Abstract
Chlorination is the most common method of water disinfection of pathogens before discharged into receiving streams, rivers, or oceans. This study evaluates chlorination efficiency in improving microbiological and physicochemical parameters in water samples available in the Sheble Bernta district, Amhara region, from May 2020 to June 2020. Water samples were collected from selected five sampling points from unprotected springs, groundwater, hand-dug well, and river in five kebele from the district. All samples were undergone sample preparation. The chlorination process was made by calcium hypochlorite and then analyzed for bacteriological and physicochemical parameters before and after chlorination using standard procedures. The results of this study before chlorination showed that total coliform (TC) of water ranged between 71 and 127,000 (CFU)/100 ml, pH = 6.89-.35, turbidity = 196–270 NTU, and total dissolved solid (TDS) = 315–404 mg/l. Then the result of the microbiological and physicochemical parameters of water after chlorination was changed into total coliform ranging between1 50 (CFU)/100 ml, pH = 7.25–8.10, and turbidity = 2.9–5.6 NTU, TDS = 93–134 mg/l. Finally, the result confirmed that the removal efficiency of chlorination in total coliform (TC), turbidity, and TDS was ranged between 98.29 and 99.99%, 97 and 92-98.61%, and 62.03 and 73.31%, respectively. It was observed that there is a significant difference in removing turbidity, electrical conductivity, and total dissolved solids (TDS) before and after chlorination, but the pH test did not show a significant difference (p < 0.05). The efficiency of chlorination in removing total coliform and impurity of water is very efficient. It plays a vital role in the bactericide effect, reducing turbidity, and water's total dissolved solids. However, chlorinated compounds' (Ca(OCl)2) increased the pH of water after treatments. This study concluded that the efficiency of chlorination in improving the quality of water is highly significant (p < 0.05) and made water under permeable level compared to the World Health Organization standard. Hence, the chlorination water treatment process in a household can be granted to get quality water at the household level.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Water covers almost 70% of the earth's surface (Technology and innovation 2015). Water continues to be needed for drinking, preparing food, bathing, cleaning, irrigation, and various other tasks (Dzwario et al. 2006). The major impurities in water are microorganisms, progeny, dissolved inorganic salts, dissolved organic compounds suspended particles, and dissolved gasses (Technology network limited Wood view 2019). Drinking water comes from ground sources such as groundwater and aquifers. It is also obtained from surface water such as rivers, streams, and glaciers. Other sources include rain, hail, snow, and biological sources such as plants and the sea through desalination (Men die 2005). Freshwater is already a limiting resource in many parts of the world. It will become even more limited in the next century due to increased population, urbanization, and climate change (Jackson 2001). According to recent estimates, the quantity of available water in developing regions of South Asia, the Middle East, and Africa is decreasing sharply while water quality is deteriorating rapidly due to fast urbanization, deforestation, and land degradation (Upadhyay et al. 2019).
The availability of safe drinking water is essential to a person’s health protection. A recent meta-analysis of field trials has suggested that household-based water quality treatment and safe storage with reducing diarrheal disease (Clasen and Boisson 2006; Clasen et al. 2007). In this context, many organizations have initiated the development of drinking water treatment systems suitable for tropical conditions in developing countries (WHO 2012).
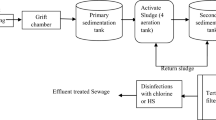
Numerous disinfection units are available for wastewater treatment applications, including chlorination, ultraviolet (UV) light, and ozonation systems (Ahmed et al. 2005). Chlorination is the most common method of wastewater disinfection and is used worldwide for the disinfection of pathogens before discharge into receiving streams, rivers, or oceans (Stover et al. 1986). Chlorine is known to be effective in destroying a variety of bacteria, viruses, and protozoa, including Salmonella, Shigella, and Vibrio cholera (Stover et al. 1986). Chlorine is effectively inactivating microorganisms in point-of-use water treatment. The application needs some serious introduction problems to this method: halogenated organic by-products such as chlorinated methane, trihalomethane, halo acetic acids, and chlorophenols activities cause cancer in human health (Yang and Shang, 2004). The chlorine properties that can control waterborne diseases' side effects require regulations and standards to limit to a minimum level.
Chlorine comes in many different forms, gas, liquid, and solid, and typical forms of chlorine used in water treatment are elemental chlorine, hypochlorite, and chlorine dioxide (Kumar, 2015). The most common disinfectants for small treatment systems are sodium and calcium hypochlorite and UV light (WHO 2008). Chlorine solution, also known as sodium hypochlorite solution or bleach, is the most affordable, easiest to produce, and most widely available chemical for household water treatment. Typically, the procedure is to add a capful of chlorine solution to a 25 l water storage container and then shake and wait for 30 min of contact time before drinking. Double dosing is advisable if the water is visibly dirty.
In many parts of the country, Ethiopia, groundwater from the borehole (deep wells), shallow wells, and Spring Rivers are an essential source of drinking water. This is especially true for rural areas as well as for towns. Most groundwater is usually consumed without any form of treatment. However, the occurrence of groundwater and spring water is not uniform because it depends on various environmental and geological factors (Dzwario et al. 2006). The parameters to take into consideration in assessing water quality are; suspended solids, pH, total dissolved solids (TDS) the level of fecal contamination (microbiological), and turbidity. The community faces the tough challenge to have of people without access to an improved water supply. Lack of access to drinking water and exposure to waterborne diseases from unsafe drinking water are problems for many people in developing countries like Ethiopia (WHO 2011).
Materials and methods
Description of the study area
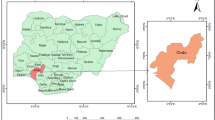
This study was conducted in the Shebel Bernta district, which is located in East Gojjam Zone, North Central Highlands of Ethiopia in the Amhara regional state (Fig. 1).
It is found about 283 km of northeast of Addis Ababa the capital city of Ethiopia and 241 km apart from Bahir Dar, the capital city of Amhara Regional State. Shebel Bernta district is bordered on the southwest by the Dejjen district, on the northwest by the Enemay district, on the north by the Enarj Enawga district, and south and southeast of Abay River. The district covers 85,499 hectares of land area and the topography feature of the district is 43.7% level (flat), 8.45% mountainous, and 48.08% valley. The major socioeconomic of the population are agricultural sectors and commercial in Yedwuha town and other small towns in the district (Figs. 2 and 3).
Shebel Bernta District is geographically located between 10°15′ latitude N and 38°30′–38°15′ longitude east. The district's total population is estimated to be 133,946 people, out of whom 63,865 are males and 70,081 are females live in the district. The districts' mean annual rainfall and community temperature vary from 400 and 1000 mm to 15 °C and 25 °C, respectively. The district's significant drinking water sources are ground, spring, hand pump groundwater, river, and tap water is water.
Apparatus and instruments
pH (PHS-25CW micro processer) meter, Micro-Siemens percent meter, Snez TDS2 meter(made in Italy, range 1/1000 ppm), multipurpose meter, autoclave, incubator, volumetric flask, measuring cylinder, micropipette, digital balance, test tube, magnetic stirrer, filter paper, polypropylene containers, and Petri dishes were used for sampling and analysis of samples.
Chemical and reagents
Commercial (70%) analytical grade by mass, granulated calcium hypochlorite (Ca(OCl)2) was an effective option for the chlorine disinfection process. Potassium chloride (KCl) and buffer solution, used for calibration purposes of pH measurement test and nutrient agar media, microorganisms grew on the surface of the pates, and distilled water for serial dilution process.
Water sample sites
Water samples were selected in five different kebeles in a purposive way as follows: Yedwuha 02, Mozegne 01, Mergech 01, Aberekuy dada Hana and Wobowerie.
Sample collection, and preparation
Five water samples were collected from five different kebeles of the Shebel Bernta district, including rural Kebeles (Aberkuy dadahana and Woboworie) and small urban areas (Yedwuha 02, Mergeche 01, Mozhegne 01). The water samples were collected purposively from lower and higher altitude geographical location areas by a simple random sampling method with the coding on tightly closed 1L pre-cleaned polypropylene containers to avoid any contamination from environmental pathogens from May 2020 to June 2020 and then transported to the microbiology laboratory in Debre Markos University. The collected water sample from the study area was stored in an icebox during transportation.
Chlorination process
Calcium hypochlorous chemical as a solid form 70% by mass concentration obtained from the regional water resource office applied in chemical water treatments. From 70% by mass concentration, 14.28 mg/l or 7.14 mg/500 ml of calcium hypochlorite Ca(OCl)2 was weighed and added into a conical flask by digital balance, which was used for undergone chlorination process. 7.14 mg of calcium hypochlorous immediately was dissolved in 500 ml of water sample in a volumetric flask in each sample site by a magnetic stirrer and was covered with aluminum foil then coded to it until 30 min of contact time then filtered it.
Serial dilution
Sample preparation for total coliform bacteria is as follows a 10 ml of water sample and the chlorinated water were mixed with 90 ml of distilled water separately and shaken appropriately for about 5 min for better homogenization. This homogenized solution was considered a stock sample. A series of six test tubes containing 9 ml of distilled water was prepared and labeled as 10–1, 10–2, 10–3, 10–4, 10–5, and 10–6. 1 ml of a water sample from the stock sample was added to first labeled 9 ml of distilled water to make 10–1 and 1 ml from 10–1 to make 10–2, from 10–2 to 10–3, from 10–3 to10−4, from 10–4 to 10–5, and finally from 10–5 to 10–6 to reduce the microbial load of the samples.
Microbial culture media process
Microorganisms, as bacteria require a suitable environment, and ingredients of culture media, ordinary media, and nutrient agar are a general-purpose nutrient medium used to cultivate microbial supporting the growth of a wide range of non-fastidious organisms. Nutrient agar is popular because it contains many nutrients needed for bacterial growth. A 16.8 g of nutrient agar media was weighed and added into a conical flask containing 600 ml of distilled water (by following the manufacturer's direction); then, the conical flask was sealed, and the media in it was boiled using a hot plate to homogenize the nutrient agar. After that, the media, Petri dishes, test tubes, pipette tips, and graduate cylinders were sterilized at 121 °C for 15 min using steam heat of the autoclave to remove all microbes. The sterilized media was poured into sterilizing Petri dishes and allowed to solidify inside the laminar flow cabinet to create aseptic conditions. Once the nutrient agar media were solidified on the plates, the plates were placed upside down to prevent the dropping of the water droplet from being formed from the hot media until the inoculation process.
Inoculation
Firstly, the Petri dishes were labeled as 10–1–10–6 using the marker, after 100 ml of each sample was collected separately in a clean, sterile glass container. Then with the help of a micropipette, 1 ml of the serially diluted water samples was added to their corresponding labeled Petri dishes (i.e., 1 ml from the test tube having 10–1 dilution, the Petri dish being labeled as 10–1 and this procedure was repeated up to the last dilution, 10–6). Remember that after the addition of a 1 ml sample to the plate, the sample was spread using a glass spreader at 45 degrees under safe conditions in the laminar flow cabinet to prevent the condensed growth of microbes for microbial enumeration, respectively. After the samples were spread on the nutrient agar plates, they were incubated in an incubator machine adjusted at 37 °C temperature for about 48 h. The spread samples in the Petri dish were incubated separately and coded before treatment (BT) and after treatment (AT); after 48 h of incubation completed, the microorganisms were grown on the surface of the petri dish plates. The microbial colonies were counted the colony-forming unit was calculated and recorded with a colony counter. The colonies express in terms of "colony-forming units" are calculated using Eq. (1): CFU/100 ml = N × 1/100 ml × DF where N = number of colony counts, ml = milliliter, and DF = dilution factor.
Chlorination test for water treatment
The analysis of physicochemical parameters such as pH test, turbidity, and total dissolved solids (TDS) was determined by APHA (1992) and Gupta (2001) before and after chlorination. Finally, the removal efficiency of these parameters was calculated by the following formulas described (Alemu and Geremew, 2019).
Statistical analysis
Data analyses were performed with the IBM SPSS Statistics version 21.0 generated to evaluate chlorination efficiency in improving microbiological and physicochemical parameters in water samples available from the Shebel Bernta district and were subjected to paired sample t-test. Paired sample t-test comparing before and after chlorination based on the level at p = 0.05.
Results and discussions
Total coliform (TC) removal efficiency
The results obtained throughout the evaluation of the efficiency of chlorination in improving the microbiological quality of water were done on the total coliform contamination on study samples. Before chlorination, the total coliform bacteria in the water sample from the five-sample site ranged from 71 to 127,000 (CFU)/100 ml in SNo-5 and SNo-1, respectively. Total coliform was recorded at a maximum of 127,000 (CFU)/100 ml, 14,900 (CFU)/100 ml, 2930 (CFU)/100 ml, 1830 (CFU)/100 ml, and 71 (CFU)/100 ml colony-forming unit resulted in SNo-1, 2, 3, 4, and SNo-5, respectively. The total coliform in water in the five-sample site in the first and second dilution factors grow up numerous total coliform before chlorination on the Petri dish indicating contaminated risk highly for humans and animals—total coliform bacteria far from WHO guideline of water quality associated risks (WHO 2017). After chlorination, SNo-1, 2, 4, and 5 ranged from 1 up to 7 (CFU)/100 ml, confirming that WHO guideline water quality drinking purpose was associated with low risk for health, but SNo-3 was 50 (CFU)/100 ml which ranged between 11 and 100 (CFU)/100 ml according to WHO quality guideline, total coliforms should be absent immediately after disinfection, and the presence of these organisms indicates inadequate treatment (WHO, 2017). The five samples SNo-1, SNo-2, and SNo-3 before chlorination were recorded with higher total coliform bacteria because the three samples of the district and kebele administration small town lived more people that contribute leave out wastage can cause contamination of water. However, SNo-4 and SNo-5 have resulted in lower total coliform bacteria; this is due to rural areas' concentration of people living far apart from each other and contributing less wastage of contamination in the water compared with the urban in the study.
The removal efficiency of chlorination to remove total coliform bacteria ranged from 98.29 to 99.99% (Fig. 4). Specifically, in SNo-1 and SNo-5, a maximum of 99.99% removal efficiency was recorded, indicating the calcium hypochlorite has killed the bacteria in this sample site, and in SNo-3 (Mergech) 98.29% was recorded as a relatively lower removal efficiency compared to the other sample site. The removal efficiency of chlorination to remove total coliform bacteria ranged from 98 to 99% (Brady et al. 2001). In this study, chlorination (by calcium hypochlorite) and water treatment were more efficient to remove the total coliform from water. The number of bacteria decreases by > 99.99% in the disinfection process (Roberto et al., 2008). The total coliform of water before and after chlorination at 95% confidence limit since the probability is greater than 0.05 were concluded different results for before and after chlorination of water treatment methods do not statistically significantly different at t value 1.195 and (p = 0.298) p > 0.05. The result of this study showed that most of the chlorination methods for this work were found to be effective in removing microorganisms from contaminated water as compared with WHO guidelines (WHO 2012).
pH test
Before chlorination, the pH of the water sample ranged from 6.89 to 7.35 and after chlorination, the pH ranged from 7.25 to 8.10. The pH value before chlorination in SNo-2, SNo-4, and SNo-5 was found to be in the acidic range of 6.89, 6.91, and 6.93, respectively, and it may be due to the pollution of water by acidic ions from farmland activity (fertilizer, pesticide, and insect side chemicals). Higher pH conditions could enhance the adsorption of positively charged ions (Alemu and Gereme 2019). In other sample sites (SNo-1 SNo-3) 7.25 and 7.35 (alkali range), pH values were recorded (Table 1). Yedwuha (SNo-1) is the administrative town of Shebel Bernta district and Mergech (SNo-3) sample site is the second administrative kebele town due to the people that contribute to the contamination by wastage residue from the market and different chemicals in metalwork garages, car washing in the roadside, and wastage from Hotel and contaminate from the town increases the alkalinity. The pH values of the water sample before chlorination were relatively lower and increased after chlorination in the entire sample size, as shown in Table 2. Normally, unpolluted water shows a pH of about 7.00 to 8.00 (WHO, 2008). After chlorination, water pH in the entire sample increased to the basic range because calcium hypochlorite is basic as the hypochlorite anion can accept a proton from the water molecule, leaving a hydroxyl anion in the solution. Moreover, it was reported that increased calcium carbonate (CaCO3) and other bicarbonates (Ca(HCO3)2) levels in the water enhance the combination with both hydrogen and hydroxyl ions to increase the buffering capacity of the water and thereby increase its alkalinity (Kemker 2013). The disinfectant process of calcium hypochlorite naturally increases the pH of water in the reaction. The permissible limits of pH for drinking water, recreation, and agricultural water use pH (6.5–8.5/9) (FDRE MoWR, 2002 and WHO, 2006). The pH of the sample before and after chlorination paired t-test at a 95% confidence interval was not a statistically significant difference at t = − 3.892 and p > 0.05 (p = 0.180) between before and after chlorination wastewater treatment. Since pH is found to affect enzymes and hormones in the body which control metabolism, growth, and development (Ukpong and Peter 2012), chlorination is needed for the alkalinization treatment of the groundwater.
Turbidity removal efficiency
The water sample's turbidity before chlorination ranged from (196–270 NTU) and the turbidity of water after chlorination was (2.9–5.6 NTU). Before chlorination of water samples SNo-1, 2, and 3, more turbid values of (270 NTU), (244 NTU), and (260 NTU), respectively, indicate relatively more coldness compare to rural sample codes SNo-4 and SNo-5.The water's turbidity value before chlorination is higher than that after chlorination. Lower values of mean annual turbidity of surface water exceeded (4.8–27.2 and 24.6 NTU), respectively (Tessema et al., 2014). After chlorination, SNo-1 was obtained, and the maximum turbidity (5.6 NTU) indicates the water sample contains more cloudiness and suspended particulate. It was more significant amounts than the WHO permissible level of drinking water standards (5 NTU) and, other samples (SNo-2, 3, 4, and 5), sites were confirmed with allowable limits of drinking water quality after chlorination (CSA, 2017).
The removal efficiency of water's chlorination for turbidity ranged from 97.92 to 98.61% as shown in Fig. 5. The more turbid in SNo-1 after chlorination confirmed that lower removal efficiency of 97.97% and the other samples recorded 98.36%, 98.61%, 98.52%, and 98.41%, respectively. Turbidity is the cloudiness caused by particulate matter present in wastewater, suspension of sediment in the distribution system, inorganic particulate matter in some groundwater, or sloughing of bio-film within the distribution system (WHO 2004). Various suspended solids like clays, sands, silt, organic and inorganic substances, and microorganisms may cause high turbidity. Turbidity in water decreases the efficiency of chlorine for disinfection (Odjadjar et al. 2012). The turbidity of water before and after chlorination showed a statistically significant difference at t = 15.697 and p < 0.05 (p = 0.000) and a positive correlation between before and after chlorination of water treatment.
Total dissolved solids (TDS) removal efficiency
TDS measures the dissolved combined content of all inorganic and organic substances present in liquid in molecular, ionized, or microgranular suspended form. The maximum TDS value was recorded at SNo-1 due to the urban area's polluted stormwater runoff from a hotel, building, and roadside washing purposes. TDS values ranged before chlorination water 315 mg/l and 404 mg/l before and after chlorination of treated water while TDS values ranged between 93 mg/l and 134 mg/l. Before treatment of water, maximum TDS value was resulted 404 mg/l, 315 mg/l, 371 mg/l, 353 mg/l, and 342 mg/l in SNo-1, 2, 3, 4, and SNo-5, respectively(Fig. 6). After treating water with chlorination was reduced to 118 mg/l, 101 mg/l, 99 mg/l, 134 mg/l, and 93 mg/l. Due to sinking, suspended solid residue in a filtered chlorinated volumetric flask surface in SNo-1, 2, 3, 4, and SNo-5, respectively. Before and after chlorination, the whole sample site was obtained below the maximum permissible limits of WHO guidelines for drinking water (WHO 2006).
The maximum removal efficiency of TDS (73.31%) was recorded in SNo-3 and minimum removal efficiency (62.03%) was recorded in SNo-4 as shown in Fig. 6 and the other sample site (SNo-1, 2, and SNo-5) was measured its removal efficiencies 70.79%, 67.93%, and 72.8% respectively. The removal efficiency of TDS levels before and after chlorination water at a 95% confidence interval showed a statistically significant difference at t = 17.497 and p < 0.05 (p = 0.000) and a positive correlation between before and after chlorination water treatment.
Conclusion
In this study, it can be concluded that chlorination has a good efficiency in removing; turbidity, electrical conductivity, and total dissolved solids (TDS). The efficiency of chlorination in removing total coliform and impurity of water is very efficient. Therefore, the chlorination method should be used in the study area to reduce human health risks by delivering safe water.
Data availability
We declare that the data and materials presented in this manuscript can be made available as.per the editorial policy of the journal.
References
Adhena A, Belay Z, Angaw K, Jemal Y (2015) Physico-chemical analysis of drinking water quality at Jigjiga City. Ethiopia Am J Environ Protect 4(1):29–32
Ahmed W, Neller R, Katouli M (2005) Evidence of septic system failure determined by a bacterial biochemical fingerprinting method. J Appl Microbiol 98:910–920
Alemu L, Geremew A (2019) Evaluation of the efficiency of ceramic filters for water treatment in Kambata Tabaro zone, southern Ethiopia. Environ Syst Res 8:1
APHA (1992) Standard methods for the examination of water and wastewater. 18th Ed.
Brady J, Garber W, Stahl JF (2001). Disinfection and Chlorination. In: Operation of Wastewater Treatment Plants, Volume II, Sacramento, CA: California State University, Sacramento Foundation, p 352
Central statistical agency of Ethiopia (CSA) in collaboration with the ministry of water, irrigation and electricity, LSMS, world bank, UNICEF, WHO, and JMP. 2017. Drinking water quality in Ethiopia
Clasen T, Boisson S (2006) Household-based ceramic water filters treatment of drinking water in disaster response: an assessment of a pilot program in the Dominican Republic. Water Pract Technol 1(2):1–9
Clasen T, Schmidt W, Rabie T, Roberts I, Cairncross S (2007) Interventions to improve water quality for preventing diarrhea: systematic review and meta-analysis. BMJ 334(7597):782
Dzwario B, Hoko Z, Love D, Guzha E (2006) Assessment of the impact of pit latrines on groundwater quality in rural areas: a case study from Marondera district. Zimbabwe, PhysChem Earth J 31:779–788
Environmental protection agency (EPA) (2010) E. coli in water by membrane filtration using membrane-thermotolerant Escherichia coli agar (mTEC), Washington DC
Gupta PK (2001) Methods in environmental analysis; water, soil and air. Updesh Purohit Agrobios, Jodhpur
Jackson RB (2001) Water Changing World. Issues in Ecology. Ecological Society of America, Washington, DC, pp 1–16
Kemker C (2013) "pH of Water." Fundamentals of Environmental Measurements, Fondriest Environmental, Inc.http://www.fondriest.com/environmentalmeasurements/parameters/waterquality/ph/, Accessed 10 August 2020
Marrero-Ortiz R, Riley KR, Karpiscak MK, Gerba CP (2009) Groundwater quality of individual wells and small systems in Arizona. J Am Water Works Assoc 101(9):89–100
Men die U (2005) The Nature of Water. The Theory and Practice of Clean Water Production for Domestic and Industrial Use. Lacto-Medals Publishers, Lagos, pp 1–21
Odjadjare EE, Igbinosa EO, Mordi R, Igere B, Igeleke CL, Okoh AI (2012) Prevalence of multiple antibiotics resistant (MAR) Pseudomonas species in the final effluents of three municipal wastewater treatment facilities in South Africa. Int J Environ Res Public Health 9:2092–2107
Technology and innovation.(2015) Publishes new double issue with a special section on Water University of South Florida (USF Innovation)
Stover EL, Haas CN, Rakness KL, Scheible OK (1986) Design Manual: Municipal Wastewater Disinfection. Environmental Protection Agency, Cincinnati
Dhār ST, Lenth Z (2011) Water quality monitoring of Prairie Lake, December 14
Technology network limited wood view (2019) Common water contaminants and how to prevent them, in Bull Lane Industrial Estate Sudbury UK
Tessema A, Mohammed A, Birhanu T, Negu T (2014) Assessment of Physico-chemical Water Quality of Bira Dam Bati Wereda Amhara Region Ethiopia. J Aquac Res Development 5:6. https://doi.org/10.4172/2155-9546.1000267
The Federal Democratic Republic of Ethiopia Ministry of Water Resources (2002) Specification for Drinking Water Quality, Addis Ababa, Ethiopia: National Meteorological Services Agency
Ukpong AC, Peter BU (2012) Physico-chemical and bacteriological analyses of drinking water in Ibeno Local Government Area of Akwa-Ibom State. Niger J Technol 31(2):116–127
Upadhyay L, Meena SR, Kharb J, Johar A (2019) Assessment of physical and chemical water quality parameters at NH-11. Int J Eng Res Technol. 08(4):76
World Health Organization (WHO) (2004) Guidelines for Drinking-water Quality, recommendations World Health Organization, 3rd edn. WHO, Geneva
World Health Organization (WHO) (2008) Guidelines for drinking-water quality [electronic resource]: incorporating 1st and 2nd addenda, Recommendations, 3rd edn. WHO Press, Geneva
World Health Organization (WHO) (2011) Guidelines for drinking water quality, 4th edn. WHO, Geneva (ISBN 978 92 4 154815 1)
World Health Organization (WHO) (2012) Joint monitoring program for water supply and sanitation estimates for the use of improved drinking-water sources in Ethiopia. WHO, Geneva
Yang and C. Shang. (2004) Chlorination by-product formation in the presence of humic acid, model nitrogenous organic compounds, ammonia, and bromide,". Environ Sci Technol 38:995–1008
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Consent for publication
All data and information are generated and organized by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Temesgen, G., Lelago, A., Assefa, E. et al. Evaluation of chlorination efficiency on improving microbiological and physicochemical parameters in water samples available in Sheble Berenta district Amhara region, Ethiopia. Appl Water Sci 13, 120 (2023). https://doi.org/10.1007/s13201-023-01922-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01922-5