Abstract
This study assessed the quality of water in the Shatt Al-Hillah River by adopting some variables of physical, chemical, and heavy metal elements. The samples have been taken at six sites along the river in 2020 (from January to December). The water quality index has been determined by using the weighted-arithmetic method which is including a series of equations. Also, the model of Inverse-Distance-Weighting in the Geographic information system was applied to create a map of the water quality in the study area. Eleven physicochemical variables and five elements of heavy metals were comprised of calcium, magnesium, dissolved oxygen, Hydrogen Ions, chloride, sulfate, total hardness, total dissolved solids, turbidity, alkalinity, electric conductivity, cadmium, copper, iron, lead, and zinc. The results showed the values of the water quality index ranged from 245 to 253 (with a category of 200–300). The water quality index was rated as very poor for the selected locations along the Shatt Al-Hillah River. The GIS result illustrated the distributing map of water quality for the Shatt Al-Hillah River for household uses. The combination of the water quality index calculations with GIS in the current study might be used as a guide for future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is an essential requirement of human and industrial development, and it is the most significant part of the environment. Water quality deals with the physical, chemical, and biological characteristics concerning all other hydrological properties (Atulegwu and Njoku 2004). The relationship between the water demand and the population is positive, for that reason, any increment in the population and human activities have a direct influence on water usage. Also, the water quality (WQ) will be low over time because of the increase in water use for both surface water and groundwater (Massoud 2012; Sahoo et al. 2015).
In a large number of countries, the WQ dropped especially the surface water which is highlighted as a dangerous problem. The policies of resource protection give great priority with very importance to WQ and its control (Simeonov et al. 2002). Recently, developing countries had a massive effort to evaluate the WQ in rivers (Kannel et al. 2007). The evaluation and analysis of WQ are the main and compulsory conditions to obey the policies of water protection. Moreover, the best use for the water sources depends on the demand. In fact, the used surface water is subject to standard norms for evaluation. Otherwise, the sources of a variety of pollution can be classified as urban, industrial, and agricultural pollution. The limitations of water use that is depending on a group of variables known by the term WQ. Mostly, the users have common specifications for these variables (Rosemond et al. 2009).
Many factors in nature are affecting the WQ like geological, hydrological, and human activities (e.g., discharging of municipal and industrial sewage water, and agricultural drainage) (Alam and Laishram 2017; Meybeck et al. 1996). The assessment of WQ is used to obtain the physicochemical and biological characteristics of the water. Also, the WQI shows one value to give an easy and comprehensive view to illustrate the data of the water (Bharti and Katyal, 2011). Hoseinzadeh et al. (2015) applied some WQ indices to investigate the Aydughmush River in Iran. The researchers obtained close results of indices except for the river pollution index. The Euphrates River is the main water resource for the Western and southern parts of Iraq. Some agricultural and industrial activities on the water stream are risky to WQ (Hassan and Shaawiat 2015).
According to Rashed (2002), the aquatic environment with its water quality is considered the main factor controlling the state of health and disease in both man and animals. Nowadays, the increasing use of chemical waste and agricultural drainage systems represents the most dangerous chemical pollution. The most important heavy metals from the point of view of water pollution are Zn, Cu, Pb, Cd, Hg, Ni, and Cr. Some of these metals (e.g., Cu, Ni, Cr, and Zn) are essential trace metals to living organisms but become toxic at higher concentrations. Others, such as Pb and Cd have no known biological function but are toxic elements.
Anazawa et al. (2004) highlight that water pollution with heavy metals becomes a question of considerable public and scientific concern in light of the evidence of their extreme to human health and the biological system. Moreover, the growing public concern over the deteriorating quality of the environment has led to generalized use when referring to trace elements, other terms such as (trace metals), (trace inorganics), and (heavy metals) are synonymous with the term trace elements (Al-Kattan 1989). Heavy metals can be used to interpret to include those metallic elements from periodic table groups IIA through VIA. They are very important to support life. However, at elevated levels they become toxic, may build up in biological systems, and become a significant detriment to aquatic life (Maryland Department of Environment 2003). Elements can be divided into two groups in terms of their density: heavy elements which are those elements with a density higher than 5 g/cm3, and an atomic number of more than 20, and light elements, which are elements with a density of less than 5 g/cm3. Heavy elements are also called trace elements because they occur in small quantities in the earth's crust which does not exceed (0.1%) (Tucker et al. 2003; Hardy et al. 2008).
This study aims to evaluate the water quality index (WQI) for the Shatt Al-Hillah River for household uses by measuring the physicochemical properties and some elements of heavy metals at six sites starting from Al-Musayyib city to the near the downstream of the river within Babylon Governorate in Al-Hashimiya city. Furthermore, produce the map of the WQI for whole the study area based on the resulting values of the WQI at each location using the interpolation method of Inverse Distance Weighted (IDW) one of the tools of the GIS.
Shatt Al-Hillah River (study area)
Shatt Al-Hillah River is a branch of the Euphrates River. It is starting in Saddat Al-Hindiya town with a discharge of 200 m3/s during summer and 245 m3/s during winter. The length of the Shatt Al-Hillah River passing through Babylon governorate is equal to 101 km. Discharge fluctuates according to the agricultural needs in the three governorates of Babylon, Qadisiya, and Al-Muthanna, with a total area of 420 thousand hectares. Thirty-six main channels are branching from both banks of the river (inside Babylon governorate boundaries) with a total length of 511.6 km (Iraqi Ministry of Construction, Housing, Municipalities, and Public Works, 2018).
This study covers the route of the Shatt Al-Hillah River which locates within Babylon Governorate that has an area of 5337 km2, including the cities of Babylon Governorate. In 2020, Babylon Governorate had a population of approximately 2,400,000 inhabitants distributed throughout sixteen cities (Iraqi Ministry of Planning 2020). The governorate is divided administratively into five major cities, referred to as districts. The five districts are Al-Hillah, Al-Qasim, Al-Musayyib, Al-Mahawil, and Al-Hashimiya.
Shatt Al-Hillah River passes through many cities and villages within Babylon Governorate and is considered the main source for different uses. The river is provided water for agricultural lands located on both banks of the river. Shatt Al-Hillah River is the main source of supplying treatment plants such as Al-Hillah Al-Jadeed and Al-Hillah Al-Kadeem water treatment plants. Moreover, the water of the river represents the main source for all industrial activities in Al-Hillah city. In addition to these main uses, the river receives many pollutants discharged by different sources, including agricultural wastes, municipal wastes, and industrial wastes.
Methodology
The main steps of methodology in this study can be shown by the flowchart in Fig. 1. The first step is to describe and show the study area by specifying the collection points. It is significant to choose and decide the location of each point to be showing a comprehensive vision of the river. After the data collection, the data input might be divided into parts (WQI calculations and export of the collected data to ArcGIS software). In ArcGIS software, some processes have been applied to create the map of the distribution for the WQI. The last stage is including the results and discussion with conclusions.
Samples collection
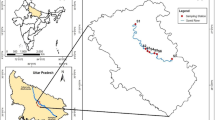
In this study, samples were collected from six locations located across the length of the river. These locations were chosen due to the potential environmental pollution by industrial, agricultural, and human activities (Fig. 2). At each location, five samples were taken at different depths (two samples on both sides and three samples at the center of the river) and then mixed to get one sample from the mixture with a (10) liters volume to represent the actual picture of the water quality in that location. Samples for physical–chemical parameters were placed in a well-sealed plastic bottle with (2) liters volume for each and (1) liter volume for dissolved oxygen to preserve the sample until it was analyzed. The samples were preserved in a cool box by putting ice around sample bottles until reaching a laboratory. Samples were acidified at the time of collection with a concentrated nitric acid (1.5 ml HNO3/l sample) to achieve a pH of 2 or less to keep the metals in solution and to minimize their adsorption on the container wall (APHA 2017).
Physicochemical analysis and metal samples pretreatment and analysis
The samples of physicochemical elements were analyzed in the laboratory of the water resources directorate, Babylon based on Standard Methods for the Examination of Water and Wastewater (APHA 2017).
For total metals analysis, a 100 ml of well-mixed sample was transferred to a beaker, 5 ml of HNO3 concentration was added and then placed on a hot plate and evaporated to near dryness, making sure that the sample does not boil. After cooling another 5 ml of acid was added, covering the beaker with a watch glass, and placing it on a hot plate. Increasing the temperature until a gentle refluxing action occurred, continuing heating, and adding additional acid as necessary until digestion is completed. Then, 1 to 2 ml of concentrated nitric acid was added and warred slightly to dissolve the residue. Washed the walls of the beaker and glass of the watch, de-ionized water, removed silicate, and other insoluble materials by filtration, and the volume was adjusted to the original volume (Baird et al. 2017). The Fe, Cu, Cd, Pb, and Zn contents were determined by an atomic absorption spectrophotometer type (BUCK/210VGP, USA). The trace metals in the collected water samples were analyzed in the laboratory of the water resources directorate, Babylon.
Water quality assessment
Along the Shatt Al-Hillah River, eleven parameters were measured from six locations selected in the year 2020 (from January until December). The selected locations are (Al-Musayyib city, Al-Hindiya, Abu Khstawyi, Al-Hillah City, Al-Maamera, and Al-Hashimiya). Table 1 shows the average concentrations, the standard deviation, and the standard error of the physicochemical elements in 2020, while Table 2 displays the average concentrations, the standard deviation, and the standard error of heavy metals in the river.
Method of calculating weights of WQI
The weighted arithmetic method (WAM) was applied to compute the water quality index (WQI). in this study, the physicochemical elements that were measured were eleven parameters including Ca, Mg, DO, pH, Cl, SO4, TH, TDS, turbidity, Alkalinity, and EC. Five elements of heavy metal were taken from the river at each location and these elements are Cd, Cu, Fe, Pb, and Zn. To estimate the water quality index (WQI), the WAM was applied to every location by implementing the following equations (Tyagi et al. 2013):
where: Sw-Ii: the sub-weight of each element (ith); IWi: the inverse value of the standard limit (STi) of each element (ith); STi: the standard limit of each element (ith) according to WHO (2017), where it was adopted corresponding units for each physicochemical and heavy metal element (mg/l) except EC with a unit of (μmhos/cm); Ci: the measured concentration value for the ith element; C0: the ideal value for each element in water with a value of zero, while the ideal value of C0 for dissolved oxygen and pH values (respectively) are equal to 14.6 ppm and 7, WQI: the water quality index.
The water quality assessment (QWA) was used to descript the situation of the Shatt Al-Hillah River for locations based on the categories of the WQI according to Alsaqqar et al. (2015), and Ali (2017), using the resulting values of the WQI (Table 3).
Based on Eqs. (1, 2, and 3), the steps of calculating the WQI for each element using the average yearly values for all selected locations can be seen in Table 4.
The steps of calculating the WQI for location 1 (as an example) are listed as follows (Table 5):
-
1.
Inserted the values of measured elements (Ci) in column (3).
-
2.
Inserted the ideal value for each element in water (C0) in column (4). Zero value was given for all elements, while the ideal values of 14.6 and 7 were given for pH and DO, respectively.
-
3.
Inserted the standard limit of each element (STi) in column (5) according to WHO (2017).
-
4.
The IWi value for each element in column (6) was calculated using Eq. 2, where that IWi represented the inverse value of the standard limit (STi) for each element.
-
5.
Calculated the Sw-Ii value for each element in column (7) (using Eq. 1). This is done by dividing the value in column (3) by the value in column (5) with considering the ideal value for each element (zero) and ideal values for dissolved oxygen, and pH that equal 14.6 and 7, respectively.
-
6.
For Eq. 3, the IWi × Sw-Ii (numerator) value for each element in column (8) was calculated by multiplying the value of IWi (column 6) by the value of Sw-Ii (7).
-
7.
Finally, to calculate the WQI using the weighted arithmetic method (Eq. 3), the summation values in column 6 (IWi × Sw-Ii) were divided by the summation values in column 8 (IWi).
GIS prediction maps using the interpolation method (IDW)
The Inverse Distance Weighted (IDW) is an interpolation method and it is used to produce the map for specific points’ values. This method is a tool of interpolation existing in the GIS. The procedure of the IDW for the prediction of the specific location is based on the group of known points to estimate the required values for unknown points, where the closer points give more accurate values compared to farther away points to predict values for unknown points (Panhalkar and Jarag 2016).
Results and discussion
Concentrations of the measured physicochemical elements along the river
The readings of Ca in the Shatt Al-Hillah River in 2020 ranged from 104 in July at the location (L.6) to 140 mg/L in February at the location (L.4). The mean value of Ca was 124 mg/L. All readings for Ca measured in the river were over the acceptable limit of 50 mg/L (WHO, 2017). Figure 3a showed that calcium concentrations were high during most seasons of the study. One of the primary causes is the presence of dissolved limestone in the riverbed or surrounding geological formations. Limestone contains calcium carbonate, which can dissolve in water and increase the calcium concentration (Saini et al. 2015). Additionally, human activities such as agriculture can also contribute to higher calcium levels in the river. Fertilizer use in agriculture can release excess calcium into the soil and water systems. Another factor that can contribute to high calcium concentrations in rivers is the natural weathering of rocks and soils, especially in areas with high rainfall and runoff. This process can release calcium and other minerals into the river, leading to higher levels (Jiang et al. 2015).
The average concentration of Mg measured in the Shatt Al-Hillah River during this year was 74 mg/L. The maximum and minimum values of Mg concentration in 2020 ranged from 28 mg/L (May) to 100 mg/L (February) at the locations (L.3 and L.5) respectively (Fig. 3b). All readings of Mg in Shatt Al-Hillah River were higher than the permissible value of 50 mg/L (WHO, 2017). This is due to the presence of magnesium-rich minerals in the watershed, such as dolomite or magnesite. These minerals can dissolve in water and contribute to higher magnesium levels in the river (De La Cruz and Mazari-Hiriart 2011). Another possible reason for elevated magnesium levels in a river could be human activities, such as discharges from wastewater treatment plants or agricultural runoff. These sources can introduce magnesium and other minerals into the river and increase their concentrations (Ghosh et al. 2019).
The maximum value of chloride (Cl) concentration in 2020 was 239 mg/L at the location (L.2) in February, and the average value was 203 mg/L in that year. During the current year, the lowest value of Cl was 180 mg/L at the location (L.4) in July. The value of chloride concentration which was measured along the river was within the allowable limit of 250 mg/L (WHO, 2017). Figure 3c showed an increase in Cl levels during winter and spring in most locations. This is due to the use of deicing salts on roads during the winter months. The runoff from these salts can flow into the river and increase Cl concentrations. In addition, increased precipitation during the winter and spring can increase river flow rates and cause more salt-laden runoff to enter the river. Another factor is geologic formations and salt deposits (Kaushal et al. 2005).
Sulfate (SO4) concentrations in 2020 varied between (341–440) mg/L at locations (L.2 and L.6) respectively, with an average value of 376 mg/L. The readings of Sulphate (SO4) for all chosen locations were more than the allowable limit of 250 mg/L (WHO, 2017) (Fig. 3d). The concentration values of Sulphate during the winter months were higher than in other months of this study. Sulfate concentrations in Shatt Al-Hillah River can increase during winter and spring months due to increased atmospheric deposition, the solubility of sulfate in water, mobilization of sulfate from soil and groundwater, and land use and land cover (Baalousha and Bond 2016; Hagedorn et al. 2016).
For the TDS, the highest reading in 2020 was 1220 mg/L and found at the location (L.6) in January, while the lowest reading was 971 mg/L at the location (L.3) in August. The average value for TDS was 1061 mg/L (Fig. 3e). In the current study, most readings of the TDS along the Shatt Al-Hillah River were over the allowable limit of 1000 mg/L (WHO, 2017). A slight difference was observed in TDS values for the samples collected from the different stations together at the same time but there was a much more difference in TDS values for the samples collected at separate times. TDS concentrations can be influenced by geological, hydrological, and land use factors, as well as meteorological conditions such as precipitation and temperature (Hartmann et al. 2017; Bartov et al. 2018). TDS concentrations in the river can increase during the winter and spring months due to decreased dilution, increased surface runoff, enhanced weathering of rocks, mobilization of dissolved salts from soil and groundwater, and anthropogenic activities such as agriculture, and urbanization. (Al-Khashman & Shawabkeh 2006; Hartmann et al. 2017).
The average value of EC was 1618 (micromhos). The highest value was 1877 (μmhos/cm) at location (L.6) in January, while the lowest value was 1494 (μmhos/cm) at location (L.3) in September. All EC values were within the acceptable limit of 2000 μmhos/cm (WHO, 2017) (Fig. 4a). High values were recorded in the winter season, due to the washing of soil by rainwater and as a result of the flow of salinity water from drainages nearby the river. This was asserted by the increase in value rates of soluble and suspended solid substances in river water (Jassim 2006).
In this study, the maximum value of the TH in 2020 was equal to 560 mg/L at (L.4) in February. The lowest value of TH was 428 mg/L, and this value was recorded at (L.6) in June (Fig. 4b). Along the Shatt Al-Hillah River, the average value in the year 2020 was 496 mg/l. The results of total hardness in January, February, March, and December at all locations were over the allowable limit of 500 mg/L (WHO, 2017). The total hardness concentrations in the river increased during the winter and spring months for various reasons. (1): an increase in the rate of rocks weathering caused by low temperatures and increased precipitation, (2): the mobilization of dissolved salts from soil and groundwater by snowmelt and rain events, (3): agricultural activities can contribute to increased TH concentrations in the river due to the use of fertilizers and pesticides, (4): the discharge of untreated sewage and other pollutants can also lead to an increase in TH concentrations in rivers (Bhat and Vaishnav 2012; Adeyemo and Adeoye 2015).
The high levels of DO were recorded at most of the locations (Fig. 4c). The highest value was 9.31 mg/L in location (L.4) in January due to good aeration, continuous mixing of water, dense zooplankton, and phytoplankton which result in high levels of dissolved oxygen. The lowest level of DO was 7.29 mg/L at the location (L.3) in July. The variations of DO levels in the river during the period of the study were attributed to the biological degradation of the organic materials or the low water level at these stations (Jassim 2006).
The highest and the lowest values of alkalinity were (respectively) 160 mg/L in January and 91 mg/L in July at the location (L.5). The average value of alkalinity was 121 mg/L. Figure 4d gives the variation of alkalinity concentration in the river water during this study. The results showed that the predominant alkaline nature of Iraqi waters was due to the presence of bicarbonates (HCO3) in water and the surrounding soil. The water level in rivers has a great effect on the alkalinity values, and this explains the monthly fluctuation of alkalinity values recorded during the present study (Jassim 2006).
The turbidity in the river ranged from 21 to 42 (N.T.U.). The highest turbidity value was 42 (N.T.U.) at the location (L.4) in July. On the other hand, the lowest value was (21) (N.T.U.) at the location (L.2) in February and (L-4) in January. Figure 5a shows the variation of turbidity concentration in the river water during the period of the study. The average value of turbidity of the Shatt Al-Hillah River was 31.4 (N.T.U.). Turbidity in the river can increase during the summer and spring seasons due to a variety of factors, including rainfall and runoff during the spring season can lead to higher turbidity levels in rivers, increased agricultural activities by irrigation of crops can lead to runoff of sediment and other pollutants into nearby the river and a variety of natural and Human development, such as construction projects and land use changes, can also contribute to increased turbidity levels in the river during these seasons.
The pH values at all locations were between 7.65 and 8.22. The highest pH value recorded was (8.22) in February at the location (L.2), while the lowest value was (7.65) in July at the location (L.6) (Fig. 5b). The pH value of water decreases as the content of CO2 increases, while it increases as the content of bicarbonate alkalinity increases. The narrow scope of pH values in rivers due to buffer capacity enables the river to resist fluctuation in pH. Therefore, there was a narrow scope in pH value in the Shatt Al-Hilla River and this agreed well with the result obtained by (Hassan and Shaawiat 2015).
Concentrations of some measured elements of heavy metals along the river
The highest value of cadmium in the river water was 7.43 µg/L at the location (L.5) IN July and the lowest value was 2.74 µg/L at the location (L.1) in January. The average readings of the Cd were 4.73 µg/L. The concentrations of Cd in Shatt Al-Hillah River water during the study period were higher than the allowable limit of 2 µg/L (WHO, 2017) (Fig. 6a). Cadmium levels increased during the summer months because high concentrations of suspended solids during summer (with a low flow rate) were caused by floating materials which increased the concentrations of trace metals in water. Chemical compounds that are used for agricultural purposes (commercial fertilizers, pesticides, and phosphate fertilizers) contain different concentrations of Cd. So, these compounds comprised one of the reasons for the increase in Cd concentration in the river.
The results showed that the average values of the Fe were 679 µg/L. The highest value of iron was 804 µg/L at location (L.3) in July, while the lowest value was 578 µg/L at location (L.2) in December with an average value of 679 µg/L. According to WHO (2017), all readings of Fe were over the permissible limit of 300 µg/L (Fig. 6b). It was noticed that the level of iron concentrations increases in the summer months compared to other months. This is due to various reasons, including changes in hydrological conditions, biological activity, and human activities. During summer months, low river flow rates and increased temperatures could result in decreased dilution and increased concentration of Fe in the water column. Additionally, increased photosynthesis and microbial respiration during the summer months led to the release of Fe from sediments and organic matter in the riverbed.
The highest value of copper was 85 µg/L at Location (L.4) in July and the lowest value was 54 µg/L at Location (L.1) in February with an average value of 68.67 µg/L. A graph of Fig. 6c for the copper concentrations with showed that all readings of Cu were within the allowable limit of 2000 µg/L (WHO, 2017). Copper concentrations increased during the summer months in the Shatt Al-Hillah River due to the discharge of industrial wastewater and sewage and agricultural runoff into it. Moreover, higher water temperatures during the summer months also led to increased concentrations of Cu in the water column. Copper is more soluble in warmer water, and as a result, the release of Cu from sediments and other sources increased with higher water temperatures.
In this study, the highest value of lead was 7.8 µg/L at the location (L.4) in July and the lowest value was 5.1 µg/L at the location (L.3) in January with an average of 6.4 µg/L. Figure 6d indicated that all concentrations of lead during the period of the study were less than the maximum level of 10 µg/L (WHO, 2017). Lead compounds in rivers resulted from increasing anthropogenic activities and lower river flow rates. Anthropogenic activities such as industrial discharges and agricultural runoff can contribute to the increased concentration of lead in the water during summer months. Moreover, lower river flow rates during the summer months led to increased concentrations of Pb in the water column. As the river flow rate decreases, the concentration of pollutants, including Pb, increased due to decreased dilution.
The average value of zinc was 69.2 µg/L in 2020. The maximum value of Zn was 86 µg/L at the location (L.5) in July, while the minimum value was 56 µg/L at the location (L.1) in January. Figure 6e showed that whole readings of Zn were lower than the acceptable limit of 3000 µg/L (WHO, 2017). The concentrations of Zn increased in the Shatt Al-Hilla River during summer months due to lower river flow rates, increased biological activity, agricultural activities, and industrial discharges. During summer months, lower river flow rates and increased temperatures contributed to decreased dilution and increased concentration of Zn in the water column. Additionally, increased photosynthesis and microbial respiration during the summer months also caused the release of Zn from sediments and organic matter in the river bed.
Water quality index
Based on the calculated values of the WQI, the water quality assessment (WQA) of the river (for each location) was rated using the categories that have been set by Alsaqqar et al. (2015). Table 6 shows the water quality value (WQI) and water quality assessment (WQA) for each location in the Shatt Al-Hillah River in 2020.
Figure 7 shows the resulting values of WQI for the Shatt Al-Hillah River. All values were within the category of (200–300) and the WQA of the Shatt Al-Hillah River was assessed as very poor.
According to Tyagi et al. (2013), The Water Quality Assessment (WQA) has been divided into five types as shown in Table 6. The water quality types are based on the calculated WQI by applying the method of weighted arithmetic. The values of WQI et al.-Musayyib (L.1), Al-Hindiya (L.2), Abu Khstawyi (L.3), Al-Hillah city (L.4), Al-Maamera (L.5), Al-Hashimiya (L.6), in 2020 were as follows: 250.8, 253.4, 248.6, 245.2, 251.7, and 250.4, respectively with a standard deviation of 2.84.
The values of the WQI (for all selected physicochemical properties and some elements of heavy metals) were within the range of (200–300) starting from the location (L.1) until the location (L.6). So, these locations have been classified within the category of very poor. It is an outcome of the number of released pollutants into the river at these locations from various sources. It has a great effect on the ecology and the water quality which is affecting positively or negatively on the WQI of the river.
The results showed that there is a slight difference in the WQI values at the selected locations along the Shatt Al-Hillah River. Also, it highlighted those similar flowing pollutants to the water river system from various resources like agricultural runoff, domestic sewers, and industrial waste discharges. Overall, the novelty of the current study includes two sections. In the first section, study the WQ by using the WQI method to cover the whole length of the Shatt Al-Hillah River.
Figure 7 shows that the WQI values in the summer were higher than the values in the winter. This is due to the measured concentrations for all selected elements in the river during the summer season were higher than the concentrations in the winter season. Consequently, the elements’ concentrations in the summer have a high effect on the WQI values.
Table 6 showed that the average yearly values of Ca, Mg, DO, pH, Cl, SO4, TDS, Turbidity, Alkalinity, Cadmium, and Iron were higher than the allowable limits of WHO, 2017. Moreover, Table 6 showed the calculated values of the WQI and WQR for each element in this study.
The second section is about creating interpolation maps for the WQI values in terms of irrigation purposes for the same river. In parallel to that, the interpolation map for the WQI has been created by using the GIS software along the Shatt Al-Hillah River 2020 (Fig. 8).
Conclusions
Shatt Al-Hillah River is the main water source for all the activities in the cities located along this river. In 2020, the water quality of the river has been evaluated because of the water crisis in the region. Eleven parameters and five elements have been measured to explain the physical and chemical and heavy metals as well as aspects of each water sample. Along the river (Shatt Al-Hillah), six locations have been used for water sampling. The parameters were Ca, Mg, DO, pH, Cl, SO4, TH, TDS, Alkalinity, turbidity, EC, Cd, Cu, Fe, Pb, and Zn. In the current study, the IDW method of the spatial analyst extension in the ArcGIS 10.5 has been utilized to create maps for the WQ including the parameters inside the catchment area. It helps to identify the locations or areas for sampling along the river, also, high impairment levels in the same area. Moreover, that will in turn assist to apply the standards and pollution control activities.
The data that shows the values for the measured parameters that have been tested during the year 2020 along the Shatt Al-Hillah River decreased gradually from the location (L.1) in Al-Musayyib to the location (L.6) in Al-Hashimiya. The measured values from location (L.4) to location (L6) showed a decrease for all selected parameters. The concentration of Ca was decreased gradually from location (L.1) to location (L.4) and then increased at location (L.5) and then returned to decrease at the location (L.6).
The outcomes of the WQI along the river for the chosen locations (from the river upstream et al.-Musayyib to the river downstream et al.-Hashimiya) present constant and stable values. The calculated values of WQI for stations (L.1 to L.6) were classified as very poor water quality. They were between 200 and 300 which is the category of very poor water quality based on the used method. The clear degradation was related to several pollutants originating in human activities and natural phenomena factors occurring along the pathway of the river. Moreover, the computed WQI values indicated that the WQ of the river at these stations was invalid to use before treating the parameter concentrations (especially) with values more than allowable limits.
Many factors can affect the pollution level of the Shatt Al-Hillah River with trace metals. These factors are the presence of agricultural areas on both banks of the Euphrates and Shatt Al-Hillah Rivers, since many of them, washed soil campaign of different chemical compounds which are used for agricultural purposes of fertilizers and pesticides, where a great amount of soil drifted into the water carrying with it different kinds of chemical compounds which are used in agricultural purposes such as fertilizers and pesticides. Pointed out that commercial fertilizers contain some traces of elements, while phosphate fertilizers contain different concentrations of cadmium ranging from (1–95) mg/kg. Other compounds, such as salts of zinc, copper, lead, and other organic metallic compounds are used as pesticides.
The river runs through several towns, suburbs, and villages such as Saddat-Al-Hindiyah, Al-Mahawil, Al-Hillah, Al-Hashimiya, and other towns. These places add more pollutants to the river caused by the discharge of domestic and factory waste materials. These materials have a direct and indirect impact on the river.
The increase in concentrations is higher in summer because of the source of water in the river of Euphrates and Shatt Al-Hillah Rivers. In this season the water is stored in dams for irrigation purposes. Thus, high concentrations of suspensions in summer and spring are attributed to floating materials.
There is a relation between the concentration of heavy elements and the water level. This was reflected clearly in the decrease or increase in concentration of elements in the Shatt Al-Hillah River during the period of the study.
Generally, in the present work, it was found that the most related parameters affecting trace metals concentration in Shatt Al-Hillah River were turbidity, temperature, hydrogen ion, dissolved oxygen, and total suspended solids, and in lower degrees with parameters chlorides, sulfates, electrical conductivity, and alkalinity, while total hardness, calcium, and total dissolved solids parameters have a low significant effect on trace metals concentrations in this study. The poor water quality problem in the study area might be improved by developing water resources management, good urban planning, and explaining the dangers of heavy metals for the local population. Also, the local governments should select expert people in the field of competence to improve the solutions. Activate the role of the local environment department and involve it in the relevant decisions.
References
Adeyemo OK, Adeoye IO (2015) Impact of industrial effluents on water quality of receiving rivers in Nigeria. J Environm Chem Ecotoxicol 7(7):70–80. https://doi.org/10.5897/JECE2015.0359
Alam W, Laishram RJ (2017) Spatial and Temporal Assessment of Water Quality of Nambul River Within Imphal City, Manipur. In: International conference on natural resources management and technology trends (ICNRM-17), at Manipur University, Imphal, pp. 108–109
Ali SK (2017) Assessment of the tigris river water quality in selected iraqi governments. Int J Sci Res 7:500–504
Al-Kattan DM (1989) Trace Elements in Tigris River and Their Impact on Drinking Water. Doctoral dissertation, MSc. Thesis, Civil engineering dept., University of Mosul, Iraq, pp 1–106)
Al-Khashman OA, Shawabkeh RA (2006) Metals distribution in soils around the cement factory in southern Jordan. Environ Pollut 140(3):387–394. https://doi.org/10.1016/j.envpol.2005.07.024
Alsaqqar AS, Hashim A, Mahdi A (2015) Water quality index assessment using gis case study: Tigris River in Baghdad City. Int J Curr Eng Technol 5:2515–2520
Anazawa K, Kaida Y, Shinomura Y, Tomiyasu T, Sakamoto H (2004) Heavy-metal distribution in river waters and sediments around A “Firefly Village”, Shikoku, Japan: application of multivariate analysis. Anal Sci 20:79–84
APHA (2017) Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, Washington
Atulegwu PU, Njoku JD (2004) The impact of biocides on the water quality. Int Res J Eng Sci Technol 1(2):47–52
Baalousha H, Bond T (2016) Sulfate dynamics in a river system during snowmelt: the role of land use and land cover. Environ Sci Technol 50(2):747–754. https://doi.org/10.1021/acs.est.5b04534
Baird RB, Eaton AD, Rice EW (2017) Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, Washington, pp 1–40
Bartov G, Shalev S, Lyakhovsky V, Gavrieli I, Guttman J, Steiner Z, Kurtzman D (2018) Seasonal variations of total dissolved solids (TDS) in a large karstic river: the case of the Jordan River. Environ Earth Sci 77(3):114. https://doi.org/10.1007/s12665-018-7233-6
Bharti N, Katyal D (2011) Water quality indices used for surface water vulnerability assessment. Int J Environ Sci 2:154–173
Bhat SA, Vaishnav MM (2012) Assessment of water quality parameters of river Jhelum. Int J Environ Sci 2(1):14–22
De La Cruz EM, Mazari-Hiriart M (2011) Magnesium in drinking water: a review. J Environ Sci Health Part A Toxic/hazard Subst Environ Eng 46(12):1285–1295
Ghosh AR, Mujumdar KK, Sinha RK (2019) Contamination of river water due to human activities: a review. J Environ Manage 231:1026–1037
Hagedorn B, Klotzli U, Cosio C (2016) Seasonal dynamics of sulfate in rivers: anthropogenic and biogenic sources. Environ Sci Technol 50(18):9887–9895. https://doi.org/10.1021/acs.est.6b02451
Hardy DH, Myers J, Stokes CE (2008) Heavy Metals in North Carolina Soils: Occurrence & Significance. NC Department of Agriculture and Consumer Services, Agronomic Division.
Hartmann J, Jemcov I, Lutz SR (2017) Seasonal variations of water quality parameters in the Upper Rhine River: a regional study. Environ Monit Assess 189(7):314. https://doi.org/10.1007/s10661-017-6034-y
Hassan FM, Shaawiat AO (2015) A contribution to the Epipelic Algal Ecology in Lotic Ecosystem of Iraq. J Environ Prot 6:85
Hoseinzadeh E, Khorsandi H, Wei C, Alipour M (2015) Evaluation of aydughmush river water quality using the national sanitation foundation water quality index (NSFWQI), River Pollution Index (RPI), And Forestry Water Quality Index (FWQI). Desalin Water Treat 54:2994–3002
Iraqi Ministry of Construction Housing (2018) Municipalities, and public works. Directorate of Water Resources, Babylon
Iraqi Ministry of Planning (2020) Records of Directorate of Census Babylon, internal reports. Iraqi Ministry of Planning, Baghdad
Jassim MS (2006) Environmental study of some possible pollutions on Euphrates river between Al-Hindia dam and Al-Kufa-Iraq, Ph. D. Thesis, College of Science, University of Babylon.
Jiang Y, Zhu M, Wang L, Li F (2015) Temporal and spatial distribution of calcium and magnesium in the Xiangxi River basin China. Environ Earth Sci 73(11):7301–7310
Kannel PR, Lee S, Lee YS, Kanel SR, Khan SP (2007) Application of water quality indices and dissolved oxygen as indicators for river water classification and urban impact assessment. Environ Monit Assess 132:93–110
Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT (2005) Increased salinization of fresh water in the northeastern United States. Proc Natl Acad Sci 102(38):13517–13520
Maryland Department of Environment (2003) Water Quality Analysis of heavy metals for the Loch Raven Reservoir impoundment in Baltimore County, Maryland. Loch Raven Reservoir Impoundment WQA. Submitted to Watershed Protection Division, U.S. Environmental Protection Agency, Region III, pp 1-20
Massoud MA (2012) Assessment of water quality along a recreational section of the damour river in lebanon using the water quality index. Environ Monit Assess 184:4151–4160
Meybeck M, Kuusisto A, Mäkelä A, Mälkki E (1996) Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes. In: Bartram J, Balance R (eds) Water quality (Chapter 2). CRC Press, Cambridge, pp 13–36
Panhalkar SS, Jarag AP (2016) Assessment of spatial interpolation techniques for river bathymetry generation of panchganga river basin using geoinformatic techniques. Asian J Geoinform 15:1–12
Rashed MN (2002) Biomarkers as indicator for water pollution with heavy metals in rivers, seas and oceans. South Valley University, Egypt
Rosemond S, Duro D, Dubé M (2009) Comparative analysis of regional water quality in Canada using the Water Quality Index. Environ Monit Assess 156:223–240
Sahoo MM, Patra KC, Khatua KK (2015) Inference of water quality index using ANFIA and PCA. Aquatic Procedia 4:1099–1106
Saini P, Kumari R, Singh AK, Sharma RK (2015) Assessment of water quality and identification of polluted sites in Betwa River Basin. India Environ Monit Assess 187(2):65
Simeonov V, Einax JW, Stanimirova I, Kraft J (2002) Environmetric modeling and interpretation of river water monitoring data. Anal Bioanal Chem 374:898–905
Tucker MR, Hardy DH, Stokes CE (2003) Heavy Metals in North Carolina Soils: Occurrence & Significance. NC Department of Agriculture and Consumer Services, Agronomic Division.
Tyagi S, Sharma B, Singh P, Dobhal R (2013) Water quality assessment in terms of water quality index. Am J Water Resour 1:34–38
World Health Organization (WHO) (2017) Guidelines for drinking water quality, first addendum, 4th edn. WHO, Geneva
Acknowledgments
Great thanks from the authors to the staff of the Babylon Directorate of Water, Resources Iraqi Ministry of Construction Housing, Municipalities, and Public Works to enable the authors to test the physicochemical properties and the heavy metal in its laboratory.
Funding
Open access funding provided by Lulea University of Technology. Open access funding is provided by Lulea University of Technology, Sweden, and Al-Mustaqbal University College, Iraq, supported by the fund of the current paper.
Author information
Authors and Affiliations
Contributions
Conceptualization: AC, UAJ; Methodology: AC, UAJ, AM; Formal analysis: AC, UAJ, AM, HSHM, HH, AAH; Writing—original draft preparation: AC, UAJ, AM, MI; Writing—review and editing: AC, UAJ, AM, NAA, HSHM, HH, AAH, MI; Funding acquisition: NAA, AM, HSHM; Supervision: NAA, HSHM.
Corresponding author
Ethics declarations
Ethics approval
The manuscript is conducted in an ethical manner advised by the targeted journal.
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent to publish
The research is scientific consent to be published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chabuk, A., Jahad, U.A., Majdi, A. et al. Integrating WQI and GIS to assess water quality in Shatt Al-Hillah River, Iraq using physicochemical and heavy metal elements. Appl Water Sci 13, 147 (2023). https://doi.org/10.1007/s13201-023-01933-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01933-2