Abstract
In this work, crash precipitation technique was employed to synthesize a visible light-responsive tungsten trioxide (WO3) photocatalyst using ammonium paratungstate as tungsten precursor. The spray-dried (120 °C) and calcined (600 °C) WO3 powder was characterized by analyzing methods of XRD, PSD, BET and BJH, Raman, FESEM/EDX, FTIR, UV–vis DRS and XPS spectroscopy. X-ray diffraction (XRD) and Raman studies confirm the well crystalline monoclinic crystal structure. Scanning electron microscopy (SEM) images showed micron-sized spherical bulks of WO3 particles with needle-like morphology. A normal distribution with a d50 (median diameter) value of 6.0 μm was observed with particle size analysis. Much enhanced BET surface area of 102 g/m2 with wide pore size 1.8 nm is measured compared to commercially available WO3 that results in increased pollutants surface adsorption. Fourier transform infrared spectroscopy (FTIR) study demonstrated that the calcined sample surface is enriched with bonded hydroxyl groups, beneficial for powder particles activity. Photocatalyst band gap was calculated by considering the absorbance measurements recorded on UV–vis diffuse reflectance spectroscopy (DRS). The energy value of 2.6 eV was calculated which lies in the visible light region while X-ray photoelectron spectroscopy (XPS) analysis showed 6 + oxidation state for tungsten. Bulk WO3 sphere photocatalytic activity was evaluated through the exertion of synthetic textile methylene blue (MB) dye and sulfamethoxazole (SMX) pharmaceutical antibiotic. The obtained activity results showed 85% and 100% degradation for MB and SMX under 100 min visible light irradiation. We expect that our work may provide a new sample for energy production (H2) through water photolysis, gas sensing and soft matter research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last two–three decades, our ecosystem is badly damaged by industrial aqueous wastes, such as heavy metals (Pb, Hg, As), textile dyes (methylene blue, methyl orange and red), organic compounds (phenol and chlorophenol), pharmaceutical drugs, etc. Thanks to the increasing awareness of communities for environmental protection, which compelled the researchers to address the irreparable damage caused by these effluents discharge into fresh water (Ghashghaie et al. 2022; Ostad-Ali-Askari 2022). Heterogeneous photocatalysis received tremendous attention to be a suitable tool for the decomposition of aqueous pollutants. Therefore, it is awarded as a green technology for the treatment of almost all type of toxic and recalcitrant contaminants sourced from industries and hospitals and discharged into water resources (Vamvasakis et al. 2015). Semiconductor photocatalyst utilization for the treatment of the environment aroused the interest of many due to its versatility in removing a plethora of contaminants, including in the gaseous and liquid phases (Ramos-Delgado et al. 2013). Among semiconductor oxides, titanium dioxide (TiO2) is one of the best studied photocatalysts, because its conduction and valance band energies are suitable for both redox processes, i.e., oxidation of oxygen and reduction of water molecules (Banerjee et al. 2015; Nagy 2016). However, there are some limitations which restrict the usage of TiO2; for example, it possesses relatively fast photoinduced electron–hole pairs recombination; in addition, its light absorption properties lie in the ultraviolet (UV) range due to its large band gap of ̴ 3.2 eV. The 3–5% of the UV radiation coming from the sun gives motivation to the researchers to work out on other semiconductors oxides (WO3, V2O5, Fe2O3, BiVO4, CdSe etc.) with the ability to be excited by visible light (containing 45% of the solar light spectrum) (Nagy 2016). Nano-structured WO3 is a promising n-type semiconductor oxide because of its suitable band gap (in range of 2.6–2.8 eV), thus making it the second most search out photocatalyst activated under visible light. In addition, it has non-toxicity, oxygen evaluation ability, strong physical and chemical stability under acidic condition in aqueous solution, no-photo corrosion, cost-effectiveness and satisfactory visible light (~ 12–15%) absorption and long minority carrier diffusion length (~ 500 nm to 6 μm) (Coridan et al. 2014; Hong et al. 2009). Therefore, high potential of this material led to its wide applications in electro-catalyst, photochromic, anticancer, gas sensing (Senguttuvan 2010), electrochemical energy storage (Shinde and Jun 2020) and in photoelectrochemical water oxidation (Zheng 2019). Among such wide range of applications, specifically, their photocatalytic ability to initiate decomposition of dyes and harmful organic compounds has stirred significant concern (Vamvasakis et al. 2015; Hunge 2016; Wicaksana et al. 2014; Zhao and Miyauchi 2008; Hayat et al. 2011; Bamwenda and Arakawa 2001; Wang et al. 2011; Murillo-Sierra et al. 2021). For such application, the synthesis of tungsten oxide (WO3) nano-structure with high crystallinity is pre-requisite, since the presence of amorphous phase assists the photoinduced electron–hole (e−/h+) pairs recombination, leading to a drastic decrease in catalytic activity decay (Usami et al. 2012). Moreover, photocatalysis is a surface phenomenon; the interaction and adsorption of the organic molecules on the WO3 surface will define its activity. The synthetic techniques followed for the preparation of WO3 are: hydrothermal, anodization, microwave, precipitation technique, chemical etching, thermal evaporation, electro-spinning, RF (radio frequency) sputtering, electro-deposition, solvothermal, pulse vapor deposition, hard templating approaches, chemical vapor deposition (CVD) and hot wire CVD (Breedon 2010; Karthik 2017; Houx et al. 2010; Tahir 2018; Jadkar, et al. 2017; Hsieh et al. 2011; Heidari et al. 2010). But each of these techniques has one or more limitations, including high energy requirement with expensive and complicated equipment requirement, vacuum-dependent, mesoporous silica as sacrificial framework requirement, complex material preparation procedure, and some of them are often unable to produce nano-crystalline WO3 morphologies. Hence, it is essential to utilize a simple protocol to construct the required nanostructures for obtaining an effective and efficient photocatalysts. For this purpose, a variety of methods were employed previously. Few of the studies involved enhancing the specific surface area which encompasses a mesoporous structure which led to the performance improvement in the catalyst (Usami et al. 2012).
In this work, ammonium paratungstate hydrate (NH4)10(H2W12O42)·4H2O) was used as the precursor of tungsten to prepare well crystalline WO3 structure. The method is considered simple and green chemical pathway owing to the lack of involving any volatile or toxic chemicals in this synthesis. The synthesis is carried out at room temperatures and pressure and its subsequent spray drying at ~ 120 °C. Besides, complete characterization of the material was conducted to understand its physicochemical properties and evaluated its photo-catalytic activity for the decomposition of model pollutants; a pharmaceutical antibiotic sulfamethoxazole (0.012 g/L) is never studied before with WO3 particles and of decomposition of methylene blue (a synthetic textile dye, 0.6 mg/L) in the presence of visible light.
Materials and methods
Chemicals
Anhydrous ethyl alcohol was provided by Commercial Alcohols (Boucherville, Quebec, Canada), and Fischer Scientific provided the hydrochloric acid (HCl, ACS plus certified). Ammonium paratungstate hydrate (99.99% trace metal basis, gravimetric) was acquired from prominent supplier, i.e., Sigma-Aldrich, Canada. All the received chemicals were employed without exercising any additional purification procedure; in addition, in this work we used laboratory de-ionized water during various experimental steps.
Catalyst preparation
We adopted a crash precipitation technique to prepare WO3 powder. Among water, HCl and ammonium paratungstate ((NH4)10H2(W2O7)6), the molar ratio was 500: 200: 0.005. Hydrochloric acid dissolved the ammonium paratungstate under strong magnetic stirring. Subsequently, the solution was added quickly to water, which resulted WO3 precipitates. After that, 3–4 times de-ionized water (6 × 100 mL) was used to wash the obtained WO3 precipitates. Finally, the stable WO3 suspension was obtained by adding de-ionized water (30 mL) to the precipitate under constant magnetic stirring for 3 h.
An experimental protocol referred by (Nooshin et al. 2017) and Yamato GB-22 dryer (equipment) was used to prepare the final WO3 material. The dried sample was calcined in programmable muffle furnace at a temperature of 600 °C for 3 h to obtain the final crystalline form of WO3.
Characterization methods
A Phillips X'PERT diffractometer was used to record the X-ray diffraction (XRD) patterns to study the crystal structure and powder crystallinity. Monochromatic high-intensity radiations (Cu-kα (λ = 1.54178 Å)) were used for scanning. The operating conditions were set as 40 mA and 30 kV and 2θ = 10–80° range at a scanning rate of 0.08°/s. The photocatalyst particle size distribution (PSD) was recorded on 950 Horiba laser diffractometer. We describe the median diameter d50 and the volume moment mean diameter \(\left[4,3\right]= \frac{{N}_{i}{d}_{i}^{4}}{{N}_{i}{d}_{i}^{3}}\), where Ni is particles number with the corresponding di diameter. The sample was degassed under vacuum at 200 °C for 20 h and N2 adsorption and desorption isotherms at 77 K were used to estimate the textural properties of the material on a Quantachrome Autosorb-1 Instrument. To investigate the catalyst surface functional groups, Bruker Tensor 27 with OPUS data collection program (V 1.1) and the reference potassium bromide was employed to note the FTIR spectra of the powder in the energy range of 5000–400 cm−1. The specific surface area was calculated with the Brunauer–Emmett–Teller (BET) method (P/Po:0.05–0.30, C constant: 50–200), and Barrett–Joyner–Hallender (BJH) theory (desorption branch, P/Po:0.15–0.995) was applied to calculate the specific surface area and to measure the mesopore size distribution. The surface morphology of the powder was examined on a field emission scanning electron microscope (FESEM-JEOLJSM-7600F, operated voltage (10 kV) and energy range of 10 keV), and attached energy-dispersive X-ray (EDX) detector was employed to testify the presence of oxygen and tungsten through elemental distribution maps. Diffuse reflectance spectra (DRS) were measured using Thermo Fisher Scientific evolution 300 PC UV–vis spectrophotometer by attaching Pike technologies EasiDiff. DRS was measured in range of 190–900 nm. In addition, potassium bromide (KBr) powder was employed as reference in the analysis. Kubelka–Munk function calculations method enables us to calculate the photocatalyst energy value from the obtained spectrum. Renishaw InVia Reflex Raman microscope using 514-nm excitation laser was used to obtain Raman spectra of the synthesized samples. Exposure time was fed as 10 s and a power of 300 mW at room temperature. The elemental oxidation states were investigated by using X-ray photoelectron spectroscopy. VG ESCALAB 3 Mark II equipment employing X-ray source of Al Kα (1486.6 eV) was used. First, a survey spectrum was obtained for the identification of all the elements in the sample. Subsequently, the identified elements were subjected to a high-resolution spectrum measurements (pass energy of 20 eV and 0.05 eV steps). For the processing of the obtained data, a VG Avantage software was utilized; all the peaks were regulated with the reference C 1 s peak at 285.0 eV, attributed to adventitious carbon.
Visible activity experiments
We tested the activity of the WO3 photocatalyst material in the decomposition of the model pollutants. In individual flasks, photocatalyst (0.1 g/L) and pollutants [MB (0.085 g/L) and sulfamethoxazole (0.012 g/L)] were suspended in laboratory de-ionized water and sonicated for an hour to lessen aggregation of photocatalyst particles. Both the pollutant solution (MB or SMX) and photocatalyst suspension were mixed in a batch reactor; more water was added to make the final volume to 0.85 L followed by magnetic stirring in the dark for 30 min to ascertain the adsorption/desorption equilibrium. Without any interruption, filtered air is supplied during the experiment.
A 180-W Heraeus single Hg medium-pressure lamp (λrange = 410–660 nm, shipped from Germany) protected in a quartz sleeve was placed vertically at the center point inside the batch reactor. A UV filter (UVPS, USA) was applied to reduce any emitted UV radiation. Each experiment under visible light illumination lasted for 70 min. The reactor suspension temperature was kept in the range of 25–30 °C by circulating cold water. Accumet TMAB15 pH meter equipped with a glass pH electrode was used to record reactor suspension pH, and throughout the activity experiments, a constant pH of 5.9–6.0 was recorded. We withdraw a 2-ml sample at regular intervals of 10 min; before the decomposition testing, the sample was filtered (Millipore filters, porosity 0.22 μm) to eliminate any possible photocatalyst particles.
For the degradation of MB, a UV–vis spectrophotometer was employed by gauging at 664 nm the MB solution maximum absorbance, and HPLC (Agilent 1100 series high-performance liquid chromatography equipped with a Zorbax Eclipse C-8column (4.6 mm × 150 mm, 3.5 μm) examination (Nasuhoglu et al. 2011) was performed to record the degradation of SMX with respect to time. The eluents consisted of acetonitrile and 20 mM NaH2PO4 (sodium dihydrogen phosphate) buffer. Acetonitrile was used such that it was varied at a uniform flowrate (0.8 mL/min) from 30% acetonitrile to 50% over 10 min. Phosphoric acid was used to maintain the pH of buffer equivalent to 2.8. Diode array detector (DAD) at 262 nm was used for the detection of SMX.
Results and discussion
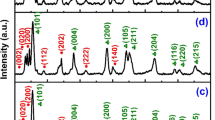
The XRD diffraction pattern for the synthesized WO3 photocatalyst is illustrated in Fig. 1a; the peak reflections are sharp and intense, indicating that the WO3 powder is well crystalline. The highest peaks indicated a monoclinic crystalline structure (ICDD ref. card no. 43–1035) which reflect that no other phase transformation took place. The diffraction peak of [2 0 0] at 2θ = 24.3° is the most intense, signifying [2 0 0] is the major growth direction. This representative peak broadening is referred to determine the crystallite size by the Scherrer formula (\(D= 0.9\lambda /\beta \mathrm{Cos}\theta\), where λ is the X-ray wavelength (15.418 nm); β is the full width at half maximum (FWHM, radians); and θ is the Bragg’s angle (degrees) approximation. The FWHM value of all the peaks was calculated on profile fitting of the noted XRD spectrum using the built-in XRD pattern processing software. The calculated crystalline size of WO3 was 24 nm (Table 1).
It was reported that the median diameter (d50) and volume moment mean diameter (D [4,3]) values were 6.0 and 6.3 μm, respectively, for calcined nanopowder in water (Fig. 1S(A), supplementary information). The values were compared with the commercially available WO3 sample; the recorded values of D[4,3] and d50 were 13.3 and 11.1 μm, respectively, illustrating that our synthesized WO3 nanoparticles are less prone to coagulation in solution. This is beneficial for the photocatalyst activity; it is obvious that aggregations of particles decrease the active surface and also have a negative effect to lessen the irradiated light access to the inner surface of the catalyst particles.
The sample textural properties (specific surface area, pore volume and size) were measured through BET and BJH analysis. As per IUPAC classification (Thommes 2015), an overlapping type 1(b)—type 2 isotherm (Fig. 1S(b), supplementary file) shows the coexistence of macro-, meso- and micropores. Materials belonging to type 1(b) have a broad micropore (< 2 nm) and a narrow mesopores (2–50 nm) size distribution, while a macroporous (> 50 nm) structure is attributed to type 2, which is the result of shortage of plateau at the maximum filling pressure. In addition, a H3 type hysteresis was observed, related to plate-like aggregates linked through a macropores network, partly filled with the adsorbate. The BET and BJH results listed in Table1 show that the prepared WO3 has much enhanced surface area of 102 m2/g in comparison with the commercial WO3 (1.8–3.0 m2/g (Li 2010; Tao 2020)). The presence of macro- and mesopores (Fig. S1b-insert) and high surface area will have greater concentration of active sites which will assist the adsorption of the pollutant molecules to decompose; as a consequence, improved photocatalytic performance may be projected (Khan and Berk 2015).
The field emission SEM (FESEM) micrographs of the calcined WO3 powder is shown in Fig. 1b. The smaller particles having needle-like structure agglomerate to form big spheres, size around 1.0 µm (Fig. 1b-insert). In addition, on close examination the micrograph also illustrates the intra-particle porous structure which is in support of the BJH analysis. It is interesting to note that regardless of the preparation technique, WO3 particles possess needle like structure, like this work stated crash precipitation technique followed by spray drying, hydrothermal method (Navarro 2014), low temperature solvothermal synthesis (Xiao 2015), combustion synthesis (Chen 2015), and chemical vapor deposition (CVD) method in which plasma bombardment of W followed by oxidation was performed (Cabrera 2012). The elemental mapping recorded with SEM–EDX (Fig. 1b-insert) clearly shows the presence of tungsten as well that of oxygen (in high proportion) supporting its stoichiometric amount in WO3.
The FTIR spectrum of WO3 nanopowder is displayed in Fig. 1c. The strong absorption peak in the range 650–1000 cm−1 corresponds to the stretching modes of O–W–O bonds (Guéry et al. 1997). The O–W, δ(H–O–H) bending mode and υ(H–O) stretching mode because of adsorption of water molecules are ascribed to the peaks at 1440, 1620 and 2075 cm−1, respectively (Jothivenkatachalam et al. 2014). The well-defined peak of W–OH stretching band is observed around 3400 cm−1 (Díaz-Reyes et al. 2008), which indicates that OH− groups bind onto the surface of the WO3 nanoparticle. During the photocatalytic process, these OH− groups play a crucial part to scavenge the photoinduced holes to generate hydroxyl radicals (OH·), which helps in the degradation of the organic pollutant molecules.
The optical property of the WO3 powder is quantified by following the procedure; first UV–vis reflectance spectrum is recorded followed by the Kubelka–Munk formulation (Khan and Berk 2015), used to convert the reflectance spectrum measurements into the equivalent absorption coefficient (α) proportional to Kubelka–Munk (F(R)) function; \(F\left(R\right)= \alpha = \frac{{(1-\mathrm{Ref})}^{2}}{2\mathrm{Ref}}\). The straight part of the Tauc plot (\({(\alpha h\vartheta )}^{n}\) versus \(h\vartheta\) (Fig. 2a), where the exponent n = 0.5) to the point α = 0 gives the band gap (Eg) energy value equal to 2.6 eV (478.0 nm) (Table 1), demonstrating the visible light spectrum absorption onset for WO3. This clearly portrays that the as-prepared WO3 powder may be effective in the visible spectrum range.
In Raman spectrum (Fig. 2b), the peak 181.2 cm−1 is ascribed to W-W bond, while the bending vibration of W–O–W bonds corresponds to 327.6, 274.3 and 137 cm−1 peaks. The O–W–O bond vibration corresponds to the peak at high wave number of 717.6 cm−1, and the stretching vibration of the crystalline WO3 of the W–O–W bending oxygen is ascribed to the peak at 807.2 cm−1. Notably as illustrated elsewhere (Chong et al. 2015; Adhikari et al. 2014), the peaks at 717.1 and 807.1 cm−1 are the two fingers print Raman peaks depicting the monoclinic crystalline WO3.
The chemical composition of the elements, carbon, oxygen and tungsten in atomic percentages (19.3, 61.0 and 19.6 at.%, respectively) is determined by conducting the X-ray photoelectron spectroscopy (XPS) analysis; in Fig. 2c the survey spectrum is displayed. We also analyzed the sample XPS spectrum by high-resolution deconvolution of the W (4f), O (1 s) and C (1 s) to evaluate the oxidation states. All peaks calibration is described to the C 1 s species, equivalent to the adventitious carbon from the XPS instrument. The peak of C 1 s (Fig. 2d on deconvolution generates two peaks indicating two carbon bonding, i.e., C–C and C–O–C at the binding energy of 285 eV and 285.8 eV, respectively. The latter is ascribed to atmospheric contamination. The high-resolution spectrum of O 1 s peak is split up into three peaks (Fig. 1S(c)—supplementary information); O–W6+ peak at 530.4 eV is ascribed to the crystal lattice oxygen, OH–W6+ bond at binding energy of 531.5 eV illustrating the surface OH− groups bonded to W and the oxygen bonded to carbon (C–O) or adsorbed water (H2Oads) peak corresponding to the binding energy at 532.9 eV. The high-resolution XPS spectrum of W4f core level is presented in Fig. 2e. It can be observed that two symmetrical peaks at 36.5 eV and 38.6 eV arise upon deconvoluting the W4f spectra which can be matched to W4f7/2 and W4f5/2 bonds, respectively. These doublet bonds energy stands for the W6+ valency state. The difference in energy value of W4f7/2 and W4f5/2 spin orbitals shows magnitude of 2.1 eV which coincides with the theoretical value of WO3, thus corroborating the reported literature (Chong et al. 2015). In summary, XPS data evidently show that the sample surface contains W in the valance state of W6+. No other valency states were noticed. On further examination, doing the O/W ratio calculation from the atomic sensitivity factor (S) and their relative peaks areas values, the ratio comes to be 2.99, signifying the stoichiometric ratio of O to W in WO3.
Photocatalytic activity tests
The photocatalytic activity of the as-synthesized WO3 powders was tested by examining the degradation of pharmaceutical antibiotic sulfamethoxazole (SMX) and methylene blue (MB) dye solution under visible light (Fig. 3). The degradation activity tests consisted of two portions: (1) Dark experiments, i.e., to permit the maximum pollutant adsorption on the surface of photocatalyst particles, which encompasses the reactor suspension (pollutant and photocatalyst) magnetically stirred for 30 min in the dark. (2) Light experiments, the reactor suspension is then subjected to visible light for the designated experimental time of 70 min. Moreover, it is compulsory to confirm the degradation of the pollutant molecules mainly done through the photocatalyst action; henceforth, it is important to separate the effect of photolysis (absence of photocatalyst material). In photolysis, the pollutant molecules are decomposed directly by absorbed photons from the light source as a result of photochemical reactions. As demonstrated in Fig. 3a, no considerable change was recorded in the concentration of MB, whereas ~ 19.0% of the SMX (Fig. 3b) was decomposed under the effect of photolysis. It is confirmed that the SMX decomposition is not likely with visible light illumination, because SMX can absorb light up to 310 nm (Nasuhoglu et al. 2011; Abellán et al. 2007). The light source used in this work radiates radiation greater than 410 nm. This clearly illustrates that the SMX decomposition, i.e., ~ 19.0% without employing WO3 catalysts, is the result of photo-oxidation rather than photolysis (Xekoukoulotakis 2011).
As illustrated in Fig. 3a, b, the enhanced activity of the as-prepared WO3 powder irradiated under visible light against the model pollutants compared to the commercial WO3 can be supported by considering the following factors. The synthesized material has improved crystallinity, large surface area via small crystallite size, wide pore size, higher degree of surface hydroxyl groups and low band gap. The photocatalytic process is a surface phenomenon: large specific surface area translates into enhanced adsorption of pollutant molecules; also, large pore size is helpful for the easy diffusion of parent pollutant and intermediate and or final product species. The WO3 powder has much wide pore diameter, illustrating the existence of meso- and macropores that lead to easy adsorption of pollutant molecules. Because the MB molecular dimension is 13.6 × 4.7 × 2.4 Å (Khan et al. 2018) and SMX molecular dimension size is 1.49 × 0.64 × 0.56 nm (Li 2022), both pollutant molecules can access pores with diameter greater than 15 Å. The good crystallinity of WO3 powder may also contribute to improved activity, because increased crystallinity minimizes the defects in the crystalline structure, whereas poorly crystalline powders have defect structure which are always prone to recombination between the photoinduced charge carriers at the defect positions. Also, charge generation, mobility and lifetime of separated charges, charge migration and trapping processes at the solution–particle interface are more superior in material possessing high level of crystallinity (Bamwenda and Arakawa 2001).
FTIR identified the high concentration of OH− groups which are advantageous for photocatalytic process activity. They consume the photoinduced VB hole (h+) to produce hydroxyl radicals (OH·), which unselectively react with pollutant species toward their degradation (Khan 2017). Moreover, low band gap value gives evidence that the photocatalyst particles are activated with longer wavelength light irradiation, i.e., visible light. The schematics shown in Fig. 3c illustrate the plausible photocatalytic mechanism; the visible light photoinduced charge carriers may be utilized in the process redox reactions. The VB holes are consumed by the OH− to produce OH• radicals, thereby used in the decomposition of the pollutant molecules. Also, the VB holes can directly degrade the pollutant species. The CB electrons are used to generate hydrogen peroxide (H2O2) which is then used to produce hydroxyl radicals for the pollutant degradation process or the H2O2 may also be used to degrade pollutant directly. In summary, the photogenerated e−/h+ pairs are successfully consumed in photocatalytic process and therefore result in the enhanced degradation of pollutant molecules, i.e., methylene blue and sulfamethoxazole.
Conclusion
We have prepared monoclinic WO3 nano-particles through a facile and economical method. The influence of WO3 nano-particles morphology and crystalline structure on its ability to photo-decompose methylene blue dye and antibiotic sulfamethoxazole using visible light was investigated. This study illustrates that the WO3 powder provides better pollutant molecules adsorption and photodegradation performance due to their superior physiochemical properties compared to commercially available WO3 powder. Higher surface area with wide pore size of the synthesized powder as depicted by the BET and BJH analysis results in enhanced pollutant molecule adsorption on the catalytic material surface; also, FTIR study portrayed greater degree of surface hydroxyl groups and adsorbed water molecule. In addition, upon visible light illumination the lower band gap of the photocatalyst helps in increased production of e−/h+ charge carriers’ pairs, which are successfully scavenged in the enhanced production of hydroxyl radials through hydroxyl ions pathway directly or indirectly through the hydrogen peroxide pathway. The hydroxyl radicals are then consumed in the degradation of the model pollutant species. In summary, we know the scientific community is struggling to develop green technologies based on more efficient and active photocatalytic structures active under visible light. The facile as-prepared WO3 powders may be scale up for practical application because it represents an attractive alternative for the decrease in the contaminants in the environment by employing a cost-effective energy source as the solar light. In addition, the prepared catalyst may also be employed in dye-synthesized solar cell, hydrogen production, low-cost gas sensing technologies, fuel cells applications, atmospheric and water pollutants degradation.
References
Abellán MN, Bayarri B, Giménez J, Costa J (2007) Photocatalytic degradation of sulfamethoxazole in aqueous suspension of TiO2. Appl Catal B Environ 74:233–241. https://doi.org/10.1016/j.apcatb.2007.02.017
Adhikari S, Sarkar D, Maiti HS (2014) Synthesis and characterization of WO3 spherical nanoparticles and nanorods. Mater Res Bull 49:325–330. https://doi.org/10.1016/j.materresbull.2013.08.028
Bamwenda GR, Arakawa H (2001) The visible light induced photocatalytic activity of tungsten trioxide powders. Appl Catal A Gen 210:181–191. https://doi.org/10.1016/S0926-860X(00)00796-1
Banerjee S, Dionysiou DD, Pillai SC (2015) Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl Catal B Environ 176–177:396–428. https://doi.org/10.1016/j.apcatb.2015.03.058
Breedon M et al (2010) Synthesis of nanostructured tungsten oxide thin films: a simple, controllable, inexpensive, aqueous sol−gel method. Cryst Growth Des 10:430–439. https://doi.org/10.1021/cg9010295
Cabrera RQ et al (2012) Photocatalytic activity of needle-like TiO2/WO3−x thin films prepared by chemical vapour deposition. J Photochem Photobiol A Chem 239:60–64. https://doi.org/10.1016/j.jphotochem.2012.05.002
Chen P et al (2015) Combustion synthesis and excellent photocatalytic degradation properties of W18O49. CrystEngComm 17:5889–5894. https://doi.org/10.1039/C5CE00995B
Chong SK, Dee CF, Rahman SA (2015) Single reactor deposition of silicon/tungsten oxide core-shell heterostructure nanowires with controllable structure and optical properties. RSC Adv 5:2346–2353. https://doi.org/10.1039/C4RA13257B
Coridan RH, Arpin KA, Brunschwig BS, Braun PV, Lewis NS (2014) Photoelectrochemical behavior of hierarchically structured Si/WO3 core-shell tandem photoanodes. Nano Lett 14:2310–2317. https://doi.org/10.1021/nl404623t
Díaz-Reyes J, Dorantes-García V, Pérez-Benítez A, Balderas-López JA (2008) Obtaining of films of tungsten trioxide (WO3) by resistive heating of a tungsten filament. Superf y Vacío 21:12–17
Ghashghaie M, Eslami H, Ostad-Ali-Askari K (2022) Applications of time series analysis to investigate components of Madiyan-rood river water quality. Appl Water Sci 12:202. https://doi.org/10.1007/s13201-022-01693-5
Guéry C, Choquet C, Dujeancourt F, Tarascon JM, Lassègues JC (1997) Infrared and X-ray studies of hydrogen intercalation in different tungsten trioxides and tungsten trioxide hydrates. J Solid State Electrochem 1:199–207. https://doi.org/10.1007/s100080050049
Hayat K, Gondal MA, Khaled MM, Yamani ZH, Ahmed S (2011) Laser induced photocatalytic degradation of hazardous dye (Safranin-O) using self synthesized nanocrystalline WO3. J Hazard Mater 186:1226–1233. https://doi.org/10.1016/j.jhazmat.2010.11.133
Heidari EK, Zamani C, Marzbanrad E, Raissi B, Nazarpour S (2010) WO3-based NO2 sensors fabricated through low frequency AC electrophoretic deposition. Sens Actuators B Chem 146:165–170. https://doi.org/10.1016/j.snb.2010.01.073
Hong SJ, Jun H, Borse PH, Lee JS (2009) Size effects of WO3 nanocrystals for photooxidation of water in particulate suspension and photoelectrochemical film systems. Int J Hydrog Energy 34:3234–3242. https://doi.org/10.1016/j.ijhydene.2009.02.006
Hsieh Y-T, Chang L-W, Chang C-C, Shih H (2011) Synthesis of WO3 nanorods by thermal CVD at various gas flow rates and substrate temperatures. Electrochem Solid State Lett 14:K40. https://doi.org/10.1149/1.3586784
Hunge YM et al (2016) Visible light catalysis of methyl orange using nanostructured WO3 thin films. Ceram Int 42:789–798. https://doi.org/10.1016/j.ceramint.2015.08.178
Jadkar V et al (2017) Synthesis of γ-WO3 thin films by hot wire-CVD and investigation of its humidity sensing properties. Phys Status Solidi (a) 214:1600717. https://doi.org/10.1002/pssa.201600717
Jothivenkatachalam K, Prabhu S, Nithya A, Jeganathan K (2014) Facile synthesis of WO3 with reduced particle size on zeolite and enhanced photocatalytic activity. RSC Adv 4:21221–21229. https://doi.org/10.1039/C4RA01376J
Karthik M et al (2017) One-step microwave synthesis of pure and Mn doped WO3 nanoparticles and its structural, optical and electrochemical properties. J Mater Sci Mater Electron 28:6635–6642. https://doi.org/10.1007/s10854-017-6354-3
Khan H (2017) Sol–gel synthesis of TiO2 from TiOSO4: characterization and UV photocatalytic activity for the degradation of 4-chlorophenol. Reac Kinet Mech Cat 121:811–832. https://doi.org/10.1007/s11144-017-1195-x
Khan H, Berk D (2015) Selenium modified oxalate chelated titania: Characterization, mechanistic and photocatalytic studies. Appl Catal A General 505:285–301. https://doi.org/10.1016/j.apcata.2015.05.030
Khan H, Jiang Z, Berk D (2018) Molybdenum doped graphene/TiO2 hybrid photocatalyst for UV/visible photocatalytic applications. Sol Energy 162:420–430. https://doi.org/10.1016/j.solener.2018.01.055
Le Houx N, Pourroy G, Camerel F, Comet M, Spitzer D (2010) WO3 nanoparticles in the 5–30 nm range by solvothermal synthesis under microwave or resistive heating. J Phys Chem C 114:155–161. https://doi.org/10.1021/jp908669u
Li J et al (2010) Synthesis of monoclinic WO3 nanosphere hydrogen gasochromic film via a sol–gel approach using PS-b-PAA diblock copolymer as template. Solid State Sci 12:1393–1398. https://doi.org/10.1016/j.solidstatesciences.2010.05.016
Li Y et al (2022) High performance removal of sulfamethoxazole using large specific area of biochar derived from corncob xylose residue. Biochar. https://doi.org/10.1007/s42773-021-00128-9
Murillo-Sierra JC, Hernández-Ramírez A, Hinojosa-Reyes L, Guzmán-Mar JL (2021) A review on the development of visible light-responsive WO3-based photocatalysts for environmental applications. Chem Eng J Adv 5:100070. https://doi.org/10.1016/j.ceja.2020.100070
Nagy D et al (2016) Photocatalytic WO3/TiO2 nanowires: WO3 polymorphs influencing the atomic layer deposition of TiO2. RSC Adv 6:95369–95377. https://doi.org/10.1039/C6RA18899K
Nasuhoglu D, Yargeau V, Berk D (2011) Photo-removal of sulfamethoxazole (SMX) by photolytic and photocatalytic processes in a batch reactor under UV-C radiation (λmax=254nm). J Hazardous Mater 186:67–75. https://doi.org/10.1016/j.jhazmat.2010.10.080
Navarro JRG et al (2014) WO3 nanorods created by self-assembly of highly crystalline nanowires under hydrothermal conditions. Langmuir 30:10487–10492. https://doi.org/10.1021/la5025907
Nooshin S, Marco GR, Daria CB, Li H, Patience SG (2017) Spray dried SiO2 WO3/TiO2 and SiO2 vanadium pyrophosphate core-shell catalysts. Powder Technol 316:434–440. https://doi.org/10.1016/j.powtec.2016.10.056
Ostad-Ali-Askari K (2022) Management of risks substances and sustainable development. Appl Water Sci 12:65. https://doi.org/10.1007/s13201-021-01562-7
Ramos-Delgado NA, Hinojosa-Reyes L, Guzman-Mar IL, Gracia-Pinilla MA, Hernández-Ramírez A (2013) Synthesis by sol–gel of WO3/TiO2 for solar photocatalytic degradation of malathion pesticide. Catal Today 209:35–40. https://doi.org/10.1016/j.cattod.2012.11.011
Senguttuvan TD et al (2010) Gas sensing properties of nanocrystalline tungsten oxide synthesized by acid precipitation method. Sens Actuators B Chem 150:384–388. https://doi.org/10.1016/j.snb.2010.06.053
Shinde PA, Jun SC (2020) Review on recent progress in the development of tungsten oxide based electrodes for electrochemical energy storage. Chemsuschem 13:11–38. https://doi.org/10.1002/cssc.201902071
Tahir M et al (2018) WO3 nanostructures-based photocatalyst approach towards degradation of RhB dye. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-017-0771-x
Tao Y et al (2020) WO3–SiO2 nanomaterials synthesized using a novel template-free method in supercritical CO2 as heterogeneous catalysts for epoxidation with H2O2. Mater Today Chem 18:100373. https://doi.org/10.1016/j.mtchem.2020.100373
Thommes M et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Usami Y, Hongo T, Yamazaki A (2012) Phosphate constituent effects on the structure and photocatalytic properties of mesoporous tungsten oxides. Microporous Mesoporous Mater 158:13–18. https://doi.org/10.1016/j.micromeso.2012.03.015
Vamvasakis I, Georgaki I, Vernardou D, Kenanakis G, Katsarakis N (2015) Synthesis of WO3 catalytic powders: evaluation of photocatalytic activity under NUV/visible light irradiation and alkaline reaction pH. J Sol Gel Sci Technol 76:120–128. https://doi.org/10.1007/s10971-015-3758-5
Wang X, Zheng YF, Yin HY, Song XC (2011) Green synthesis and catalytic function of tungsten oxide nanoparticles. J Nanosci Nanotechnol 11:2501–2505. https://doi.org/10.1166/jnn.2011.3593
Wicaksana Y, Liu S, Scott J, Amal R (2014) Tungsten trioxide as a visible light photocatalyst for volatile organic carbon removal. Molecules 19:17747–17762. https://doi.org/10.3390/molecules191117747
Xekoukoulotakis NP et al (2011) Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices. Catal Today 161:163–168. https://doi.org/10.1016/j.cattod.2010.09.027
Xiao B et al (2015) Low-temperature solvothermal synthesis of hierarchical flower-like WO3 nanostructures and their sensing properties for H2S. CrystEngComm 17:5710–5716. https://doi.org/10.1039/C5CE00870K
Zhao Z-G, Miyauchi M (2008) Nanoporous-walled tungsten oxide nanotubes as highly active visible-light-driven photocatalysts. Angew Chem Int Edit 47:7051–7055. https://doi.org/10.1002/anie.200802207
Zheng G et al (2019) Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 11:18968–18994. https://doi.org/10.1039/C9NR03474A
Acknowledgements
The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through the project number PSAU/2022/01/23514.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aldrees, A., Khan, H., Alzahrani, A. et al. Synthesis and characterization of tungsten trioxide (WO3) as photocatalyst against wastewater pollutants. Appl Water Sci 13, 156 (2023). https://doi.org/10.1007/s13201-023-01938-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01938-x