Abstract
Groundwater is a significant source of plant water use since groundwater consumption of plants reduces the volume of surface irrigation water. In this study, groundwater table effect on hard red spring wheat (Triticum aestivum L.) growth and yield parameters were investigated using a lysimeter technique in a controlled environment. Three different groundwater table depths including 30, 60, and 90 cm and a control treatment with surface irrigation were tested. The results showed that water consumption in the 90 cm water table depth was 11% and 31% lower than the water consumption in the 60 and 30 cm water table depths, respectively. Consequently, the groundwater table depth increased, and crop water consumption decreased. Similarly, with the increasing water table depth from 30 to 90 cm in the lysimeter, the crop water use efficiency and crop yield increased by 79% and 71%, respectively. The 90 cm water table produced the highest crop yield, above-ground biomass, and kernel quality compared to 30 and 60 cm water table depths. Quality analysis of the wheat kernels indicated that the kernels from the 90 cm depth had relatively higher starch content, pasting properties, and gluten proteins compared to the kernels from other water table depths.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is an essential element for plant growth. High quality and sufficient quantity of freshwater resources are important for the healthy growth of plants. Climatic changes over the years have resulted in seasonal fluctuations in groundwater table levels through natural and anthropogenic processes including discharge, recharge, and usage of water resources. These seasonal variations in groundwater table affect the crop water availability, stages of crop growth, properties of soil, salinity level of the soil, time and period of irrigation applications, and further lead to a reduction in crop production (Stromberg 2001; Yang et al. 2007; Huo et al. 2012; Bai et al. 2020). In many areas of intensive agriculture, water supply by groundwater is safer and more accessible than that by irrigation canals. Groundwater is also typically less prone to pollution compared to surface waters.
While groundwater-based irrigation has undoubtedly promoted agricultural growth, failure to effectively regulate groundwater withdrawals results in the overuse of groundwater. Over-withdrawal of groundwater drastically changes the groundwater discharge and recharge rates. The optimum groundwater level increases crop yield and crop water use efficiency (Bai et al. 2020). In some cases, shallow water table enhances the water utilization by plants. However, a shallow water table may cause waterlogging, root anoxia, and soil salinization. Hence, a controlled drainage system is the best way to maintain the groundwater level (Wesström and Messing 2007; Nosetto et al. 2009; Ghamarnia et al. 2012).
Since every crop’s root development and water needs are different, lysimeter studies need to be conducted for each crop to investigate groundwater’s impact on crop production. The lysimeter system is used to simulate field conditions under a controlled setting, making it easy to adequately study independent variables associated with groundwater usage for crop production (Luo and Sophocleous 2010). Several studies were conducted to explore the effects of the water table depth and water use efficiency on several crops’ yields. It was found that an optimum crop yield was achieved at a water table depth of 150–200 cm for wheat, corn, sunflower, berseem, sorghum, and sugarcane (Kahlown and Ashraf 2005). Mejia et al. (2000) conducted a 2-year-long lysimeter study to determine the yield and water consumption of corn (Zea mays L.) and soybean (Glycine max (L.) in 50 and 75 cm water table depths. A freeflow drainage system was installed at 100 cm below the soil surface to serve as the control. During the first season of the study, by comparing with the control treatment, the corn produced 13.8 and 25% greater yields for 50 and 75 cm water table depths, respectively, while soybean produced 8.5 and 12.9% greater yields for 50 and 75 cm water table depths, respectively. In the second season, corn produced 6.6 and 6.9% greater yields, and soybean produced 37.3 and 32.2% greater yields in the 50 and 75 cm water table depths compared to the control plots, respectively. Therefore, 75 cm water table depth was concluded to be the most efficient depth for corn and soybean growth (Mejia et al. 2000).
In a study conducted on winter wheat by Kiremit et al. (2022), a water table depth of at least 42 cm was recommended to ensure higher wheat yields and seed quality. Nosetto et al. (2009) found that crop yields were the highest at the water table depths of 70–165 cm for hard wheat, 120–220 cm for soybeans, and 140–245 cm for maize, respectively. Ghamarnia et al. (2012) discovered that raising the water table depth (60, 80, and 110 cm) increased groundwater contributions between 4 and 12%, depending on the irrigation treatment. Liu and Luo (2011) investigated the water table's impact on winter wheat's yield and water use under rainfed conditions and reported that the yield, water use efficiency, and root development of winter wheat increased when the water table depth was between 110 and 150 cm. The effects of various water table depths (30, 50, 70, and 90 cm) on soybean growth were examined by Fidantemiz et al. (2019). In their water table experiments without surface irrigation, both 70 and 90 cm water table depths were found optimum for soybean in terms of water use efficiency, grain yield, and root distribution. In another study using lysimeters under a controlled environment, Kadioglu et al. (2019) observed that groundwater contribution to canola plants was the highest in a 90-cm-water table depth lysimeter.
There are seven wheat varieties grown in the USA including hard red winter, hard red spring, soft red winter, soft white, hard white winter, hard white spring, and durum. Hard red spring wheat is mostly grown in the Northern Plains (states of Minnesota, Montana, North Dakota, and South Dakota), Idaho, and Washington in the USA, and in Canada. Hard red spring wheat grows best in warm temperatures between 15 and 23 °C and requires precipitation ranging between 12 and 18 inches. It is used for bread flour and blending due to its high protein content, strong gluten, and high water adsorption properties (Atwell and Finnie 2016; AgMRC 2022). All the studies mentioned above provide a comprehensive understanding of the water table effect on crop growth. However, as of our knowledge, this current study is the first lysimeter study to investigate the water table effect on hard red spring wheat (Triticum aestivum L.) growth and yield parameters. There are a few studies available to determine the effect of water table depth on wheat; however, no study is available on the hard red spring wheat variety, which is commonly grown in cold climatic regions. Besides, none of the previous studies investigated the seed quality in different water table depths. Hence, this study used the lysimeter technique to address the effects of different water table depths (30, 60, and 90 cm) on the growth, yield, root distribution, seed quality, and water use of hard red spring wheat.
Materials and methods
Preparation of lysimeters and experimental setup
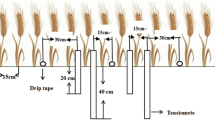
The experiments were carried out in a climate-controlled greenhouse located in Fargo, ND. Polyvinyl chloride pipes were used as lysimeters with a diameter, wall thickness, and height of 15.24, 0.5, and 127 cm, respectively (Fig. 1). A total of thirty-two lysimeter columns were used in this study with three different constant water table depths including T30 = 30 cm; T60 = 60 cm; and T90 = 90 cm and control treatment, TControl, with no water table depth. Eight lysimeters were used for controlled treatment while the remaining lysimeters were used for the water table treatments. A total of eight replicates (R1 to R8) with a randomized complete block design were employed. The amber color Mariotte bottles (4.0 L each) were placed on adjustable table platforms, and the heights of the platforms were set to the desired water table depths. The bottles were attached to the bottom of the lysimeters with a tubing system to feed the lysimeters. Water levels in the lysimeters were monitored continuously throughout the experiment, and the water consumption of the plants was calculated. The Mariotte bottles supplied water for each lysimeter to the system continuously depending on the plant’s water utilization performance. Whenever the water level was reduced in the bottles, it was replenished back to keep enough water in the bottles. The water consumption of the hard red spring wheat from the bottles was calculated with the reduction in the water volume in the bottles for each lysimeter. Tap water was used for the entire experiment.
Soil packing and sensor system
Bulk field loam topsoil was obtained from a farm in Fargo (ND, USA), and the soil was air-dried. The soil’s textural properties were measured at NDSU Soil Testing Laboratory (Fargo, ND). The soil is made of sand (43%), silt (35%), and clay (22%). The other characteristics of the soil are as follows: Field capacity is 0.32 cm3/cm3, readily available water (50%) is 0.26 cm3/cm3, the permanent wilting point is 0.21 cm3/cm3, and bulk density is 1.43 g/cm3. The soil was mixed with sand (300 g of sand for every 1.0 kg of soil) in the mixer before packing the lysimeters to increase the soil aeration capacity so that enough air can be supplied to the plant in the lysimeters. The lysimeters were filled with 5.0 cm of gravel and 5.0 cm of sand to prevent clogging. Then, 100 cm of sandy soil was added to the lysimeters. All the lysimeters (total of 32) were packed uniformly. The bottom of the lysimeters was sealed with caps.
Three soil water potential sensors (TEROS-21, Meter Inc., WA, USA) were inserted in each TControl lysimeter to determine irrigation amounts and times at three different soil depths including 15, 45, and 75 cm from the soil surface, respectively. In order to monitor the soil moisture content in the lysimeters: (i) One sensor was placed in T30 at a depth of 15 cm; (ii) two sensors were inserted in the T60 at depths of 15 and 45 cm; and (iii) three sensors were inserted in the T90 at a depth of 15, 45, and 75 cm, respectively. A total of 15 sensors, including the replications, were inserted into the lysimeters. All the sensors were connected to three multichannel data loggers (ZL6, METER Group, Inc., Pullman, WA, USA).
A Vp-4 sensor (Meter Inc., WA, USA) was also placed in the greenhouse to measure indoor air temperature and humidity. To determine reference crop evapotranspiration (ET0), an atmometer (ETgage model E, C&M Meteorological Supply, CO, USA) was placed in the greenhouse and monitored daily using data loggers (Onset Computer, Bourne, MA, USA). Similarly, the field capacity, permanent wilting point, bulk density, and readily available water of the experimental soil were obtained using the HYPROP-FIT software and the WP4 method (Roy et al. 2018).
Planting and harvesting
Once all the lysimeters were packed with soils, then they were filled with water to ensure uniform water curve conditions for all treatments before planting. Then, the valves at the bottom of the lysimeters were opened for 36 h to make sure the sufficient amount of water drained out. All the Mariotte bottles that were connected to the lysimeters were filled with water and the valve on the Mariotte system opened to drain the water into the lysimeters from the bottom. Eight hard red spring wheat seeds (ND VitPro hard red spring wheat, PI 682660) were sown in each lysimeter, and six successfully germinated seeds were kept in the lysimeters. The germination and growing stages of the plants throughout the experiments were monitored using the sensors installed into the lysimeters. Following the germination, a 12-h photoperiod was applied to the plants for the first 4 weeks, then followed by 16 h after 2 and 6 weeks. The greenhouse was set at a constant temperature of 21 °C during the day and 15 °C at night, with a light intensity of 775 mM m−2 s−1. The plant was harvested once it reached the physiological maturity stage.
Plant growth parameters, yield, and root distribution
After the harvesting, above-ground plant biomass, yield, and root biomass were determined for the mature wheat plant in each lysimeter. The wheat plant was cut at the point right above the soil surface, and the wheat kernels were extracted by threshing the plant spikelets. The portion of plants without kernels was weighted on a scientific balance to determine the fresh plant weight. The plants were oven-dried for 24 h at 80 °C and weighed again to calculate the dry weight of above-ground biomass. The plant yield was calculated as the total weight of the wheat kernels per plant. For the determination of root distribution, the soil cores from each lysimeter were extracted at three depths from the top of the lysimeter (0–40 cm, 40–70 cm, and 70–100 cm). The extracted soil cores were washed to separate coarse roots in the soil. After washing, the plant roots were dried in the oven and weighed to determine the below-ground biomass.
Hard red spring wheat kernel quality was analyzed at the NDSU Wheat Quality & Carbohydrate Research Laboratory. A digital caliper (Mitutoyo America Co., Aurora, IL) was used to measure wheat grains’ length and width. The starch grains of the wheat endosperm were examined morphologically by scanning electron microscopy (SEM). The wheat grains coated with gold–palladium were positioned on aluminum supports using Balzer’s sputter coater SCD030. The JEOL JSM -6300 SEM system was used to image the starch grains.
Wheat starch pasting properties were studied using Rapid Visco-Analyzer (RVA) to generate a complete pasting curve as a function of temperature (°C) and time (min) according to the American Association of Cereal Chemists (AACC) Approved Method 76-21.02. Thermoview software was used to record initial viscosity (cP centipoise), maximum peak viscosity (cP), final viscosity (cP), breakdown, and setback. Briefly, 3.5 g (14% mb) of wheat flour was weighed into an RVA canister, followed by 25 mL of deionized water. Then, the sample was placed on the RVA instrument to start controlled heating at a rate of 12 °C/min until 95 °C was reached, followed by a controlled cooling process until 50 °C was reached. The heating and cooling cycle was completed in 13 min.
Moreover, wheat proteins were studied following Gupta et al. (1993) and Ohm et al. (2009) protocols with minor modifications. First, 10 mg (12% mb) of wheat flour was suspended in 1.0 mL of the pH 6.9 extraction buffer (0.5% sodium dodecyl sulfate (SDS) and 0.05 M sodium phosphate). The mixture was placed in the Eppendorf ThermoMixer C for 5 min at 2,000 rpm. Then, the samples were centrifuged using the Eppendorf Centrifuge 5424 R at 17,000 g for 15 min. After centrifugation, the supernatant was saved to analyze the SDS extractable proteins fraction. Furthermore, the solid residue was utilized to obtain the SDS unextractable proteins fraction. Therefore, 1.0 mL of the extraction buffer was added to the solid residue. Then, the mixture was sonicated using a Sonic Dismembrator Model 100 (Fisher Scientific, Waltham, MA) for 30 s at 10 W. The sonicated mixture was centrifuged, and the supernatant was saved to analyze the SDS unextractable proteins fraction. The saved supernatants were filtered through a 0.45-mm PVDF membrane followed by a final heating step (80 °C for 2 min) to inactivate any enzymatic activity as described by Larroque et al. (2000). The Agilent 1100 system (Agilent Technologies, Santa Clara, CA) with an Agilent 1200 photodiode array detector (PAD) (Agilent Technologies, Santa Clara, CA) was utilized to run the Size Exclusion-High Performance Liquid Chromatography (SE-HPLC) analysis using a Yarra 3.0 µm SEC-4000 column (300 × 4.6 mm, Phenomenex, Torrance, CA) coupled with a BioSep SEC-s4000 guard cartridge (Phenomenex, Torrance, CA). The SE-HPLC analysis was carried out by injecting 10 μL of the sample that flows through the columns at a rate of 0.5 mL/min. The eluting solution was 0.1% trifluoroacetic acid (TFA) in 50% aqueous acetonitrile. The UV absorbance was measured at 214 nm (Larroque and Bekes 2000; Ohm et al. 2009).
Determination of crop water use, irrigation rate, and water use efficiency
After harvesting, three randomly selected lysimeters from each treatment were vertically cut to calculate the groundwater consumption of the crop by using the equation explained earlier in the literature (Ritchie 1998). Therefore, a total of 12 lysimeters, which are 3 lysimeters from each treatment, were cut. Since the experiments were conducted in a controlled environment, there were no precipitation, irrigation, deep percolation, or runoff. Therefore, the equation of soil water balance (Eq. 1) can be simplified by taking into account the controlled environment (Hillel 1998).
where ET is evapotranspiration (mm), Cr is the capillary inflow of water (mm), S1 represents the initial storage of water, and S2 is the final storage for soil water in the lysimeters. Sensors and soil moisture release curves were employed to determine the soil profile’s initial moisture content. The initial soil moisture content was determined using the 360 and 168 mm water table and the control treatments. In the control treatment, 50% of the total available moisture was assumed to be readily available water threshold. The required irrigation depth (d) was calculated using Eq. 2 (Majumdar 2001).
where Fci and Mbi are defined as the percentage by weight of the soil layer’s field capacity and water content, respectively. Asi is the apparent specific gravity (bulk density), Di denotes the depth of each soil layer, and n represents the soil layer numbers. Water use efficiency was computed for both water table depth and control treatments according to the method explained in Sinclair et al. (1984).
Water use efficiency (g/cm) was calculated for each water table treatment and the control treatments separately (Bai et al. 2020). For the water table treatment, the water use efficiency was calculated as wheat grain yield (Y, g/plant) per water consumed by the wheat in each water table lysimeter (Etc) to produce the yield (Eq. 3). For the control treatment, the water use efficiency was calculated as grain yield (Y, g/plant) divided by the amount of irrigation applied to the wheat in the control treatment columns (Eti) to produce the yield (Eq. 4). WUE in the equations denotes water use efficiency.
Statistical analysis
One-way analysis of variance (ANOVA) at a 95% confidence interval (P ≤ 0.05) was performed using the SPSS statistical software (Version 27., IBM Corp., NY, USA) to assess the significance of differences between treatment averages. For comparing all the treatment-means, the Fisher Least Significant Difference (LSD) method was used. The relations between water table depths and plant parameters, such as yield, above- and below-ground biomass, and water use, were examined at a probability level of P ≤ 0.05, using Pearson partial correlation and multiple regression.
Results and discussion
Environmental conditions of the greenhouse and crop evapotranspiration
Greenhouse air temperatures were measured daily during the growing season of hard red spring wheat from September 24, 2020 (seeding), to December 24, 2020 (harvest), and the average temperatures varied between 16.9 and 19.8 °C. The atmometer results showed that the daily reference crop evapotranspiration (ET0) values ranged from 3.9 mm (at germination) to 7.6 mm (at anthesis). ET0 refers to the rate of evapotranspiration from a reference surface. The reference surface is a hypothetical grass reference crop with specific characteristics. The reference evapotranspiration concept was introduced to assess the evaporative requirement of the atmosphere independent of crop type, crop development, and crop management practices. As water is abundantly available at the reference evapotranspiring surface, soil factors do not affect the evapotranspiration (ET). The only factors affecting ET0 are climatic parameters. Therefore, ET0 is a climatic parameter that can be computed from weather data. It expresses the evaporating potential of the atmosphere at a specific location and time of the year, without considering the crop characteristics and soil factors (Allen et al. 1998). The ET0 in our study was determined by the atmometer placed in the greenhouse, and the data were extracted from the data loggers. The cumulative ET0 during the experiments (92 days from seeding to harvesting) was calculated as 591.53 mm. The uniform temperature in the greenhouse prevented ET0 from being visibly affected by air temperature due to controlled environmental conditions (Fig. 2).
Irrigation application and soil moisture content for the control treatments
Over the course of the growing season, variations in soil moisture content (Fig. 3a) and irrigation regime (Fig. 3b) were observed in the control treatment. The soil moisture content was maintained between the field capacity and readily available moisture content throughout the growth period to prevent excessive or insufficient irrigation from causing stress to the plants (Fig. 3a). Based on the rooting depth of the plants at different growth periods, the amount of irrigation water required for the control treatment at each growth stage was calculated (Fig. 3b).
Hard red spring wheat root development was estimated to be 30 cm depth in the first 30 days after sowing, 6 cm depth between 30 and 60 days, and 90 cm depth after 60 days until harvesting (Thorup-Kristensen et al. 2009). Plant water requirements were calculated, and irrigation was applied at the indicated rooting depths.
Grain yield and growth parameters
The statistical differences between treatment groups for plant height, grain yield, above-ground biomass, 1000-kernel weight, average kernel weight, and number of kernels were significant (P ≤ 0.05). The results proved that the growth parameters are influenced by the depth of the water table (Table 1). The lowest values of above-ground biomass, kernel number, plant height, and grain yield were found in plants from T30 treatment. The plants in the control treatment had the lowest 1000-kernel weights and average kernel weight while T90 treatments were recorded as the highest values. As with growth parameters, grain yield increased with increasing the depth of the water table from 30 to 90 cm. Similar results were obtained by Noshadi and Karimi (2021) who also reported that wheat grain yield increased as the water table depth increased from 60 to 90 cm. Zhang et al. (2000) and Bai et al. (2020) reported that soil moisture in the root zone is linked to plant growth and yield, which explains the findings of this study. When the water table was below 70 cm from the surface, soil aeration in the root zones was reduced, which also reduced the root growth and consequently grain yields (Wesstrom et al. 2001).
The roots biomass distribution in the lysimeter profiles
In both water table depth and control treatments, root biomass was maximum at the top of the lysimeter profile (0.0–40 cm) (Table 2). In the Tcontrol, T30, and T60 treatments, plant root biomass reduced with increasing soil depth, while in T90 it decreased slightly with soil depth, but not significantly at 40–70 cm and 70–100 cm (Fig. 4). While root development is significantly impacted by soil saturation, some of the roots are still able to penetrate the saturated soil profile (although highly constrained) leading to reduced crop performance. Therefore, most of the root biomass is usually concentrated in the zones with lesser soil moisture content. The root distribution results in Kadioglu et al. (2019) (Table 6) and Bai et al. (2020) (Figure 6) are a few literatures to show that plant roots can penetrate saturated soils as indicated by the root biomass values in depths beyond soil saturation depths in both studies. The lowest and highest total average root biomasses were recorded in T30 and T90 water table depths, respectively (Table 2). Similarly, the average root biomass was slightly higher than the control treatment; however, there is no significant difference (P ≤ 0.05) between the two treatments in terms of root biomass values. Fidantemiz et al. (2019) reported similar results with root biomass being the highest at 90 cm depth compared to 30 cm depth for a different crop. These results proved that the crops grown at 30 cm water table depth were affected by waterlogging in the soil profile, resulting in poor root development and low crop yields (Kahlown and Azam 2002; Fidantemiz et al. 2019). This can also be explained by the fact that grain yield and root biomass were lowest at T30 and highest at T90.
Crop water use efficiency
Before planting, the moisture content of the soil (field capacity) was measured as -33 kPa in all the lysimeters. After planting, the water consumption of the plants was monitored and found that ETc values decreased with the increase in water table depths in all water table treatments (no irrigation) (Table 3). The highest and lowest ETc values were recorded in T30 and T90 treatments, respectively. These results showed that hard red spring wheat consumed the highest amount of water from 30 cm water table depth compared to 60 and 90 cm depth. In the control experiments (irrigation experiments), the water consumption was the highest with ETi values of 721 ± 2.16 mm compared to all treatments, which means it was even higher than T90 treatment (Fig. 5a). Statistically significant differences in plant water use (P ≤ 0.05) were observed between T90 and T30, but no significant difference between T30 and Tcontrol treatments was observed. For the water use efficiency, the highest and lowest values were recorded in treatment T30 and T90, respectively (Fig. 5b). Similarly, the control treatment had the lowest water use efficiency. It was concluded that a high-yielding crop of spring wheat can be grown without supplementary irrigation when an optimal water table is provided (Ghamarnia and Farmanifard 2014). Kahlown and Ashraf (2005) found that groundwater contributes the most to plants at low water tables and that as water table depth increases, groundwater contribution decreases. It was also reported by Noshadi and Karimi (2021) that water use efficiency was optimum at 90 cm depth of water table.
Seed quality
The SEM images of wheat kernels from different water table depths and control treatments revealed that the starch granules can be able to categorize as large (A-granules) and small (B-granules) (Fig. 6). The large A-granules starch granules are recognized by the characteristic disk-like shape, while the B-granules possess a spherical shape (Singh et al. 2003), with both granules exhibiting a smooth surface (Magallanes Lopez et al. 2019). The SEM images of the wheat kernels across the treatments showed no noticeable difference, although some damaged starch granules were detected across the treatments. However, none of the observed damage was attributed to the differences in water table depth. Water stress during germination and fungal infections are some of the main factors known to affect starch composition (Gou et al. 2017; Jackowiak et al. 2005). The hard red spring wheat plant was not exposed to either water stress or diseases in this study. This may help explain why there were no noticeable differences in the starch content across treatments based on the SEM images.
Figure 7 shows the SE-HPLC chromatograms for the SDS buffer extractible and unextractable protein fractions of the hard red spring wheat kernels from the water table and control treatments. The chromatograms are divided into four fractions as follows: F1—high molecular weight (MW) polymeric proteins (3.5–4.7 min), F2—low MW polymeric proteins (4.7–5.2 min), F3—gliadin (5.2–5.8 min), and F4—albumin and globulins (5.8–7.4 min). Breadmaking characteristics have been reported to be associated with a molecular weight distribution of wheat proteins (Ohm et al. 2010). Specifically, Ohm et al. (2010) reported that SDS-unextractable fraction 1 (F1) had positive correlations with breadmaking properties, and SDS-extractable F1 had negative correlations among hard red spring wheat genotypes. The absorbance area (AA) represents a quantity of protein fraction based on flour weight, and the area % represents a percent of the protein fraction based on total protein (Fig. 7). Similar to the starch component analysis results, the gluten protein fractions analyses did not show any noteworthy differences across the treatments. Although there were no noticeable differences across the treatments, the wheat kernels from the control treatment were higher F1 for both extractable and unextractable proteins compared to the other treatments. This may have occurred because the control treatment received an adequate and timely water supply throughout the experiment. These results provide the potential for future experiments to study the association between wheat protein fractions and water stress.
Size-exclusion HPLC separation of a extractable and b unextractable protein fractions of hard red spring wheat seeds harvested from plants grown in different treatments. The chromatograms are divided into four fractions as follows: F1—high molecular weight (MW) polymeric proteins (3.5–4.7 min), F2—low MW polymeric proteins (4.7–5.2 min), F3—gliadin (5.2–5.8 min), and F4—albumin and globulins (5.8–7.4 min)
Conclusions
Lysimeters were used in this study to examine the effects of water table depth on yield, seed quality, water use, and root distribution of hard red spring wheat. The results showed that different water table depths produce different growth conditions in the plant. With increasing depth from 30 to 90 cm, plant growth parameters and water use efficiency increase, while crop water use decreases. Root development of the plant in the lysimeters was affected by the water table depth. It was found that plant root biomass was higher in the upper part of the soil profile (0–40 cm depth) in all treatments. The root biomass of plants in T30, T60, and Tcontrol treatments decreased as the soil depth increased, while the root biomass of the plants in T90 slightly decreased across the soil depths, with no substantial reduction at 40–70 and 70–100 cm soil depths. No significant difference (P ≤ 0.05) was observed between the average root biomass of the plants in T90 and Tcontrol treatments, with T90 having the highest total average root biomass values among the treatments.
The assessment of seed quality showed that the depth of the water table did not influence the quality of hard red spring wheat. One possible explanation is that the wheat plants were not subjected to fungal diseases and/or water stress, which are known to adversely affect seed quality. Overall, results from yield and growth parameters showed that 90 cm water table depth is the optimum depth for low water consumption with a high yield and high root development. For future studies, all these studies could be repeated in field conditions with different soil and wheat varieties. Water table depths higher than 90 cm could be applied.
References
AgMRC (2022) Wheat. https://www.agmrc.org/commodities-products/grains-oilseeds/wheat. Accessed 12 Mar 22
Allen R, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration—guidelines for computing crop water requirements. FAO irrigation and drainage paper 56. Food and Agriculture Organization of the United Nations, Rome
Atwell W, Finnie S (2016) Wheat flour, 2nd edn. AACC International, Inc, San Diego
Bai S, Kang Y, Wan S (2020) Winter wheat growth and water use under different drip irrigation regimes in the North China PlainWinter wheat growth and water use under different drip irrigation regimes in the North China Plain. Irrig Sci 38:321–335
Fidantemiz YF, Jia X, Daigh AL, Hatterman-Valenti H, Steele DD, Niaghi AR, Simsek H (2019) Effect of water table depth on soybean water use, growth, and yield parameters. Water 11(5):931
Ghamarnia H, Farmanifard M (2014) Yield production and water-use efficiency of wheat (Triticum aestivum L.) cultivars under shallow groundwater use in semi-arid region. Arch Agron Soil Sci 60:1677–1700
Ghamarnia H, Golamian M, Sepehri S, Arji I, Rezvani V (2012) Groundwater contribution by safflower (Carthamus tinctorius L.) under high salinity, different water table levels, with and without irrigation. J Irrig Drain Eng 138(2):156–165
Guo H, Liu Y, Li X, Yan Z, Xie Y, Xiong H, Zhao L, Gu J, Zhao S, Liu L (2017) Novel mutant alleles of the starch synthesis gene TaSSIVb-D result in the reduction of starch granule number per chloroplast in wheat. BMC Genom 18:358
Gupta RB, Khan K, Macritchie F (1993) Biochemical basis of flour properties in bread wheats. I. Effects of variation in the quantity and size distribution of polymeric protein. J Cereal Sci 18:23–41
Hillel D (1998) Environmental soil physics: fundamentals, applications, and environmental considerations, 1st edn. Elsevier, Amsterdam
Huo Z, Feng S, Huang G, Zheng Y, Wang Y, Guo P (2012) Effect of groundwater level depth and irrigation amount on water fluxes at the groundwater table and water use of wheat. Irrig Drain 61(3):348–356
Jackowiak H, Packa D, Wiwart M, Perkowski J (2005) Scanning electron microscopy of Fusarium damaged kernels of spring wheat. Int J Food Microbiol 98(2):113–123
Kadioglu H, Hatterman-Valenti H, Jia X, Chu X, Aslan H, Simsek H (2019) Groundwater table effects on the yield, growth, and water use of canola (Brassica napus L.) plant. Water 11(8):1730
Kahlown MA, Ashraf M (2005) Effect of shallow groundwater table on crop water requirements and crop yields. Agric Water Manag 76(1):24–35
Kahlown MA, Azam M (2002) Individual and combined effect of waterlogging and salinity on crop yields in the Indus basin. Irrig Drain J Int Comm Irrig Drain 51(4):329–338
Kiremit MS, Arslan H, Sezer İ, Akay H (2022) Evaluating and modeling of the seedling growth ability of wheat seeds as affected by shallow-saline groundwater conditions. Gesunde Pflanzen 74(2):357–369
Larroque OR, Bekes F (2000) Rapid size-exclusion chromatography analysis of molecular size distribution for wheat endosperm protein. Cereal Chem 77:451–453
Larroque OR, Gianibelli MC, Gomez SM, MacRitchie F (2000) Procedure for obtaining stable protein extract of cereal flour and whole mean for size-exclusion HPLC analysis. Cereal Chem 77:448–450
Liu T, Luo Y (2011) Effects of shallow water tables on the water use and yield of winter wheat (Triticum aestivum L.) under rain-fed condition. Aust J Crop Sci 5:1692–1697
Luo Y, Sophocleous M (2010) Seasonal groundwater contribution to crop-water use assessed with lysimeter observations and model simulations. J Hydrol 389(3–4):325–335
Magallanes López AM, Manthey FA, Simsek S (2019) Wet milling technique applied to deoxynivalenol-contaminated wheat dry-milled fractions. Cereal Chem 96:487–496
Majumdar DK (2001) Irrigation water management: principles and practice. PHI Learning Pvt. Ltd
Mejia MN, Madramootoo CA, Broughton RS (2000) Influence of water table management on corn and soybean yields. Agric Water Manag 46(1):73–89
Nosetto MD, Jobbagy EG, Jackson RB, Sznaider GA (2009) Reciprocal influence of crops and shallow ground water in sandy landscapes of the inland pampas. Field Crop Res 113:138–148
Noshadi M, Karimi S (2021) The influence of groundwater depth and nitrogen levels on wheat yield, water productivity and nitrogen losses. Agric Res. https://doi.org/10.1007/s40003-021-00603-6
Ohm JB, Hareland G, Simsek S, Seabourn B (2009) Size-exclusion HPLC of protein using a narrow-bore column for evaluation of breadmaking quality of hard spring wheat flours. Cereal Chem 86(4):463–469
Ohm JB, Hareland G, Simsek S, Seabourn B, Maghirang E, Dowell F (2010) Molecular weight distribution of proteins in hard red spring wheat: Relationship to quality parameters and intrasample uniformity. Cereal Chem 87:553–560
Ritchie JT (1998) Soil water balance and plant water stress. In: Understanding options for agricultural production. Springer, pp 41–54
Roy D, Jia X, Steele DD, Lin D (2018) Development and comparison of soil water release curves for three soils in the red river valley. Soil Sci Soci Am J 82:568–577
Sinclair TR, Tanner C, Bennett J (1984) Water-use efficiency in crop production. Bioscience 34(1):36–40
Singh N, Singh J, Kaur L, Sodhi NS, Gill BS (2003) Morphological, thermal, and rheological properties of starches from different botanical sources. Food Chem 81:219–231
Stromberg JCJJOAE (2001) Restoration of riparian vegetation in the south-western United States: importance of flow regimes and fluvial dynamism. J Arid Environ 49:17–34
Thorup-Kristensen K, Salmerón Cortasa M, Loges R (2009) Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 322(1):101–114
Wesstrom I, Messing I (2007) Effects of controlled drainage on N and P losses and N dynamics in a loamy sand with spring crops. Agric Water Manag 87:229–240
Wesstrom I, Messing I, LinneAr H, LindstroEm J (2001) Controlled drainage effects on drain outflow and water quality. Agric Water Manage 47:85–100
Yang J, Wan S, Deng W, Zhang G (2007) Water fluxes at a fluctuating water table and groundwater contributions to wheat water use in the lower Yellow River flood plain, China. Hydrol Process Int J 21(6):717–724
Zhang XY, Pei D, You MZ (2000) Response of leaf water potential, photosynthesis, and stomatal conductance to varying soil moisture in four crops: winter wheat, corn, sorghum, and millet. China J Plant Ecol 24:280–280
Acknowledgements
The funding for this research was provided by the ND Wheat Commission and ND Water Resources Research Institute. The authors gratefully acknowledge all these supports. Any opinions, findings, and conclusions explained in this study are those of the authors and do not necessarily reflect the views of the funding agencies.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this research.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Odili, F., Bhushan, S., Hatterman-Valenti, H. et al. Water table depth effect on growth and yield parameters of hard red spring wheat (Triticum aestivum L.): a lysimeter study. Appl Water Sci 13, 65 (2023). https://doi.org/10.1007/s13201-023-01868-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01868-8