, Cd2+, Cu2+, Ni2+, Pb2+, and Zn2+ concentrations were measured using an Atomic Absorption spectrophotometer.
Water quality indices
The water quality index (WQI) provides a number that reflects overall water quality at a certain station and period, based on several results of water quality parameters. The water quality index tries to simplify complex water quality data by converting it into a single number that accurately and simply expresses the state of water quality at each sample station (Galal Uddin et al. 2021).
Weighted arithmetic water quality index
Weighted arithmetic water quality index (WAWQI), is a widely used simple method to assess the water quality for a specific purpose based on the index value of the water body (Menberu 2021) as well as to classify water quality according to the degree of purity (Goher et al. 2014). The acceptability of Wabe river water for household use was determined using the WAWQI, which estimates water quality based on purity using water quality criteria, and the computation was performed using Eq. 1.
where, Qi = the sub-index of ith parameter; Wi = unit weight of ith parameter, and n is the number of parameters (Goher et al. 2014).
The sub-index score of each inclusion parameter was calculated by using Eq. 2.
where Qi is the sub-index value of the ith parameter; Vi = Observed values of the ith parameter; Vo = ideal value of the ith parameter, and Si = standard permissible value of the ith parameter.
The optimal pH and DO values are 7 and 14.6, respectively. For the rest of the parameters, zero was chosen as the optimal value (Goher et al. 2014). The following empirical connection was also used to determine the unit weight of each variable.
where K is the proportionality constant and was determined by applying Eq. 4.
The unit weight of each parameter for drinking purposes was determined using World Health Organization (WHO 2017) and European Communities (EC 1989) drinking water quality standards. Turbidity, pH, TDS, DO, BOD, COD, TH, Ca2+, Mg2+, NO3−, PO4−2, SO4−2, Cl−, K+, and Na+ were all used to assess the Wabe River's suitability for drinking (Goher et al. 2014).
PERI for evaluating heavy metals risk on aquatic ecosystem
The Potential Ecological Risk Index (PERI) was developed to assess the contamination of heavy metals based on the characteristics and environmental behaviour (Smitom et al. 2021). Furthermore, the PERI method has an advantage over other methods, such as geo-accumulation index, contamination index, and regression analysis, because it involves knowledge of the toxic response factor for a given contaminant, whereas other indicators look at pollutant concentrations while ignoring toxicity to humans and other variables (Marara and Palamuleni 2019). In the current study, Cadmium, Copper, Zinc, Lead and Nickel were analyzed to verify the potential risk to the aquatic ecosystem. The procedure for determining the risk level of heavy metals in the aquatic ecosystem was performed by applying the following steps.
-
Computation of single contamination factors,
-
Computation of comprehensive contamination measure or degree of contamination, and
-
Determination of PERI.
The contamination factor for single heavy metal is obtained by dividing the heavy metal concentration by the guideline value (Ojekunle et al. 2016).
where, Cf = contamination factor; Cm = measured concentration; Cb = background concentration.
The summation of individual contamination factors will give the degree of contamination and was calculated by applying Eq. 6.
where Cd is the level of contamination and Cf is the contamination factor of individual heavy metals.
Finally, each metal contamination factor was multiplied by its toxic response factor to calculate the risk posed by each heavy metal to the aquatic ecosystem. The value of the prospective ecological risk index was the sum of each individual heavy metal risk. Heavy metals including zinc, copper, cadmium, nickel, and lead have hazardous reaction factors of 1, 5, 30, 5, and 5, respectively (Rahman et al. 2014).
Low, Moderate, relatively high and Very high are the Classes for level of contamination Cd < 8, 8 < Cd < 16, 16 < Cd < 32, and Cd ≥ 32, respectively. On the other hand, if the single heavy metal risk or ecological risk factor (Er) is less than 40, it is classified under low risk. While, if it is in the range of 40 to 80, it will fall under the moderate class. In addition, if the Er lies in the middle of 80 to 160, the risk is considerable. Whereas, Er which ranges from 160 to 320 is categorized as high risk and it is dangerous if Er is more than 320. Similarly, according to PERI, the risk level was categorized as low (PERI < 150), moderate (150 to 300), strong (300 to 600), and very high (PERI > 600) (Ojekunle et al. 2016). Potential Ecological Risk and contamination factor scaling was mentioned in Table 6.
Metal index (MI)
The additive effects of heavy metals on human health are determined by MI, leading to a fast assessment of the overall quality of drinking water. The lower the water quality, the higher the metal concentrations are in comparison to the maximum permissible amount. The ‘threshold of warning’ category in any water sample is MI > 1, indicating unsuitability for intended purpose (Goher et al. 2014).
MI to examine the cumulative effect of each heavy metal was determined by using Eq. 7.
Where, Ci is the concentration of each heavy metal and MAC is the maximum permissible limit of each indicator (Goher et al. 2014).
Pollution index (PI)
The pollution index is an important tool for assessing the state of water quality. This method can directly link contamination levels to whether or not a river can be used for a given purpose, as well as the values of specified parameters (Suwari 2021). In this work, PI was used to assess the potential contaminants in river water, and it was calculated using Eg.8
where PI is the pollution index, Ci is the concentration of water quality parameters, Lij is the permissible limit of water quality parameter for designated water use, (Ci/Lij) m is the maximum value of Ci/Lij, (Ci/Lij)r is the average value of Ci/Lij.
Water has no effect if the PI value is less than one; it has a moderate effect if the PI value is between one and two. Water quality is stated to be moderately damaged if the PI value falls between 2 and 3. In addition, if the PI value is between 3 and 5, heavy metals have a significant impact on water quality. Furthermore, if the PI value is greater than5, the water body will be severely harmed (Goher et al. 2014; Sedeño-Díaz et al. 2019).
GIS for mapping water quality status in Wabe river
To visualize the spatial extent of water quality status for drinking, aquatic life, and risk related to heavy metals on the aquatic ecosystem. The computed values of WAWQI, MI, and PERI were interpolated in ARC GIS 10.3 software using the inverse distance weight interpolation method.
Statistical analysis
The primary data was analyzed and interpreted by using different computer programs. Descriptive correlation and ANOVA tests were done with SPSS. Descriptive statistics were used for mean computation. A correlation test was carried out to assess the relations of physicochemical and biological characteristics of the river with its metal characteristics. Origin 8.5 was employed for graphical illustrations.
Results and discussion
Water quality results (wet and dry seasons)
In the current study, the analyzed water quality parameters, pH, TDS, Hardness, Chlorides (Cl−), Sulfates (SO42−), Carbonates (CO32−), Nitrates (NO3−), Sodium (Na+), Calcium (Ca2+), Magnesium (Mg2+), Potassium (K+), Dissolved Oxygen (DO), Copper (Cu2+), zinc (Zn2+), Lead (Pb2+) and Nickel (Ni2+) were within the acceptable limit for drinking purposes according to WHO standards. During the research period, majority of the sampling stations had turbidity, COD, BOD5, phosphorus (PO43−), and cadmium (Cd2+) levels that were over the safe drinking water permissible limits (Fig. 2). Furthermore, in most of the sample points, NO3−, Cu2+, Cd2+, and Zn2+ levels were beyond the CCME (Canadian Council of Ministers of the Environment) permissible limits, indicating aquatic toxicity.
Parameters above acceptable limit according to WHO drinking standards
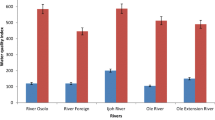
In the present analysis, the turbidity values were in the range of 111.67 NTU (T4) to 278.3 NTU (W6) and 13.3 NTU (T2) to 26 NTU (W5) during the wet and the dry seasons, respectively, which are above the permissible limit of 5 NTU (Leta and Dibaba 2019; WHO 2017; Goher et al. 2014; CCME 2007) (Fig. 2d). In downstream river parts, highest value of turbidity was observed. This could be owing to the presence of suspended particles in the river water, such as silt, plankton, clay, organic matter, and microorganisms/decomposers (Fig. 5a) (Ameen 2019). Furthermore, the discharge of urban waste such as household waste, solid waste, and toilet waste from both Wolkite and Gubre towns that join the river, as well as surface runoff, might be the cause for elevated turbidity values on the river's downstream stream side. A similar finding was made on the Rebu River, where the mean turbidity ranged from 10.7 to 800 NTU, exceeding the maximum allowable drinking level. This was due to the presence of a soap and detergent industry (Tadesse et al. 2018). Anthropogenic impacts such as indiscriminate solid waste disposal in the river could explain the highest value reported during the dry season (Nartey et al. 2012).
The phosphate concentrations during the wet and dry seasons were in the range of 0.12 mg/l (T4) to 0.615 mg/l (W5) and 0.065 mg/l (T1) to 0.24 mg/l (W5), respectively (Fig. 2c). During the rainy season, sampling stations W4 (0.57 mg/l), W5 (0.615 mg/l), and W6 (0.524 mg/l) were all above the European community's proposed ambient standard (0.5 mg/L) (EC 1989). Unless in extremely high concentrations, phosphorus does not constitute a human or health risk (Leta and Dibaba 2019). During the wet season, downstream sampling stations had higher phosphate content than upper and middle river portions. Domestic waste discharge from urban areas (Wolkite and Gubre town), agricultural land runoff (fertilizers), and leachate (illegal dumping along the riverbank) joined the river water along with runoff during the rainy season, was the possible reason for the maximum concentration (Menberu et al. 2021). The Wabe River watershed has a high slope, which can be linked to both anthropogenic influence and topographic conditions that promote soil erosion from the catchment to the river. During the dry season, sampling site W5 has a high phosphate concentration, which could be attributable to the local populations' usage of detergents and soaps for laundry, bathing, and car cleaning. However, the phosphate values in the current study were comparable to the Awetu River results, which varied from 0.023 to 0.063 mg/l (Leta and Dibaba 2019).
The BOD5 values were in the range of 13.07 to 26.13 mg/l and 4.2 to 12.74 mg/l during the wet and dry seasons, respectively (Fig. 2a). During the rainy season, all sampling locations were above the permissible level of 5 mg/l, and during the dry season, all sampling stations except T4 and W3 were above the permissible limit of 5 mg/l (Leta and Dibaba 2019; WHO 2017). According to the CCME, however, BOD has no criterion value for aquatic life (Goher et al. 2014). During the rainy season, the highest levels of 26.13 and 24.06 mg/L were reported from Sample point W4 and T3, respectively (Fig. 2a). In the dry season, highest levels of 12.74 and 10.6 mg/L were recorded at W4 and W5, respectively (Fig. 2a). The organic matter contamination of river water was due to waste from urban (Wolkite Town) and agricultural runoff from neighboring rural regions during the wet season. Direct dumping of solid waste into rivers could also generate a large quantity of BOD5 level in the river water sample. High BOD5 levels in river water cause stress, asphyxia, and death of aquatic organisms due to a lack of oxygen (Bhateria and Jain 2016). The current study findings were lower than the results found by Leta and Dibaba (2019), which range from 700 to 1000 mg/l on the Awetu River. This could be as a result of the Awetu River having greater organic matter contamination than the Wabe River.
Parameters in above acceptable limit according to CCME aquatic life standards
Copper concentrations were in the range of 0.18 mg/l (T1) to 0.6 mg/l (W4) and 0.11 mg/l (T1) to 0.32 mg/l (W5) during the wet and the dry seasons, respectively (Fig. 2f). The maximum permissible limit of copper for drinking purposes according to WHO and aquatic life according to CCME is 2 and 0.004 mg/L (WHO 2017; Goher et al. 2014), respectively. Copper concentrations were under the permitted range for human consumption (WHO 2017), but above the acceptable limit for aquatic life (CCME 2007). Higher levels of copper concentration present in the aquatic ecosystem can affect the existence and growth of aquatic organisms (e.g. fish) (Patrick and Baawain 2019). The higher values during the wet season were possibly due to domestic wastewater, runoff from adjacent farmland that carries copper-containing fertilizer & fungicide and a nearby quarry site wastewater that joins the river water (Vasiliu et al. 2020). On the other hand, the maximum values observed during the dry season were possible because of the dumping of solid waste at the river bank and into the river (Ghannam 2021).
Zinc concentrations were in the range of 0.08 mg/l (T4) to 0.271 mg/l (W5) and 0.048 mg/l (T1) to 0.214 mg/l (W5) during the wet and dry seasons, respectively (Fig. 2g). All Sample points, with the exception of Sample points T1 (0.048 mg/l) and T4 (0.05 mg/l) during the dry season alone, had zinc values above the permissible level in both seasons. The maximum permissible limit of zinc concentration for drinking and aquatic life is 3 mg/l (WHO 2017) and 0.05 mg/l (Goher et al. 2014), respectively. During the rainy season, zinc concentrations were higher than in the dry season. It was also higher near the town than it was at the upper and intermediate stream sampling spots. The highest values were found in the rainy season probably due to municipal waste, as well as leachate from the landfill site (Mekonnen et al. 2020) and the runoff from agricultural land that brings artificial fertilizers, and fungicides into the river (Xiao et al. 2019). Alternatively, during the dry season, the highest value was reported at downstream sampling stations, possibly due to zinc desorption from bottom sediments into the water column. At high quantities in water bodies, zinc is harmful for the existence of aquatic life (Li et al. 2018; Zhong et al. 2018). During both seasons, zinc concentrations in the river were under the acceptable limit for drinking, but not safe for aquatic life, with the exception of T1 and T4 in the dry season.
Nitrate concentrations were in the range of 19 mg/l (T4) to 44.5 mg/l (W4) and 2.3 mg/l (T1) to 6.17 mg/l (W4) during the wet and dry seasons, respectively (Fig. 2b). The maximum nitrate permissible limits are 50 mg/l (WHO 2017) and 2.93 mg/l (Goher et al. 2014) for drinking and aquatic life, respectively. During both seasons, nitrates were below the permissible limit for drinking in all Sample points. Except for T1 and T2 Sample points during the dry season, all nitrate concentrations were above the acceptable limit of 2.93 mg/l for aquatic life in all Sample points for both seasons. In the current study, the observed nitrate content was raised at downstream sample spots than in upper and middle sections of the river during both seasons. The highest values during the rainy season were probably due to domestic sewage from both rural and urban areas (Wolkite and Gubre) as well as runoff from surrounding farmland that carries nitrogen-containing fertilizers (Tadesse et al. 2018). The highest nitrate concentration recorded at sample point W4, were possibly be attributed to indiscriminate disposal of solid waste into the river and near the river bank, as well as nitrate release from the river’s bottom deposits (Suteja and Purwiyanto 2018).
Parameters in above acceptable limit according to WHO and CCME
The COD concentrations were in the range of 27.32 mg/l (T2) to 64 mg/l (W4) and 12.3 mg/l (T4) to 26.1 mg/l (W5) during the wet and dry seasons, respectively (Fig. 2a). The COD permissible limits are 10 mg/l (WHO 2017) and 7 mg/l (Goher et al. 2014) for drinking purposes and aquatic life’s, respectively. COD values were higher than the permissible limit in all Sample points for drinking purposes and aquatic life. The higher values that were conceivable might be due to increased chemical pollutants from the nearby agricultural land and domestic waste from towns (Wolkite and Gubre) that enter along with runoff. The higher COD level at sampling station W5 during the dry season was due to direct dumping of solid waste into the river and near to the riverbank (Mustapha et al. 2013). During all study periods, however, the observed mean COD concentration surpassed the maximum legal limit for drinking and aquatic life in all sample points. COD levels above a certain threshold can endanger both humans and aquatic life by diminishing dissolved oxygen in the water (Hamlat et al. 2017).
Cadmium concentrations were in the range of 0.005–0.032 mg/l and 0.003–0.01 mg/l during the wet and dry seasons, respectively (Fig. 2e). According to WHO and CCME, the maximum cadmium permitted levels for drinking and aquatic life are 0.003 mg/l (WHO 2017) and 0.001 mg/l (Goher et al. 2014), respectively. The highest value during the wet season was probably due to the inflow of domestic sewage from both towns, leachate from the nearby landfill sites and runoff from agricultural land carrying agrochemicals and fertilizers (Eliku and Leta 2018; Sahle et al. 2019). Furthermore, increased cadmium levels could be due to leaching from nickel–cadmium-based car batteries and other metal wastes from garages that mix with river water. The highest value reported during the dry season was possibly due to a decrease in river water volume and an increase in metallic ion content (Edokpayi et al. 2020). Besides, the highest result during the dry period may be attributable to the following release of cadmium ions from bottom sediments into the water column (Chen et al. 2021). Cadmium is hazardous to freshwater aquatic life even in tiny concentrations, and to humans as causing damage to the kidneys, liver, skeletal system, cardiovascular system, eyesight, and hearing capacity (Patrick and Baawain 2019).
During both seasons, the cadmium content was above the maximum permissible limit for drinking, with the exception of T2 and T4 in the dry season. The values in the current study were lower, especially during the dry season, compared to the results found by Bushero et al., (2022) on the Akaki River, which range from 0.05 to 0.14 mg/l. In the case of the Little Akaki River, this might be because industrial wastewater is present. Surface runoff and the leachate entry might be the reason for higher concentrations of cadmium in the case of Wabe River water quality results during the wet season. Higher Cadmium concentrations in water are not safe for aquatic life sustainability.
Water quality index (wet and dry seasons)
Evaluation of river water quality for drinking purposes
The Weighted Arithmetic Water Quality Index (WAWQI) and Metal Index (MI) methods were used to evaluate the Wabe river water suitability for drinking purposes. The WAWQI was calculated using parameters like as pH, DO, TDS, BOD5, COD, NO3−, PO43−, Cl−, SO42−, Na+, Ca2+, K+, Mg2+, TH, and Turbidity throughout the evaluation process. During both seasons, BOD5, COD, Turbidity, and PO43− were all above the permissible level for drinking in most of the sample points. Heavy metals such as Cd2+, Cu2+, and Zn2+ were used in the MI calculation for aquatic life appropriateness and drinking purposes. Except Sample points T2 and T4 during the dry season, cadmium was the only element that exceeded the drinking water limit in all sample points during both seasons. Metals such as Pb2+ and Ni2+, on the other hand, were not included in the MI calculation since they were not within the instrument's detection limits in all sample points during both seasons.
The calculated WAWQI found that the river water quality for drinking purposes was inadequate in all sampling stations during the rainy season (> 100) (Table 1). The highest WAWQI value was found at sample point W6 (487.8) (downstream portion of dump site), which could be related to greater organic matter, turbidity, phosphorus, and metal concentrations. During the wet season, the downstream sample locations W4 (427.4), W5 (465.9), and W6 (487.8) had the highest WAWQI readings comparing with the upstream Sample points T4 (205.4), T2 (213.2), and T1 (263.6). However, in general, all Sample points fall in the ‘unsuitable’ category in the rainy season, which are not suitable for drinking purposes. The classification of the water quality status according to WAWQI was presented in Table 1.
During the dry season, the WAWQI revealed that the Wabe River's water quality ranged from good to unsuitable (Table 1). During the dry season, sampling locations W5 and W4 are classified as "Unsuitable" and "Very Poor," respectively, and require sufficient treatment before use. W6 (59.4), W3 (58.9), W2 (62.9), W1 (58.8), T4 (53.2), T3 (66.4), and T1 (56.5) are all in the "Poor" category, which means they are unfit for drinking purposes (Table 1). T2 sample points are classified as "Good" (26–50), which suggests they are safe to drink throughout the dry season (Menberu et al. 2021). Lower results during the dry season could be attributed to reduced sediment and leachate influx into the river, as well as the self-purification mechanism (Mekuria et al. 2021).
According to MI's output (Table 2), all sample points fell into the "threshold of warning" category, with rainy season values greater than dry season values. It could be caused by silt incursion and leachate from disposal sites. Copper and zinc concentrations were found to be 'no effect' at all sampling stations during both seasons, according to the Pollution Index results. PI cadmium data revealed that Sample point W4 was categorized as "seriously" and "moderately" during the wet and dry seasons, respectively. T3, W2, W3, and W6 were categorized as "Moderately affected" during the wet season. T1, T2, T4, and W1 have been categorized as "Slightly Affected". W2 was categorized as "Strongly Affected" (Table 3). Sample stations W4 and W5 were categorized as "Moderately affected" during the dry season. T1, T3, W1, W2, W3, and W6 have been categorized as "slightly affected" sampling locations. T2 and T4 sampling locations have been categorized as having "No effect" (Table 3).
The IDW map depicted the spatial variations of both WAWQI and MI (Fig. 3). Wabe river water was unfit for human consumption without treatment, according to WAWQI and MI findings. The Wabe river water deterioration is caused by soil erosion, runoff from urban areas and agricultural fields, leachate from traditional dump sites, swimming, washing clothes, and cars.
Appraisal of river water quality for aquatic life and aquatic ecosystem
The Metal Index was used to determine whether river water was suitable for aquatic life. During the wet and dry seasons, the computed MI values ranged from 53.9 to 186.1 and 33.5 to 94.3, respectively (Table 2). Because of the shift in heavy metal content, the estimated MI values had disparities in both spatial and seasonal outcomes (Guo et al. 2022). The IDW approach was used to interpolate the spatial fluctuation of the MI value in order to indicate the extent of spatial pollution (Fig. 4). Accordingly, all of the Sample points fell in the category of "thresholds of warning," indicating that the river water is unsafe for aquatic life.
The Wabe river water's suitability for aquatic life was determined using the Pollution Index (PI). Copper concentrations fell in the category of “Seriously affected” at all sample points during both seasons. This could be caused to silt, leachate, or residential wastewater input (USEPA 2007). Cadmium concentrations have been categorized as "seriously affected" at all sampling locations throughout the wet season. During the dry season, sample sites W4 (6.08) and W5 (7.36) were designated as "seriously affected," sample points T1, T3, W1, W2, W3, and W6 were categorized as "strongly affected," and sample points T2 and T4 were categorized as "moderately affected." Higher concentrations at Sample points W4 and W5 could be attributed to sediment influx and urban wastewater (Kasa et al. 2022). Concentration of zinc During the wet season, pollution index findings at sample points W4 and W5 were "strongly affected," sample points T3, W1, W2, and W6 were "Moderately affected," and sample points T1, T2, T4, and W3 were in the "Slightly affected" category. During the dry season, W5 was categorized as "Strongly affected", W2 and W4 as "Moderately affected", T2, T3, W1, W3 and W6 as "Slightly affected", and T1 and T4 as "No effect". Higher zinc concentrations may be related to soil erosion (Kasa et al. 2022) (Table 3). Accordingly, the Wabe river water was highly contaminated and unsafe for aquatic life.
Potential risk of heavy metals on aquatic ecosystem
The degree of contamination (Cd) and ecological risk factors (Er) were utilized to calculate the grade of danger posed by toxic heavy metals to the aquatic life. The calculated degree of contamination ranged from 53.9 to 186.14 during the wet season and 33.46 to 94.28 during the dry season (Table 5). During the wet and dry seasons, the Cadmium (Cd) metal contamination factors (Cf) were highest at W4 (32 & 8.4) and lowest at T2 (5 & 3), respectively. During the wet and dry seasons, copper (Cu) metal contamination factors (Cf) were highest at W4 (150) & W5 (80), and lowest at T1 (45 & 27.5). These highest results might be attributed to the inflow of leachate from dumping sites, which are adjacent to these sample positions. During both seasons, the contamination factor is classified as "heavy" (> 3) at all sampling stations (Astatkie et al. 2021).
The highest levels of Cd were found during the wet season compared to the dry season samples. Similarly, during the rainy season, the degree of heavy metal contamination was higher at the downstream sample sites than in the intermediate and upper river sections, such as in the series W4 > W5 > W3 > W6 > W2 > T3 > T2 > T4 > W1 > T1. During the dry seasons, the estimated Cd values were in the order W5 > W4 > W3 > W2 > T3 > W6 > T4 > T2 > W1 > T1. During both seasons, however, the degree of contamination was characterized as very high (Cd > 32) for each sample site.
Ecological risk factors and PERI
The ecological risk factor (Er) for Zinc, Copper, and Cadmium during the wet season in this study was in the range of 1.6–5.4 (low risk), 225–750 (high risk to dangerous), and 150–960 (significant to dangerous), respectively (Table 6). The Er for Zinc, Copper, and Cadmium during the dry season was ranged from 1 to 4.3 (low), 137.5 to 400 (significant-dangerous), and 90 to 300 (significant-high risk), respectively. Copper had a higher level of pollution and a higher risk factor than Cadmium and Zinc at all sample stations during both seasons, with the exception of W4 and T1. During the wet and dry seasons, the computed PERI was in the range of 436.9–1714.1 (strong–very high risk level) and 288.5–704.3 (Moderate–very high risk level), respectively (Table 6). The IDW approach was used to interpolate the seasonal and spatial fluctuation in risk value (Fig. 4). As a result, heavy metals, mainly copper and cadmium, had significantly degraded the water quality of the Wabe River. This might have been caused by pollutant runoff from the watershed, indiscriminate solid waste disposal, soil erosion, leaching, and weathering (Gu 2018).
Sources and influencing factors for pollution
Natural and human causes altered the ion composition of river water samples. The effect of these factors on the key chemical composition of river water can be intuitively judged using the Gibbs diagram (Gibbs 1970). The atmospheric precipitation zone is in the lower right of the Gibbs diagram, when the TDS of the water sample point is low and Na+/ (Na+ + Ca2+) or Cl/ (Cl + HCO3) is high. The rock weathering zone lies to the left of the centre, with a water sample point with a medium TDS and a Na+/(Na+ + Ca2+) or Cl/(Cl + HCO3) ratio of about 0.5. The evaporation concentration zone is in the upper right with a high TDS and a high Na+/ (Na+ + Ca2+) or Cl/ (Cl + HCO3) value.
All of the samples from the Wabe River were concentrated in the dominating area of rock weathering, indicating sediment influx, according to the current study Gibbs diagram in the wet season samples (Fig. 5a). The Gibbs diagram during the dry season, on the other hand, revealed two types of patterns. During the dry season, all sample points showed in between the rock dominance zone and evaporation zone except T2, W1, and T1 in the left side diagram (Fig. 5b). It exhibits a mixed regulating mechanism when sample points fall between rock dominance and evaporation (i.e. the influencing factor was the combination of both rock weathering and evaporation). Also, T2, W1, and T1 sample points were concentrated outside of the broken line. Similarly, all sample points on the right-hand graphic were concentrated outside of the broken line (Fig. 5b). When sample points fall outside of a broken line, anthropogenic activities are the source of contaminants.
Hydro-chemical composition of Wabe river water
Analytical values were plotted on a piper diagram for both dry and rainy seasons to determine the hydro-chemical composition of river water and water type (Fig. 6a, b). Two triangles and one diamond make up the piper diagram. The two triangles, each with four zones, have the same goal to demonstrate which cations and anions have dominated in the study area. The diamond-shaped surface, on the other hand, is capable of accentuating the study area's water type and contains six separate zones (I-VI) that depict diverse water kinds, as noted in the diagram's description section.
During both sampling periods in this investigation, all water sample points were concentrated in zone II (Fig. 6a, b), indicating Strong acids had a higher content than weak acids, while alkaline earth metals had a larger content than alkalis. As a result, the Wabe River's hydro-chemistry type was mixed Calcium—Magnesium—Chloride during both the rainy and dry sample events. During the rainy season, the left and right-side triangles revealed that all sample points were in the "no dominant type" zone, indicating that no cations or anions were present.
Conclusion
The Wabe River's water was gathered from ten sampling locations for a total of 60 samples during both the rainy and dry seasons, and various indices including WAWQI, MI, PI, and PERI were calculated. Turbidity, organic matter (COD, BOD5), phosphorus (PO43−), and cadmium (Cd2+), as well as nitrates (NO3−), copper (Cu2+), cadmium (Cd2+), and zinc (Zn2+), were all above the permissible limits for drinking purposes and aquatic life. Piper diagrams confirm the calcium—magnesium—chloride content in Wabe River water. Since all sampling stations showed WAWQI values greater than 100 and MI values greater than 1, especially during the wet season. This is due to the influx of organic matter, nutrients, and metals into the river. The Wabe River water is deemed to be "seriously" to "slightly" polluted for drinking purposes by the Pollution Index (PI), due to cadmium contamination. According to the Metal Index and Pollution Index data, the river water is contaminated with cadmium, copper, and zinc, making it dangerous for aquatic life. Ecological risk factors and PERI both rate river water as "very high-risk" and "high-risk to dangerous. According to Gibb's plots, the main causes of the elevated amounts of organic matter, nutrients, and metals in the Wabe River are agricultural runoff, landfill leachate, and sediment influx. According to the findings of this study, the Wabe River water is inadequate for drinking and aquatic life sustainability. Therefore, river restoration is essential for the sustainability of local communities and the preservation of aquatic life. The study recommended relocating the landfill site. Further studies should determine point and nonpoint sources, fate, and transport of pollutants by modeling studies and controlling contamination from urban and agricultural runoff.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ameen HA (2019) Springwater quality assessment using water quality index in villages of Barwari Bala, Duhok, Kurdistan Region Iraq. Appl Water Sci 9(8):1–12. https://doi.org/10.1007/s13201-019-1080-z
APHA (2012) Standards methods for the examination of water and wastewater, 23 edn. American Public Health Association
Asnake K, Worku H, Argaw M (2021) Integrating river restoration goals with urban planning practices: the case of Kebena river, Addis Ababa. Heliyon 7(7):e07446. https://doi.org/10.1016/j.heliyon.2021.e07446
Astatkie H, Ambelu A, Mengistie E (2021) Contamination of Stream sediment with heavy metals in the awetu watershed of Southwestern Ethiopia. Front Earth Sci 9:658737. https://doi.org/10.3389/feart.2021.658737
Bhateria R, Jain D (2016) Water quality assessment of lake water: a review. Sustain Water Resour Manag 2:161–173. https://doi.org/10.1007/s44840899-015-0014-7
Bougherira A, Hani A, Djabri L, Toumi F, Chaffai H, Haied N, Nechem D, Sedrati N (2014) Impact of the urban and industrial wastewater on surface and groundwater, in the region of Annaba, (Algeria). Energy Procedia 50:692–701
Bushero DM, Angello ZA, Behailu BM (2022) Evaluation of hydrochemistry and identification of pollution hotspots of little Akaki river using integrated water quality index and GIS. Environ Challenges 8:1–11. https://doi.org/10.1016/j.envc.2022.100587
Canadian Council of Ministers of the Environment (CCME) (2007) A Protocol for the Derivation of Water Quality Guidelines for the Protection of Aquatic Life. In Canadian Environmental Quality Guidelines. 1999. Winnipeg, Canadian Council of Ministers of the Environment. http://documents.ccme.ca/
CESI (2009) Gibe III hydroelectric project—environmental and social impact assessment. Mid-day international consulting engineering, 1–347. https://www.afdb.org/fileadmin/uploads/afdb/Documents/Environmental-and-Social-Assessments/G3 ESIA
Chen L, Little SH, Kreissig K, Severmann S, McManus J (2021) Isotopically light Cd in sediments underlying oxygen deficient zones. Front Earth Sci 9:623720. https://doi.org/10.3389/feart.2021.623720
Edokpayi JN, Makungo R, Mathivha F, Rivers N, Volenzo T, Odiyo JO (2020) Influence of global climate change on water resources in South Africa: toward an adaptive management approach, Chapter 5. In: Singh P, Milshina Y, Tian K, Gusain D, Bassin J (eds) Water Conservation and Wastewater Treatment in BRICS Nations. Elsevier, Amsterdam, pp 83–115
Egbueri JC (2022) Predicting and analysing the quality of water resources for industrial purposes using integrated data-intelligent algorithms. Groundwater Sustain Dev 100794
Eliku T, Leta S (2018) Spatial and seasonal variation in physicochemical parameters and heavy metals in Awash River, Ethiopia. Appl Water Sci 8(6):1–13. https://doi.org/10.1007/s13201-018-0803-x
Europian Communities (1989) Quality of surface water intended for abstraction of drinking water regulation
Galal Uddin M, Nash S, Olbert AI (2021) A review of water quality index models and their use for assessing surface water quality. Ecol Indicators. https://doi.org/10.1016/j.ecolind.2020.107218
Ghannam HE (2021) Risk assessment of pollution with heavy metals in water and fish from River Nile, Egypt. Appl Water Sci 11(7):1–10. https://doi.org/10.1007/s13201-021-01449-7
Gibbs RJ (1970) Mechanism controlling world’s water chemistry. Science 170:1080–1090
Goher ME, Hassan AM, Abdel-Moniem IA, Fahmy AH, El-Sayed SM (2014) Evaluation of surface water quality and heavy metal indices of Ismailia Canal, Nile River, Egypt. Egypt J Aquatic Res 40(3):225–233. https://doi.org/10.1016/j.ejar.2014.09.001
Gu Y-G (2018) Heavy metal fractionation and ecological risk implications in the intertidal surface sediments of Zhelin Bay South China. Mar Pollut Bull 129(2):905–912. https://doi.org/10.1016/j.marpolbul.2017.10.047
Guo W, Zou J, Liu S, Chen X, Kong X, Zhang H, Xu T (2022) Seasonal and spatial variation in dissolved heavy metals in Liaodong Bay, China. Int J Environ Res Public Health 19:608. https://doi.org/10.3390/ijerph19010608
Hamid A, Bhat SU, Jehangir A (2020) Local determinants influencing stream water quality. Appl Water Sci 10(24):1–16. https://doi.org/10.1007/s13201-019-1043-4
Hamlat A, Guidoum A, Koulala I (2017) Status and trends of water quality in the tafna catchment: a comparative study using water quality indices. J Water Reuse Desalination 7(2):228–245. https://doi.org/10.2166/wrd.2016.155
Imneisi IB, Aydin M (2016) Water quality index (WQI) for main source of drinking water (Karaçomak Dam) in Kastamonu City, Turkey. J Environ Anal Toxicol 6:407. https://doi.org/10.4172/2161-0525.1000407
Okullo JO, Moturi WN, Ogendi GM (2017) Open defaecation and its effects on the bacteriological quality of drinking water sources in Isiolo County, Kenya. Environ Health Insights 11:1–8. https://doi.org/10.1177/1178630217735539
Kasa T, Bassa AL, Negatu GT, Sahile ZA, Reddythota D (2022) Investigation of Wabe River water fitness for agricultural and industrial purposes. Heliyon 8(12):e11865. https://doi.org/10.1016/j.heliyon.2022.e11865
Leta MK, Dibaba WT (2019) Assessment of physico-chemical parameters of Awetu River. J Water Sustain 9(1):13–21. https://doi.org/10.11912/jws.2019.9.1.13-21
Li XF, Feng PFWCL, Chen DQLJK, Wu FC (2018) Acute toxicity and hazardous concentrations of zinc to native freshwater organisms under different pH values in China. Environ Contam Toxicol 103:120–126. https://doi.org/10.1007/s00128-018-2441-2
Lin L, Yang H, Xu X (2022) Effects of water pollution on human health and disease heterogeneity: a review. Front Environ Sci 10:880246. https://doi.org/10.3389/fenvs.2022.880246
Marara T, Palamuleni LG (2019) An environmental risk assessment of the Klip river using water quality indices. Phys Chem Earth 09(1):1–8. https://doi.org/10.1016/j.pce.2019.09.001
Mekonnen B, Haddis A, Zeine W (2020) Assessment of the effect of solid waste dump site on surrounding soil and river water quality in Tepi Town. Southwest Ethiopia. J Environ Public Health 2020:5157046. https://doi.org/10.1155/2020/5157046
Mekuria DM, Kassegne AB, Asfaw SL (2021) Assessing pollution profiles along Little Akaki River receiving municipal and industrial wastewaters, Central Ethiopia: implications for environmental and public health safety. Heliyon 7(7):e07526. https://doi.org/10.1016/j.heliyon.2021.e07526
Menberu Z, Mogesse B, Reddythota D (2021) Evaluation of water quality and eutrophication status of Hawassa Lake based on different water quality indices. Appl Water Sci 11:61. https://doi.org/10.1007/s13201-021-01385-6
Mustapha A, Aris AZ, Juahir H, Ramli MF, Kura NU (2013) River water quality assessment using environmentric techniques: a case study of Jakara River Basin. Environ Sci Pollut Res Int 20(8):5630–5644. https://doi.org/10.1007/s11356-013-1542-z
Nartey VK, Hayford EK, Ametsi SK (2012) Assessment of the impact of solid waste dumpsites on some surface water systems in the Accra Metropolitan Area, Ghana. J Water Resour Protect 4:605–615. https://doi.org/10.4236/jwarp.2012.48070
Ojekunle OZ, Ojekunle OV, Adeyemi AA, Taiwo AG, Sangowusi OR, Taiwo AM, Adekitan AA (2016) Evaluation of surface water quality indices and ecological risk assessment for heavy metals in scrap yard neighbourhood. Springerplus 5(560):1–16. https://doi.org/10.1186/s40064-016-2158-9
Patrick A, Baawain MS (2019) Effects of pollution on freshwater aquatic organisms. Water Environ Res 90:1272–1287
Preisner M (2020) Surface water pollution by untreated municipal wastewater discharge due to a sewer failure. Environ Process 7(3):767–780. https://doi.org/10.1007/s40710-020-00452-5
Rahman MS, Saha N, Molla AH (2014) Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ Earth Sci 71(5):2293–2308. https://doi.org/10.1007/s12665-013-2631-5
Reckendorf A, Siebert U, Parmentier E, Das K (2023) Chemical pollution and diseases of marine mammals. In: Brennecke D, Knickmeier K, Pawliczka I, Siebert U, Wahlberg M (eds) Marine mammals. Springer, Cham. https://doi.org/10.1007/978-3-031-06836-2_5
Reddythota D, Timotewos MT (2022) Evaluation of pollution status and detection of the reason for the death of fish in Chamo Lake Ethiopia. J Environ Public Health 2022:5859132. https://doi.org/10.1155/2022/5859132
Sahle M, Saito O, Fürst C, Yeshitela K (2019) Quantifying and mapping of water-related ecosystem services for enhancing the security of the food-water-energy nexus in tropical data–sparse catchment. Sci Total Environ 646:573–586. https://doi.org/10.1016/j.scitotenv.2018.07.347
Sedeño-Díaz JE, López-López E, Mendoza-Martínez E, Rodríguez-Romero AJ, Morales-García SS (2019) Distribution coefficient and metal pollution index in water and sediments: proposal of a new index for ecological risk assessment of metals. Water 12(1):29. https://doi.org/10.3390/w12010029
Smitom SD, Ajay SB, Kalamdhad S (2021) Heavy metal pollution and potential ecological risk assessment for surficial sediments of Deepor Beel. India Ecological Indicators 122:107265
Suteja Y, Purwiyanto AIS (2018) Nitrate and phosphate from rivers as mitigation of eutrophication in Benoa bay, Bali-Indonesia. Earth Environ Sci 162:1–9
Suwari (2021) Analysis of water quality status using method of water pollution index: a case study on the Dendeng River. Int J Res 9(5):200–218. https://doi.org/10.29121/granthaalayah.v9.i5.2021.3937
Tadesse M, Tsegaye D, Girma G (2018) Assessment of the level of some Physico-chemical parameters and heavy metals of Rebu river in Oromia region Ethiopia. J Biol Med 2(4):99–118. https://doi.org/10.15406/mojbm.2018.03.00085
Theodore HT (2018) John Snow, Cholera, the Broad Street Pump; Waterborne Diseases Then and Now. Case Studies in Public Health 2018:77–99
USEPA (2007) Aquatic life ambient freshwater quality criteria—Copper 2007. U. S. Environmental Protection Agency Office of Water Office of Science and Technology Washington, DC. http://www.epa.gov/waterscience/criteria/aqlife.html
Ustaoğlu F, Taş B, Tepe Y, Topaldemir H (2021) Comprehensive assessment of water quality and associated health risk by using physicochemical quality indices and multivariate analysis in Terme River, Turkey. Environ Sci Pollut Res 28(44):62736–62754
Vadde KK, Wang J, Cao L, Yuan T, McCarthy AJ, Sekar R (2018) Assessment of water quality and identification of pollution risk locations in Tiaoxi River (Taihu Watershed), China. Water 10(183):1–18. https://doi.org/10.3390/w10020183
Vasiliu D, Bucse A, Lupascu N (2020) Assessment of the metal pollution in surface sediments of coastal Tasaul Lake (Romania). Environ Monit Assess 192(749):1–16
Weldeyohanis YH, Aneseyee AB, Sodango TH (2020) Evaluation of current solid waste disposal site based on socio-economic and geospatial data: a case study of Wolkite town, Ethiopia. GeoJournal. https://doi.org/10.1007/s10708-020-10268-3
WHO (2017) Guidelines for drinking-water quality, 4th edn. World Health Organization, pp 1–518
World Health Organization (WHO) (2014) Global status report on noncommunicable diseases 2014. Geneva, Switzerland: WHO; 2014. http://www.who.int/nmh/publications/ncd-status-report-2014/en/
Xiao J, Wang L, Deng L, Jin Z (2019) Characteristics, sources, water quality and health risk assessment of trace elements in river water and well water in the Chinese Loess Plateau. Sci Total Environ 650:2004–2012. https://doi.org/10.1016/j.scitotenv.2018.09.322
Zhong W, Zhang Y, Wu Z, Yang R, Chen X, Yang J, Zhu L (2018) Health risk assessment of heavy metals in freshwater fish in central and eastern North China. Ecotoxicol Environ Saf 157:343–349. https://doi.org/10.1016/j.ecoenv.2018.03.048
Acknowledgements
The authors are grateful to the 2Ministry of Water, Irrigation & Energy, Addis Ababa, Ethiopia, for providing major secondary data.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Tilahun Kasa: Sampling and Analysis, Data Collection, Writing-draft preparation, Visualization, Investigation, Software, Validation. Daniel Reddythota: Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Writing- Reviewing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kasa, T., Reddythota, D. Investigation of the Wabe River water’s suitability for drinking purposes and aquatic life and detection of pollution sources. Appl Water Sci 13, 154 (2023). https://doi.org/10.1007/s13201-023-01952-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01952-z