Abstract
Analytical isotherm models such as Langmuir isotherm, Freundlich isotherm, and other linear isotherms are commonly used for modeling adsorption datasets for a wide range of adsorption studies. Most of these studies consider pH to be fixed. However, pH is an important parameter that varies widely. Hence, the model parameters developed for one set of experiments cannot be used in another scenario where the pH is different. Surface complexation models that can simulate pH changes are complex, multi-parameter models that are difficult to use. The modified Langmuir–Freundlich (MLF) isotherm developed earlier by us could simulate pH-dependent adsorption on goethite-coated sands. However, it has only been tested for arsenic adsorption on goethite-coated sands. Therefore, chromium adsorption datasets were considered to extend this MLF isotherm for other metal ions. Two different adsorbents, viz. coconut root activated carbon (CoAC) and palm male flower activated carbon (PaAC), were selected for the adsorption modeling of Cr(VI) using the MLF isotherm model. An improved modeling strategy was developed for fitting the MLF isotherm, which required only a single pH versus adsorption dataset, instead of several isotherms at different pH values. The new methodology could simulate the pH-dependent adsorption satisfactorily for various experimental datasets. The maximum adsorption capacity was 88.64 (mg/g) and 100.1 (mg/g) for PaAC and CoAC, respectively. The affinity constant for this model (Ka) was found to be 0.007 (L/mg) for PaAC dataset and 0.0106(L/mg) and 0.004 (L/mg) for the CoAC dataset. The average R2 values of fitting were calculated and found to be 0.98 for PaAC and 0.85 for CoAC. The average root mean square error (RSME) of the fitting of the model was 0.07 (less than 10%). This modeling strategy required less experimental data and did not require advanced characterization studies. Therefore, this study indicates that the MLF isotherm can be extended to other contaminants and for different adsorbents to model the pH-dependent adsorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium is a toxic heavy metal (Tumolo et al. 2020) naturally present in water and soil. Anthropogenic activities such as tanning, textile industries, and the production of various pigments used in anti-corrosion processing increase chromium contamination in natural resources (Vareda et al. 2019). Between the two forms that chromium exists, viz. trivalent Cr (III) and hexavalent Cr(VI), the latter poses higher toxicity to human beings as opposed to the former (Avudainayagam et al. 2003). It is because Cr(VI) can overcome the cellular barrier and perform degenerative activities and is thus recognized as a carcinogen and mutagen (Wang et al. 2017). Chromium can exhibit high surface mobility amongst a broad range of pH values making its treatment challenging (Violante et al. 2010). While many methods exist to remove heavy metals, the challenge arises when the contaminants are present in trace levels. Therefore, adsorption can be the most viable method to remove these substances (Satapathy and Natarajan 2006).
Studies using activated carbon to remove metal ions such as Cr, Cu, and Pb are gaining traction due to their cost-effectiveness and sustainability. Several studies that have been conducted to study the removal of Cr(VI) commonly used Langmuir isotherm models. A study detailing chromium adsorption combined with the medical drug levofloxacin using vanadium pentoxide showed a Langmuir isotherm trend (Mahmoud et al. 2021). Similarly, a novel supramolecular network of graphene dots enhanced the removal of hexavalent chromium along with malachite green dye (Mahmoud et al. 2021). Modeling of adsorption was performed only at a single pH value of 3 and showed the best fit for Langmuir model isotherm, suggesting a monolayer adsorption. A simple google scholar search with the keyword combination of “Hexavalent Chromium”, “activated carbon”, and “adsorption” placed between the years 2017 and 2021 will render around 16,000 research articles that were published. It is an indication that adsorption studies trends are abounding and relevant. A 2019 study (Islam et al. 2019) detailed different types of adsorbents commonly used to remove Cr(VI), comparing activated carbon obtained from organically producing charcoal, clay materials, metal oxides, and zeolites. The study comparing different operating conditions, isotherm models, and kinetic studies shows that no studies effectively correlate pH as a function of adsorption. Most research on adsorption studies on inorganic contaminants such as chromium, arsenic, or lead, fit the adsorption data into well-established models such as the Freundlich or the Langmuir isotherm model. A common observation among these studies is that although pH-dependent studies are carried out, pH-dependent adsorption models are not available. It means that adsorption data are fitted at a certain fixed pH using empirical models rather than integrating the pH and the adsorption values at several pH conditions. Studying adsorption at different pH values is imperative because pH is crucial in determining the adsorption rate and extent.
Surface complexation models (SCM) can be used to model the adsorption mechanism. The most common adsorption mechanisms are the outer sphere complexation mechanism and the inner sphere complexation mechanism. An internal surface bond indicates a more potent albeit slower attachment mechanism due to covalent or ionic bonds. The outer sphere bonds occur faster but are weaker and generally can be reversed. In this type of mechanism, a surface functional group is involved that holds the adsorbing species in weaker forces such as columbic effect, Van Der Waal's force, or hydrogen bonds. (Goldberg 1992).
The SCM model is based on defining the equilibrium constant equations for each surface complex that have a well-defined mass balance equation (Bompoti et al. 2016; Hiemstra and Riemsdijk 1996).
The fate of heavy metal transport heavily depends upon adsorption capacity of the adsorbent. A previously performed study that modeled the chromate adsorption using the SCM model elucidated the intrinsic spatial modeling requirements, which relied heavily on rigorous theoretical and experimental work (Bompoti et al. 2016). Modeling these datasets requires several characterization methods, mass balance equations and equilibrium studies to understand the charge distribution, ion changes, and surface species which tends to be cumbersome and resource extensive (Hiemstra and Riemsdijk 1996).
Jeppu et al. (2010) modified the existing Langmuir Freundlich isotherm model so that pH variations could be modeled. It was abbreviated as the modified Langmuir–Freundlich (MLF) isotherm model. The MLF isotherm equation in Jeppu and Clement (2012) showed that the MLF isotherm model was used to predict adsorption dataset on arsenate on pure goethite and goethite-coated sand, the equation for which is given in Eq. (1)
Qm is the maximum adsorption capacity of the system (mg sorbate/g sorbent), Ce is the aqueous phase concentration at equilibrium (mg/ L), and Ka is the affinity constant for adsorption (L/mg). Furthermore, n is the index of heterogeneity. By applying model Eq. (1) in the previous study, Ka associated with the pH of the solution had given a linear equation as expressed in Eq. (2)
Therefore, a relationship between the affinity constant given as a Ka and pH was produced. This study also compared predictions based on surface complexation models (SCM) and the analytical modeling isotherm. The SCM assumes that a single site density value represents maximum adsorption capacity between the adsorption systems. In contrast, other studies have shown that this is not the case (Anderson et al. 1976; Ghosh and Yuan 1987; Wankasi 2010), where different adsorption capacity values were obtained for different pH conditions. So far, there have been limited analytical adsorption models that can capture the pH-dependent adsorption of heavy metal ions similar to the SCM model frameworks.
A summary of adsorption isotherm modeling parameters for Cr(VI) adsorption using various adsorbents is listed in Table 1.
As gathered from Table 1, it is observed that adsorption studies rely heavily on single pH-based models such as Freundlich and Langmuir isotherms, even though the experimental studies conducted are based on varying pH conditions. For modeling these datasets, a single pH value at a constant temperature is chosen, thereby failing to accurately provide the pH data studies for different pH values. The MLF isotherm equation given in Ghosh and Yuan (1987) was developed to remedy this gap, where a pH edge was constructed using the MLF isotherm model to make predictions.
Activated carbon (AC) is the most widely used adsorbent as it is a low-cost adsorbent that can be easily obtained (Jjagwe et al. 2021). However, none of the studies has focused on pH-dependent adsorption of Cr(VI) on AC. A modified MLF model fitting procedure was developed by fitting readily available pH versus adsorption datasets instead of adsorption isotherms at different pH. Cr(VI) adsorption on activated carbon derived from Coconut root plant and palm male flower activated carbon was chosen for this study. The study's objective was to apply the MLF isotherm to chromium adsorption datasets for chromium adsorption derived from these two separate activated carbon sources.
Modeling methodology
Langmuir and Freundlich isotherm models are the most commonly used isotherms for adsorption studies. The Freundlich isotherm model is an empirical model which relies heavily on theoretical data that describe the extent of adsorption directly with concentration or pressure. (Jamshidi et al. 2015). It accounts for multilayer adsorptions of a heterogeneous system that assumes that different binding sites have various adsorption affinity associated with it. On the other hand, the Langmuir isotherm model assumes that maximum adsorption occurs when a saturated monolayer of adsorbate molecules is present on the adsorbent surface, such that the adsorption energy is constant. Also, there is no migration of molecules in the surface plane or interaction between the adsorbate (Al-Ghouti and Da’ana, 2020). The MLF isotherm model is an extension of the Freundlich isotherm model and Langmuir isotherm model. The limitations of the former two isotherm models are overcome by introducing an extra parameter which provides greater accuracy in predicting the adsorption results.
The modified Langmuir–Freundlich isotherm equation is represented by Eq. (1)
Since a relationship between Ka and pH was established as demonstrated in Eq. (2), Eq. (1) can be rewritten as below
such that
Qe model is the adsorption predicted by the model (mg sorbate/g sorbent), Qm is the maximum adsorption capacity of the system (mg sorbate/ g sorbent), Ce is the aqueous phase concentration at equilibrium (mg/L), and Ka is the affinity constant for adsorption (L/mg). Additionally, there is another parameter called 'n,' which is the index of heterogeneity. The Langmuir–Freundlich isotherm was used as the base model to describe the obtained chromium adsorption data. This method of fitting the isotherm will be addressed as Method (I) for brevity. The density function for heterogeneous systems uses a heterogeneity index 'n,' which varies in the MLF isotherm, typically between 0 and 1. This is because the value of n for a homogeneous material is 1, and n for heterogeneous materials is less than 1 (Turiel et al. 2003). In the above equation, the affinity constant (Ka) value can be varied to account for pH-dependent effects. In Method (I), pH edge experiments are conducted, following which the isotherm model is applied. The modeling methodology is summarized in Fig. 1. The previous study in which the MLF model was developed revealed a linear fit between adsorption constant and pH. The analysis was performed on arsenic using pure goethite and sand-coated goethite (Jeppu and Clement 2012). However, this MLF isotherm has not been further extended to other adsorbents and adsorbate systems.
Flow chart diagram of the modeling methodology (Method I) of MLF isotherm fitting, Jeppu and Clement (2012)
MLF isotherm constants are fitted as given in Eq. (1) using MS Excel solver, and the values of Ka and n are noted for each pH values. In the previous MLF isotherm fitting Method (I), Jeppu et al. (2010) used several isotherms available at five different pH values. In the new MLF isotherm fitting Method (II) in Fig. 2, a single pH Vs Qe curve was used instead of five pH Vs adsorption curves. It requires less time and is more accessible to construct the MLF model.
The R2 values used in this method are calculated below
SSE is the sum square of the errorsSST is the sum square of the total
The RSME is calculated using the formula given in Eq. (5).
J = number of data points.
Two datasets were considered for chromium adsorption on activated carbon (AC) in this modeling study. One dataset was for activated carbon from the male flower of the Palm tree, PaAC, for Cr(VI) removal, and another set of data that used AC derived from the roots of coconut root (CoAC), both of which were carried out by our team earlier (Prabhu et al. 2016) and (Corda 2019).
Experimental methodology
Case study 1: palm male flower activated carbon (PaAC) as adsorbent for chromium (VI)
Experimental results for palm male flower activated carbon (PaAC) (Dataset I)
In our previous study (Corda 2019), agricultural waste from the male flower of the toddy palm was upcycled to make an activated carbon adsorbent. The PaAC was activated using orthophosphoric acid and a series of characterization techniques were performed. A BET analysis determined the pore volume as 0.3601 (cc/gm) with a surface area of 761.93 (Sq.m/gm). Particle size distribution was also conducted and found to have a mean diameter of 47.88 µm. The point zero charge, pHZPC, was achieved at pH 5.6. The detailed characterization can be found in the supplementary section, table S.2 The adsorption mechanism was concluded to follow pseudo-second-order kinetics, with Langmuir isotherm giving the best fit. The adsorption of Cr(VI) was investigated under different experimental conditions such as varying initial concentration C0, pH of the solution, the dosage of the adsorbent S, temperature, and agitation speed. These data were taken to model the adsorption using the MLF model fitting Method (II) as detailed in Fig. 2.
The experiments were conducted with 100 mL of Cr(VI) solution; the initial concentration, C0 of Cr (VI), was 100 mg/L. The adsorbent dosage, S, was maintained at 0.25 g/mL. The pH vs Qe (chromium in adsorbed phase) is plotted in Fig. 4, and Qe was calculated based on Eq. (6).
where Qe is the amount of chromium adsorbed per unit weight expressed in mg/g, V is the volume of solution treated in liters, C0 is the initial concentration of chromium in mg/L, Ce is the equilibrium concentration mg/L, and S is the mass of the adsorbent dosed in g/L. The data from the experiments are shown in Fig. 3 (closed triangles).
Model development for chromium adsorption on PaAC
Since isotherms at different pH values were not available that were required for the MLF isotherm Method (I), the available pH vs adsorption data (Fig. 3) were used to fit the MLF isotherm parameters as described in the MLF isotherm fitting Method (II), shown in Fig. 2.
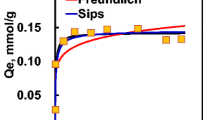
The MLF Eq. (1) uses parameters such as Ka, n, and Qe. The value of Qe was calculated from Eq. (6), and the values of Ka and n were calculated using Microsoft Excel solver by varying the values of Ka and minimizing the sum of squared errors (SSE) between the modeled Qe model and the actual Qe, which was available from the experiment. The value of Qm was taken from the maximum value of experimental data, which was 88.64 mg/g (Corda 2019). The solver-derived values of Ka, and n, and experimental Qm are tabulated in Table 2. As shown in Fig. 4, pH Vs the adsorption dataset is presented to construct the new modified MLF model. The triangles indicate experimental data, and the line indicates the fitted Ka values, achieved using the excel solver. When plotted Vs pH, the Ka values showed a power law type of relationship (Fig. 5).
The relationship can be represented as
The values of Ka from Table 2 are plotted in Fig. 4.
Following this, a validation of the model fit was done using the model to predict the adsorption isotherm data at pH 2. The model prediction and the experimental data are given in Fig. 6.
Validation study of MLF model for PaAC dataset for chromium adsorption
The MLF isotherm constructed above was validated using independent datasets from the same experimental report. In Fig. 5, Ka values fitted using the MLF model (Method (II)), were used for predicting the adsorption at pH 2 for an experimental adsorption isotherm for the same PaAC and Cr(VI) system.
Substituting the equation derived from the model fit equation, we can deduce that
We can say that the affinity constant is a function of pH of the solution. Therefore,
Substituting Eq. (7) into Eq. (3), we derive a relationship that is expressed in Eq. (9), such that
Using this equation, we derived a set of predictions for the adsorption systems to validate our model.
The isotherm predicts the adsorption very well with a R2 value of 0.95. This R2 value indicates that the fit is good.
Applying Eq. (5) to Fig. 6, the RSME calculated was 0.07, and using the Freundlich Langmuir values shown in Table 2, the Qmax was 88.64. This RSME value showed a very close fit between the experimental values and modeled Qe model.
Case study 2: coconut roots activated carbon (CoAC) as adsorbent for Cr(VI))
Experimental dataset for CoAC (Dataset II)
The second dataset for applying the MLF isotherm model was taken from our previous study for adsorption of Cr(VI) on coconut roots activated carbon (CoAC) (Prabhu et al. 2016). The roots of the coconut palm were activated using sulfuric acid, and a set of characterization techniques were carried out. The surface area was found to be 2.81 (m2/g), and the particle’s mean diameter was 27.9 µm. The pHPZC was found to be at 3.67. The details of the characterization of this experiment can be found in the supplementary section, table S.3 The adsorption mechanism can be summarized as exothermic and follows a pseudo-first-order kinetic model. In this paper, pH-based adsorption studies were carried out. The isotherm modeling that was used was Freundlich, Langmuir, Toth, Redlich–Peterson, and Dubinin–Radushkevich isotherms. However, no isotherm correlating the pH-based dataset to the adsorption dataset was performed. Therefore, the data were taken to model the adsorption using the MLF model fitting Method (II) that is detailed in Fig. 2. In this study, data for the effect of pH on chromium adsorption were generated as shown in Fig. 7. Therefore, the MLF model as discussed can be applied to this dataset to model the adsorption with pH variation.
Model development for chromium adsorption on CoAC
Since isotherms at different pH were not available as required for the MLF isotherm Method (I) (Jeppu and Clement 2012), the available pH vs adsorption data (Fig. 7) were used to fit the MLF isotherm parameters as in the MLF isotherm fitting Method (II) shown in Fig. 2. The MLF Eq. (1) uses parameters such as Ka, n, and Qe. The value of Qe was calculated from Eq. (6), and the values of Ka and n were calculated using Microsoft Excel solver by varying the values of Ka and minimizing the sum of squared errors (SSE) between the modeled Qe model and the actual Qe, which was derived from the experiment. The value of Qm was taken from the maximum value of experimental data (Prabhu et al. 2016) which was reported to be 100.1 mg/g.
The values of Ka, n derived from the solver and Qm as reported are shown in Table 3.
As shown in Fig. 8, pH Vs the adsorption dataset is presented to construct our MLF model. The triangles indicate experimental data, and the line indicates the fitted Ka values obtained using the excel solver. The Ka, n values are shown in Table 3. The Ka values, when plotted Vs pH, showed a power law type of relationship as shown in Fig. 9.
The initial concentration, C0, Qm, and adsorbent dosage S were available from the experimental data published in the study (Prabhu et al. 2016). Equation (3) is applied to the collected data for each pH to find the respective Qe value. A modified method of fitting of MLF isotherm was used.
Hence, the relationship between Ka and pH was confirmed as expressed in Eq. (2).
The Ka Vs. pH followed a power trend expressed as a function of pH, given in Eq. (10). It follows a similar pattern as Eq. (8), which is depicted in Fig. 9
The model equation was then validated using the model to predict the adsorption isotherm data at pH 2, which is detailed in the next section. The model prediction and the experimental data are described in the following section.
Validation of MLF model for CoAC dataset
To further validate our model, another set of data collected from our previously published study (Prabhu et al. 2016) was considered. The solution's initial concentration, consisting of the adsorbent C0, varied, while the pH remained fixed. A similar approach was applied as described in Fig. 2, where steps 1 to 3 were followed. Figure 9 shows the validation of modeled MLF isotherm for predicting the dataset without additional parameters.
From the previous section, the power relationship between Ka and pH was derived and substituted in Eq. (3) to render us with a new Eq. (11). Similarly, a power law relation was obtained with CoAC dataset that when applied to Eq. (3) gave us the following equation which was then used to make predictions to validate our model.
The dataset for varying initial concentration C0, between 10 and 100 ppm for a fixed pH 5, was modeled by obtaining Qe from the data and using MLF Eq. (11) to obtain the modeled Qe. As it is seen in Figs. 9 and 10, the data and the model trends are similar. However, a slight deviation exists between predicted and experimental data, as shown in Fig. 10. This is because the MLF model fitting Method (II) uses less data than MLF model fitting method(I) and therefore has a lesser quality of predictions.
The R2 values of the fitting were 0.7 and 0.76, respectively, for Figs. 10 and 11. The RSME was calculated for the results obtained from the MLF isotherm model and was found to be 0.001 and 0.0174 for Figs. 10 and 11, respectively, indicating a satisfactory fit. The calculated and average RSME values for both PaAC and CoAC are presented in Table 4.
Discussions
The modified MLF isotherm was applied to two datasets to show the isotherm modeling for Cr(VI). The RSME and R2 were calculated to check for the goodness of fit, as reported in Table 4. The parameter estimation was robust by giving us converging values while applying the excel solver feature for the experimental data. It was observed that at lower pH, a power-law relationship was attainable, while at higher pH values, a linear Ka vs pH relationship can be observed (Table 5).
Cr (VI) adsorption increases when pH decreases in the above-observed data. For the CoAC data derived from the literature, the maximum adsorption occurred at pH 2 and decreased as the pH increased. Similarly, with the experimental data for Cr(VI) adsorption using AC, the maximum adsorption was achieved at pH 2. The rate of adsorption decreased with an increase in the pH of the solution, which could be attributed to the fact that the surface of the adsorbent becomes positively charged as the pH decreases. Chromium exists as chromate (CrO42−), dichromate (Cr2O72−), and or hydrogen chromate (HCrO4−). At lower pH, HCrO4− is the most predominant speciate. As the pH levels increase, CrO42− and Cr2O72− become the more dominant ions. This can be attributed to the fact that there is a decrease in the electrostatic force of attraction between sorbent and sorbate ions with an increase in pH. The decline in adsorption beyond pH 4 or 5 is due to the less positively charged surface of the adsorbent (Verma et al. 2006).
It can also be observed that to get a more accurate affinity constant (Ka) that relates to the adsorption affinity, more data are required rather than just the pH edge. When a limited set of data is available, the modified procedures shown in Method (II) can be applied as it requires a single pH vs adsorption data, as shown in this paper. This is more readily available in most cases. The Method (I) previously developed requires more adsorption isotherms at multiple pH values, which may be difficult to obtain or not readily available in some cases. The trendline of the curve can predict the linearity or non-linearity of the dataset, showing us how the pH affects adsorption. From this, predictions of adsorption ability and how it occurs can be predicted.
From the literature, it is already known that [H+] directly correlates to the acidic or basic nature. The relationship is described in Eq. (12)
From this equation, it is apparent that with the increase in pH, as shown in Fig. 12, the available [H+] ions decrease logarithmically. Thus, this makes adsorption negligible as described; when the dominant anion speciates of chromium become less, the maximum adsorption capacity also decreases. The adsorption ability can be defined by the affinity constant, Ka. A plot of Ka vs. H+ ions demonstrates how the Ka value associated with those changes or increases with a solution's pH increase. This could be the reason for getting a mathematical relationship between pH and Ka as given in Eq. (7). In Fig. 12, for the PaAC and CoAC data, the H+ ions from the range of 1 to 10 were plotted, i.e., from 10−2 to 10−9.
Conclusions
An improved fitting procedure was used to fit the MLF isotherm to the adsorption datasets for chromium(VI) adsorption on activated carbon obtained from two different sources (Corda 2019) and (Prabhu et al. 2016). A new modified (Method (II)) was developed for fitting the MLF isotherm, which required only a single pH Vs. adsorption dataset instead of several isotherms at different pH (Method (I)). The previously developed method predicted a relationship between the pH and affinity constant in a linear trend. In contrast, the newly developed MLF model predicted the adsorption conditions in a power trend format. This could be because the experiments were conducted in lower pH conditions, giving a power trend line. The new method mentioned in this paper requires a single isotherm plot and therefore saves time and effort for modeling pH-dependent adsorption. Additionally, the MLF model that has been presented in this paper can predict adsorption data with minimal tools and less effort and uses simple software such as MS Excel. However, it does pose a set of limitations; for example, it cannot compute multiple ion adsorption modeling for a given system. It cannot account for surface charge changes that occur due to the change in ionic strengths of a solution. For a given experimental dataset, Qm cannot be transferable and must be individually assessed for each experiment. There may also be scope for applying this modeling to various other heavy metal ions.
The constructed MLF isotherm was validated by predicting three independent datasets from the same experiments. The MLF isotherm was able to fit the independent datasets satisfactorily. The MLF isotherm successfully simulated chromium adsorption at different pHs with an average RSME value of 0.07, i.e., less than 0.1 (< 10% error). The R2 was calculated for both datasets, and the average R2 value was 0.89, suggesting a good fit. This indicates that the MLF model fitting Method (II) can be used for fitting adsorption data, when limited data are available and when very high accuracy is not desired. When high accuracy of fit is required, MLF model fitting Method (I) (which needs more input experimental data) can be used. The overall study also indicates that the MLF isotherm can be extended to other contaminants and for different adsorbents to model the pH-dependent adsorption that may function with limited pH values.
References
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383. https://doi.org/10.1016/j.jhazmat.2020.122383
Anderson MA, Ferguson JF, Gavis J (1976) Arsenate adsorption on amorphous aluminum hydroxide. J Colloid Interface Sci 54(3):391–399. https://doi.org/10.1016/0021-9797(76)90318-0
Attia AA, Khedr SA, Elkholy SA (2010) Adsorption of chromium ion (VI) by acid activated carbon. Braz J Chem Eng 27(1):183–193. https://doi.org/10.1590/S0104-66322010000100016
Avudainayagam S, Megharaj M, Owens G, and Kookana RS (2003) Chemistry of chromium in soils with emphasis on tannery waste sites chemistry of chromium in soils with emphasis on tannery waste sites
Bompoti N, Chrysochoou M, Machesky M (2016) Advances in surface complexation modeling for chromium adsorption on iron oxide. Geo-Chicago. https://doi.org/10.1061/9780784480168.001
Corda NC (2019) Removal of heavy metals using Borassus flabellifer : equilibrium, kinetics and statistical studies submitted by Department of Chemical Engineering
Dula T, Siraj K, Kitte SA (2014) Adsorption of hexavalent chromium from aqueous solution using chemically activated carbon prepared from locally available waste of bamboo (Oxytenanthera abyssinica ). ISRN Environ Chem 2014:1–9. https://doi.org/10.1155/2014/438245
Ghosh MM, Yuan JR (1987) Adsorption of inorganic arsenic and organoarsenicals on hydrous oxides. Environ Prog 6(3):150–157. https://doi.org/10.1002/ep.670060325
Goldberg S (1992) Use of surface complexation models in soil chemical systems. Adv Agron 47(C):233–329. https://doi.org/10.1016/S0065-2113(08)60492-7
Hiemstra T, van Riemsdijk WH (1996) A surface structural approach to ion adsorption the charge distribution (CD) model. J Colloid Interface Sci 179:488–508
Hlungwane L, Viljoen EL, Pakade VE (2018) Macadamia nutshells-derived activated carbon and attapulgite clay combination for synergistic removal of Cr(VI) and Cr(III). Adsorpt Sci Technol 36(1–2):713–731. https://doi.org/10.1177/0263617417719552
Islam MA, Angove MJ, Morton DW (2019) Recent innovative research on chromium (VI) adsorption mechanism. Environ Nanotechnol Monit Manag 12:100267. https://doi.org/10.1016/j.enmm.2019.100267
Jamshidi M, Ghaedi M, Dashtian K, Hajati S, Bazrafshan A (2015) Ultrasound-assisted removal of Al 3+ ions and Alizarin red S by activated carbon engrafted with Ag nanoparticles: central composite design and genetic algorithm optimization. RSC Adv 5(73):59522–59532. https://doi.org/10.1039/c5ra10981g
Jeppu GP, Clement TP (2012) A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J Contam Hydrol 129–130:46–53. https://doi.org/10.1016/j.jconhyd.2011.12.001
Jeppu GP, Clement TP, Barnett MO, Lee K-K (2010) A scalable surface complexation modeling framework for predicting arsenate adsorption on goethite-coated sands. Environ Eng Sci 27(2):147–158. https://doi.org/10.1089/ees.2009.0045
Jjagwe J, Olupot PW, Menya E, Kalibbala HM (2021) Synthesis and application of granular activated carbon from biomass waste materials for water treatment: a review. J Bioresour Bioprod 6(4):292–322. https://doi.org/10.1016/j.jobab.2021.03.003
Kan C-C, Ibe AH, Rivera KKP, Arazo RO, de Luna MDG (2017) Hexavalent chromium removal from aqueous solution by adsorbents synthesized from groundwater treatment residuals. Sustain Environ Res 27(4):163–171. https://doi.org/10.1016/j.serj.2017.04.001
Kobya M (2004) Adsorption, kinetic and equilibrium studies of Cr(VI) by hazelnut shell activated carbon. Adsorpt Sci Technol 22(1):51–64. https://doi.org/10.1260/026361704323150999
Labied R, Benturki O, Eddine Hamitouche AY, Donnot A (2018) Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): kinetic, equilibrium, and thermodynamic study. Adsorpt Sci Technol 36(3–4):1066–1099. https://doi.org/10.1177/0263617417750739
Liu Z, Chen G, Xu L, Hu F, Duan X (2019) Removal of Cr(VI) from wastewater by a novel adsorbent of magnetic goethite: adsorption performance and adsorbent characterisation. ChemistrySelect 4(47):13817–13827. https://doi.org/10.1002/slct.201904125
Liu H, Zhang F, Peng Z (2019) Adsorption mechanism of Cr(VI) onto GO/PAMAMs composites. Sci Rep 9(1):1–12. https://doi.org/10.1038/s41598-019-40344-9
Mahmoud ME, Mohamed AK, Salam MA (2021) Self-decoration of N-doped graphene oxide 3-D hydrogel onto magnetic shrimp shell biochar for enhanced removal of hexavalent chromium. J Hazard Mater 408:124951. https://doi.org/10.1016/j.jhazmat.2020.124951
Mahmoud ME, Fekry NA, Abdelfattah AM (2021) Novel supramolecular network of graphene quantum dots-vitamin B9-iron (III)-tannic acid complex for removal of chromium (VI) and malachite green. J Mol Liq 341:117312. https://doi.org/10.1016/j.molliq.2021.117312
Mahmoud ME, Amira MF, Azab MMHM, Abdelfattah AM (2021) Effective removal of levofloxacin drug and Cr(VI) from water by a composed nanobiosorbent of vanadium pentoxide@chitosan@MOFs. Int J Biol Macromol 188:879–891. https://doi.org/10.1016/j.ijbiomac.2021.08.092
Prabhu KB, Kini MS, Sarovar A (2016) Equilibrium, kinetic, and thermodynamic studies on the removal of chromium using activated carbon prepared from Cocos nucifera roots as an adsorbent Int J Appl Environ Sci 1–25 ISSN 0973,http://eprints.manipal.edu/id/eprint/146653
Satapathy D, Natarajan GS (2006) Potassium bromate modification of the granular activated carbon and its effect on nickel adsorption. Adsorption 12(2):147–154. https://doi.org/10.1007/s10450-006-0376-0
Sharma YC, Srivastava V, Weng CH, Upadhyay SN (2009) Removal of Cr(VI) from wastewater by adsorption on iron nanoparticles. Can J Chem Eng 87(6):921–929. https://doi.org/10.1002/cjce.20230
Tumolo M et al (2020) Chromium pollution in European water, sources, health risk, and remediation strategies: an overview. Int J Environ Res Public Health 17(15):5438. https://doi.org/10.3390/ijerph17155438
Turiel E, Perez-Conde C, Martin-Esteban (2003) A Assessment of the cross-reactivity and binding sites characterisation of a propazine-imprinted polymer using the Langmuir-Freundlich isotherm. Analyst 128(2):137–141. https://doi.org/10.1039/b210712k
Vareda JP, Valente AJM, Durães L (2019) Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review. J Environ Manag 246(May):101–118. https://doi.org/10.1016/j.jenvman.2019.05.126
Verma A, Chakraborty S, Basu JK (2006) Adsorption study of hexavalent chromium using tamarind hull-based adsorbents. Sep Purif Technol 50(3):336–341. https://doi.org/10.1016/j.seppur.2005.12.007
Violante A, Cozzolino V, Perelomov L, Caporale A, Pigna M (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr 10(3):268–292. https://doi.org/10.4067/S0718-95162010000100005
Wang Y, Su H, Gu Y, Song X, Zhao J (2017) Carcinogenicity of chromium and chemoprevention: a brief update. OncoTargets Therapy 10:4065–4079. https://doi.org/10.2147/OTT.S139262
Wankasi D (2010) Studies on the effect pH on the sorption of Pb(II) and Cu(II) ions from aqueous media by nipa palm (Nypa fruticans Wurmb). J Appl Sci Environ Manag. https://doi.org/10.4314/jasem.v12i4.55240
Yadav S, Srivastava V, Banerjee S, Weng C-H, Sharma YC (2013) Adsorption characteristics of modified sand for the removal of hexavalent chromium ions from aqueous solutions: kinetic, thermodynamic and equilibrium studies. CATENA 100:120–127. https://doi.org/10.1016/j.catena.2012.08.002
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira, S.K., Kini, S., Prabhu, B. et al. A simplified modeling procedure for adsorption at varying pH conditions using the modified Langmuir–Freundlich isotherm. Appl Water Sci 13, 29 (2023). https://doi.org/10.1007/s13201-022-01800-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01800-6