Abstract
The concentrations of heavy metals and health risks associated with the consumption of domestic water sources around a dumpsite in Enugu (southeastern Nigeria) were assessed by atomic absorption spectrophotometry and multivariate analysis. The results showed the dumpsite leachate mean pH value of 4.1, Mn+ of 11.4 µg/l, Fe3+ 4.1 µg/l, Cd+ 0.3, As+ 6.03 µg/l, Pb+ 2.47 µg/l, Ni+ 14.13 µg/l, and Cr3+ 1.48. The groundwater and surface water samples had mean heavy metal values as follows: Mn+ 5.23 and 0.75 µg/l, Fe3+ 1.49 and 0.15 µg/l, Cd+ 0.08 and 0.10 µg/l, As+ 1.90 and 0.15 µg/l, Pb+ 1.01 and 0.35 µg/l, Ni+ 0.37 and 0.05 µg/l, and Cr3+ 0.03 and 0.05 µg/l, respectively. Comparison with maximum permissible limits indicated that the mean heavy metal concentrations of the tested samples were lower than the limits for drinking water, except for Mn+, As+, and Pb + , which were marginal above recommended limits. Principal component analysis results showed two principal components with the first principal component having high loadings of Mn+, Fe3+, As+, Pb+, Ni+, and Cr3+, which are statistically correlated. Probable sources of these contaminants include automobile parts and tools, batteries and electrical appliances and electronics heaped at the dumpsite. Analysis of variance results indicates a significant difference in datasets between and within each and both water sources. Human health risk assessment indices indicated that the water sources are moderate to highly polluted and could pose both non-carcinogenic and carcinogenic threats when consumed untreated, with children being more exposed than adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growth in human population in most countries' rural and urban areas has resulted in an equal increase in waste created daily from domestic, industrial, and commercial operations. Unlike in most developed countries, where proper waste management mechanisms such as controlled incineration and well-designed landfills that meet safety standards are in place, wastes in most developing countries are mostly disposed of indiscriminately by dumping them around residential areas, markets, farms, roadsides, and open dumps or dumpsites (Abd El-Salam and Abu-Zuid 2015; Coker et al. 2016). The lack of proper supply of sanitary landfills, which is considered a fundamental obligation of the government, is one of the main reasons for these options being used in underdeveloped nations (Mor et al. 2006; Onyekwelu 2016; Mgbenu and Egbueri 2019).
A liquid component known as leachate, which is mostly generated by the breakdown of chemical compounds in plastics, electronics, foodstuffs, vehicle parts, plastics, and batteries dumped in dumpsites, is a major source of concern. Chemical, physical, and biological pollutants are frequently present in leachate. Among the chemical contaminants in dumpsites are heavy metals (Hoffmann et al. 1991; Afolayan et al. 2012). Heavy metals such as Mn, Fe, Cd, As, Pb, Ni, and Cr, which are metalloids, transition metals, basic metals, lanthanides, and actinides with specific gravity > 5.0, are known to be toxic and harmful to the environment, including surface and groundwater sources, and could be carcinogenic when ingested by human beings (Nriagu 1989; Nwigwe 2008; Nagaiyoti et al. 2010; Omaka et al. 2017). Because heavy metals in water sources have a high potential to cause health problems, monitoring water quality and its influencing variables is essential for water resource management and sustainability across the world (Onyekuru et al. 2010; Okogbue et al. 2012; Pawari and Gawande 2015).
Despite the fact that residents of developing countries rely heavily on natural water sources due to a lack of adequate or better alternatives provided by local authorities, research has revealed that only about 4.9% (Annapooorna and Janardhana 2015) of surface water worldwide is fresh and suitable for human consumption and other purposes (Nweke et al. 2013; Itumoh et al. 2015; Aghamelu et al. 2022). Researched and published works abound on the quality and impacts of heavy metals (which usually migrate through infiltration and runoff into the hydrological cycle) on natural water sources by dumpsite leachates and the possible health risks they pose in different parts of the world (for example Beaven and Walker 1997; Hoffman et al. 1991; Esakku et al. 2003; Khan et al. 2013; Pawaj and Gawande 2015) and in parts of Nigeria (for example; Afolayan et al. 2012; Coker et al. 2016; Onyekwelu and Aghamelu 2019). To the authors' knowledge, no published article or available documented work had considered the possibility that human health challenges reported daily in the Enugu Metropolis of southeastern Nigeria (see Fig. 1) are linked to heavy metals from a dumpsite, which was established at the Ugwuaji area of Enugu metropolis more than four decades ago (Onyekwelu 2016).
This research is one of the first of its kind undertaken by the authors, with the primary goal of determining the heavy metals-related health hazards to the general public, particularly among those who consume and utilize natural water sources inside the dumpsite's impact zone in the Enugu city. To achieve the goal, heavy metal concentrations in water resources were assessed using standard laboratory testing procedures and data analysis using integrated multivariate analytical and health indexical tools designed for evaluating the interrelationship, possible source apportionment of heavy metals, and associated human health risks. Although previous studies, such as US-EPA (1997, 1999), Lim et al. (2008), Li et al. (2016), and Zang et al. (2018), had successfully deployed health indexical tools in other parts of the world and Nigeria (Ukah et al. 2019) to achieve similar objectives, they are being used for the first time in the Enugu area for a dumpsite related research. The findings of this study could serve as a benchmark for authorities in the area and elsewhere in terms of understanding the potential health risks posed to children and adults who drink water from a dumpsite influence zone, as well as developing a proper waste management strategy to avoid current and future health threats.
Study area description
Location and accessibility
The Enugu metropolis is located between longitudes 7° 28′ 36″ E and 7° 37′ 43″ E and latitudes 6° 2′ 46″ N and 6° 22′ 10″ N in the southeastern geopolitical zone of Nigeria, as shown in Fig. 2. The metropolis is surrounded by the Nkanu West, Nkanu East, and Udi local area councils. Accessibility through the location is enhanced by a good network of roads such as the Akpugo–Agbani, the Enugu–Port Harcourt, and the Enugu–Aninri Highways. The access roads include the Enugu–Port Harcourt Expressway, Akpugo–Agbani, and Enugu–Aniri Roads. There is also an international airport at the Emene area to aid international travels. The hinterlands are accessed through unpaved roads and footpaths.
Geology
The Enugu metropolis is geologically underlain by two formations, the Coniacian Agbani Formation and Campanian Enugu Shale (Fig. 3). The Agbani Formation dips in the west-northwest direction at 30–120 and comprises predominantly medium to coarse-grained sandstones that are moderately consolidated, with lenticular shales and beds of grits and pebbles observed in some locations during field studies. The Enugu Shale conformably overlies the Agbani Formation and comprises dark grey shales with an alternating sequence of thin sandstone and sandy shale layers. It dips in the west-southwest direction at an amount ranging between 4 and 8°. The Enugu Shale covers over 70% of the area and spans the western and central portions.
Hydrology and climate
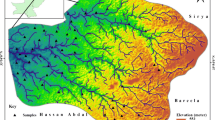
The metropolis area is drained by a network of ephemeral streams (see Fig. 2), rivers and springs recharged mainly by rainfall. The perennial nature of surface waters in the area is attributed to base flow from the upper unconfined aquifer system and intense evaporation along its tributaries. Two climatic seasons are prevalent in the area, a rainy (or wet) season that starts around April and ends around November, and a dry season that lasts from December to March (Aghamelu et al. 2013). The two seasons are predominantly controlled by the southwest trade winds from the Atlantics and the northeastern wind which blows across the Sahara (Mgbenu and Egbueri 2019). The dry season is characterized a relative dryness and moderately high temperature. Temperature values reaches up to 32 °C with an average relative humidity at times drops to 20 °C (Egboka 1993). The vegetation is that of a rainforest with a predominance of shrubs and trees in the farmlands (see Fig. 4).
Hydrogeology
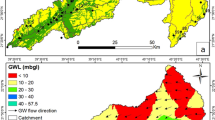
Two distinct aquifer systems have been identified in the area. They are the confined and unconfined aquifers (Aleke et al. 2016). While the unconfined aquifers sit on the Agbani Sandstone members as well as the weathered parts of the Enugu Shale, the confined aquifers occur in the Enugu Shale. The groundwater flow system of the area is defined by the structural and stratigraphic framework of the area, trending mainly in the northeast to southwest direction, as shown in Fig. 5. The infiltration pattern is in the radial form due to the topographic outline of the site (Onyekwelu and Aghamelu 2019). The groundwater flow direction is shown in Fig. 6.
Description of the dumpsite
The dumpsite is a situated within the Ugwuaji Nike settlement of Enugu metropolis. It is at about 1.6
km off the Enugu–Port Harcourt expressway and west of the Enugu-Udi escarpment (Fig. 5). The Ugwuaji dumpsite serves as the final ground for waste disposal in the Enugu metropolis housing a variety of wastes ranging from domestic to industrial to agricultural wastes as shown in Fig. 7. The dumpsite slopes downward in all directions away from its centre and has a thickness that ranges from 3 to 8 m. It is worthy to note at this point that the dumpsite which was originally designed as a landfill produces about 200 L of leachates per day, according to Onyekwelu and Aghamelu (2019) which leach freely into the surface waters and infiltrates the underlying groundwater system contaminating them in the process because the bottom of the dumpsite is not lined for leachate containment. Previous studies by Obasi et al. (2013) and Onwe et al. (2016) had listed the factors that influence the infiltration of water in some geological formations in the southeastern part of Nigeria to include grain size, porosity, and viscosity of the water type. Figure 8 shows the pH values distributions in the study area.
Materials and methods
Sampling
A total of 12 samples were collected, three from leachate generated at the dumpsite, seven from hand-dug wells, and two samples from surface water bodies within a 300 – 1000 m radius of the dumpsite. The coordinates of sample points were taken with a Garmin (GPSMAP model 78S) portable Geographical positioning system (GPS) device. The hand-dug wells are all located between 20 – 1,000 m radius of the dumpsite that was considered as the ‘influence zone’ with high potentials of being contaminated by the dumpsite. The sample locations and GPS readings are presented in Table 1, and the locations are shown in Fig. 2.
As a precaution, the polyethene sample bottles used in collecting the water samples were flushed with a phosphate-free detergent, rinsed with de-ionized water and rinsed again with the sampled water before collection. A few drops of concentrated HCl acid were added to preserve the samples for the analyses and stored in ice-crested coolers while being transported, following standard procedures recommended by the American Public Health Association (APHA 2005) to the laboratory for chemical analysis. To ensure the validity of results, analysis was completed within a period of 48 h of sample collection.
Laboratory analysis
The laboratory analyses of the water samples were carried out in Simuch Analytical Laboratory, Nsukka, following the APHA (2005) standard methods. The heavy metals tested include Fe3+, Mn+, Pb+, and Ar+,
Cd+, Cr3+ and Ni+. They were analysed using the Buck Model 210/211 GF graphite furnace and 220 Atomic Absorption Spectrometer. pH was measured in situ using a handheld digital pH metre (HACH HQ11D model).
Statistical analyses
Statistical analyses carried out were descriptive statistics, principal component analysis, a single-factor analysis of variance (ANOVA), agglomerative hierarchical cluster analysis (AHCA), and Pearson correlation analysis. These were done following guidelines outlined by Olobaniyi and Owoyemi (2006), Jenn et al. (2007), and Engelmann et al. (2018). The computer software used for the data analyses and graphical display of results included the ArcGIS 9 (version 9.3), and Corel Draw (version 11), and XLSTAT (version 2022.1.2). Surfer 16 (2019 edition).
Human health risk analysis
Environmental and exposure assessment
In measuring the heavy metal content in the area, the heavy metal evaluation index (HEI), heavy metal pollution index (HPI), and contamination index (CI) assessment techniques were used. The following equations can be used to compute these indices (Wagh et al. 2018; Ukah et al. 2019):
where HEI = Heavy metal evaluation index, \({H}_{c}\) = monitored value; \({H}_{MAC}\) = maximum admissible concentration (MAC) of the ith parameter.
where HPI = Heavy metal pollution index, HMC = heavy metal concentration in water sample; AL = allowable limit; and n = number of analysed heavy metals.
where CI = Contamination index, Cfi = contamination factor for the ith parameter; CAi = analytical value for the ith component; and CNi = upper permissible concentration of the ith parameter (N denotes the normative value).
Non-carcinogenic health risk assessment
The hazard quotients (HQ) and hazard indices (HI) are both considered and calculated for each water sample when evaluating for non-carcinogenic health risks. After computing the CDI as indicated in Eq. 5, the HQ is evaluated as seen in Eq. 6 (both equations given by US-EPA 1997):
where CDI is the chronic daily intake and is referred to as the exposure dose (mg/kg/day); Cw is the contaminant concentration in water (mg/L); IRW signifies the water ingestion rate (IRW is equal to 1 L for children and 2L for adults); EF denotes the exposure frequency (EF is equivalent to 365 days per year); ED is the exposure duration (adult ED = 70 years while children ED = 6 years); BW is the body weight (equivalent to 70 kg and 15 kg for adult and children, respectively); whereas AT signifies the average exposure time (equivalent to 25,550 days and 2,190 days for adult and children, respectively), according to Ukah et al. (2019).
The hazard index (HI) is the last stage of the non-carcinogenic risk assessment. It is the summation of the hazard quotient values and given by Lie al. (2016) and Zhang et al. (2018) as follows:
where RfD denotes the reference dose of a specific element (mg/kg/day). The RfD values for the heavy metals considered are; 0.046 (Mn), 0.7 (Fe), 0.001 (Cd), 1.5 (Cr), 0.0003 (As), 0.0035 (Pb), and 0.02 (Ni), according to Barzegar et al. (2018), and Ukah et al. (2019).
Carcinogenic health risk assessment
Rahman et al. (2018) had used probability of cancer risk (PCR) to assess the tendency of an individual to develop cancer throughout a lifetime by exposure to potential carcinogens in drinking water. Equation 8 (US-EPA 1999; Lim et al. 2008) was used in determining the PCR of each carcinogenic metal.
where SF is the slope factor (mg/kg/day).
A good PCR value is ≤ 1 × 10−6which suggests that about 1 in 1,000,000 people will get cancer as a result of being exposed to a carcinogen, according to Lim et al. (2008) and Adamu et al. (2015). Risks in the range of 1 × 10–6 to 1 × 10–4, on the other hand, have been considered to be tolerable by US-EPA (1999), Yang et al. (2012) and Rahman et al. (2018). Cd, Cr, As, Pb and Ni amounts in water were used to calculate the PCR for this investigation. For Cd, Cr, As, Pb, and Ni, SF values of 0.38, 0.05, 1.5, 0.0085, and 0.91 were employed, respectively (Naveedullah et al. 2014; Taiwo et al. 2020; Ukah et al. 2019).
Result and discussion
pH
The pH value results are presented in Table 2. The results show that leachate samples were very acidic with pH that ranged from 3.8 to 4.3, while samples from groundwater and surface water samples were moderately acidic with pH that varied from 4.6 to 6.2. The low values of pH in the tested water samples within the dumpsite area may be a reflection of an acid-producing stage of the decomposing wastes. The low pH values in all tested leachate samples may indicate that the leachate was undergoing methanogenic or an anaerobic phase. As earlier observed by Fatta et al. (1999), the first stage of leachate formation is characterized by low pH values. The contour plot of pH shows that pH increases in groundwater sources in the eastern and southern directions, suggesting that the direction of surface water flow and neutralization reaction might have influenced the pH results.
Heavy metal concentration
Mn +
The results of the heavy metal analysis are summarized in Table 2, and their concentrations are compared.
in Fig. 9. The table shows that Mn+ ranges from 3.8 to 4.3 µg/l for leachate samples, 0.6 to 9.8 µg/l for groundwater samples, and 0.4 to 1.1 µg/l for surface water samples. The Mn+ enrichment in leachates and water sample from Ebenezer Anglican Church, with Mn+ higher than the drinking water limit of 5 µg/l (0.005 mg/l) given by WHO (2011) (see Table 3), may be attributed to automobile parts and tools from the dumpsite. Other possible sources of Mn+ would include agricultural activities such as extensive use of fungicides and fertilizers. As shown in Fig. 10a, the concentration of Mn+, similar to pH, increases in groundwater sources in the eastern and southern directions. This supports the inference that the direction of surface water flow had water quality.
Fe 3+
Fe3+, as shown in Table 2, ranges from 4.0 to 4.3 µg/l for leachate samples, 0 (below detection) to 4.0 µg/l for groundwater samples, and 0 to 0.3 µg/l for surface water samples. Fe is one of the most abundant metals in the earth's crust and normally exists in form of oxides. In addition to the high availability of Fe in the earth's crust, Fe oxides are important constituents of plastics and paints which probably led to its concentration within the study area. Although Fe is essential for human health, its high concentration would impair the potability of drinking water (WHO 2003). Excess Fe content in water resources could result in "inky" flavour, "red hot" disease, promotion of microbial growth, bitter and astringent taste, scaling in pipes, cloth and plumbing fixtures discolouration (Ukah et al. 2019). High Fe3+ content may cause the formation of iron bacteria leading to an increase in slimy coatings on water distribution systems. The highest amount of Fe3+ was recorded in leachates, while the lowest was recorded in groundwater samples, which mostly have their Fe3+ below the detection limit. Fe3+ increases farther away from the dumpsite, towards the eastern part of the study area, as shown in Fig. 10b. All the tested samples had their mean Fe3+ below the WHO (2011) maximum permissible limit of 5 µg/l (0.005 mg/l) for drinking purposes. However, relatively high values in the leachate samples are a typical evidence of dumping of iron and steel scrap wastes at the dumpsite (see Fig. 7).
Cd +
Table 2 indicates that among the tested samples, the highest concentration (12.2 µg/l) of Cd+ was observed in sample no. 3 (leachate at the dumpsite), while the surface water samples had their Cd+ concentrations below the detection limit. The Cd+ of the groundwater samples ranged between zero (below detection) and 0.2 µg/l. Cd+ in leachates and groundwater samples are significantly higher than the WHO (2011) maximum permissible limit for drinking water. The high Cd+ concentrations in leachate and samples bodies may attribute to batteries and appliances dumped at the site. The recommended limit of Cd is 3 μg/l (or 0.003 mg/l), and the levels for drinking water should be less than 1 μg/l (WHO 1993, 2003). The results indicated that Cd+ in all the tested samples were all below the permissible limit of 5 µg/l (or 0.005 mg/l) recommended by WHO (2011) for drinking purposes. The relative enrichment in leachates was due to closeness to the contaminants source or sources.
As +
The results show that As+ ranges between 5.1 and 7.3 µg/l in leachate samples, 0 and 5.1 in groundwater and 0 and 0.3 µg/l in surface water. As+ is highest in a leachate sample (sample no. 1) at the dumpsite, while no As+ was detected at Nyo River and in groundwater samples from a hand-dug wells at Ike Ekweremadu Street and Thinkers Corner. The rest of the samples such as Asata River, Vision Company, Umunaji Ngene and Obeagu shallow hand-dug wells contained minimal As+ concentrations ranging from 0.3 to 0.8 µg/l, and below recommended limit of 1 µg/l (or 0.001 mg/l) in drinking water (WHO 2011). Distance away from the dumpsite the risk of pollution by As+ decreases, as shown in Fig. 10d.
The occurrence of high As+ concentrations in leachate samples is relatively associated with the presence of clay and shale minerals, Fe, Mn oxides and organic matter which can influence the sorption, solubility and rate of oxidation of As species (Plant et al. 2000). Electrical appliances disposed of at the site also play a vital role in the high concentrations of As+. This suggests that the occurrence of As+ in the area, although presently lower than the recommended standard for drinking water, started long after other contaminants have accumulated.
Pb +
Results in Table 2 indicate that Pb+ has mean values of 2.47 µg/l in leachate samples, 1.90 µg/l in groundwater samples, and 0.35 µg/l in surface water samples. Pb+ is very poisonous, even at low concentrations. In infants, Pb could cause severe neuro-development problems and intelligence quotient (IQ) loss. In adults, it causes nervous system disorders, anxiety, kidney problems, hypertension, failure of reproductive organs and cancer (Nigerian Industrial Standard 2007; Brown and Margolis 2012; Ukah et al. 2019). Pb+ concentration is observed to be evenly distributed within the study area with its highest concentration in a leachate sample (sample no. 3). However, Ebenezer Anglican Church and Goshen Estate shallow hand-dug wells near the dumpsite have concentrations of Pb+ ranging from 2.3 µg/l – to 2.4 µg/l. Vision Company and Thinkers Corner shallow hand-dug wells showed an absence of Pb+ (Fig. 10e).
The amount of Pb+ in the dumpsite depends on several factors including pH, and battery standing time of the dumpsite, with soft, acidic water being the most solvent. High concentrations of Pb suggest that Pb is being leached from multiple sources such as those from batteries and plumbing systems due to oxygenated water, increased temperature and low pH (Schock 1989, 1990). Pb contamination can be very severe in young children when ingested because they absorb it five times more than adults, especially when other nutrients such as iron and calcium are not available (Nazarpour et al. 2019). Generally, Pb + tend to be more mobile in the leachates than in shallow hand-dug wells and surface water bodies, tending to be leached out of the former and to form residual concentrations in the latter. The WHO (2003, 2011) guideline has recommended 1 µg/l (or 0.01 mg/l) as the maximum permissible limit for drinking water. Comparison with this recommended limit indicates that the groundwater with mean Pb+ value of 1.01 µg/l is slightly polluted. Thus, Pb+ is a potential environmental pollutant in the study area, especially in the areas northeast, east, and southeast of the dumpsite (see Fig. 10e).
Ni +
Ni+ in leachate samples ranges from 13.8 to 14.4 µg/l, groundwater 0 to 1.1 µg/l, and surface water 0 to 0.1 µg/l. As expected, the highest Ni+ was recorded in a leachate sample (sample no. 3), while it was below the detectable limit in River Nyo and hand-dug wells from Umunaji Ngene, Obeagu and Ike Ekweremadu Street, samples nos. 7, 8, 9, and 10, respectively. The maximum permissible limit of Ni+ set by WHO (2011) for drinking water is 7 µg/l (or 0.07 mg/l). A comparison of the results shows that the mean Ni+ value (0.37 µg/l) of the groundwater samples is lower than the recommended maximum limit, indicating a no risk or low potential for groundwater contamination by Ni+. Probable sources of Ni+ in the dumpsite and the adjoining areas include batteries, electrical appliances and electronic gadgets. Allergic contact dermatitis is the most prevalent effect of toxicity of Ni+ in the general population (WHO, 2005). According to Plant and Raiswell (1983), Ni+ deficiency results in decreased plasma cholesterol, increased liver cholesterol, ultrastructural changes in the liver cells, rough hair, impaired reproduction and poor growth of offspring. Contour plot of Ni+ concentration in Fig. 10f indicates that its impact is most within the locality of the dumpsite with a low tendency of polluting the water bodies, distance apart from the site.
Cr 3+
Results show that Cr3+ in the tested samples ranges as follows: leachate 0.2 to 2.5 µg/l, groundwater and surface water both 0 to 0.1 µg/l. The highest Cr3+ of 2.5 µg/l was in a leachate sample (sample no. 2) while it is below detected in all groundwater samples except two samples from Ebenezer Anglican Church (sample no. 5) and Ike Ekweremadu Street (sample no. 9). The mean Cr3+ value (0.03 μg/l) in groundwater is less than the maximum permissible limit of 5 µg/l (or 0.005 mg/l) for drinking water given by (WHO 2011), indicating very low contamination by Cr3+, especially for drinking and domestic purposes. A high amount of Cr3+ in drinking water would lead to gastrointestinal tract infection and in the long-term would have effects on sensitive populations, causing diseases such as Wilson disease and other metabolic disorders like Cr homeostasis (WHO 2005). The presence of Cr3+ in the leachate samples may have originated from the automobile exhaust, diesel tanks and vehicles which are dumped within the vicinity of the dumpsite, with the environmental impacts felt mostly within less than a 100 m radius of the dumpsite.
Statistical analysis
Principal component analysis
The principal component analysis result, showing the number of principal components, the loading of.
variables on each component and the percentage of data variance in the dataset, is presented in Table 4. As shown in the table and Fig. 11, two components accounted for 89.1% of the total variance in the dataset, with their eigenvalue < 1. In these two principal components, only variables with factor loading ≥ 0.60 were used in the analysis.
Principal component 1: This component has high loading factors of Mn+, Fe3+, As+, Pb+, Ni+, and Cr3+. The loading factors in this principal component accounted for a total variance of 74.8% of the dataset. The heavy metals are related activities and substances dumped at the dumpsite. Principal component 1 can, therefore, be attributed to the dumpsite activities and substances.
Principal component 2: This principal component accounts for 14.3% of the total variance. The loading factors in this principal component include Cd+ and Cr3+. These two heavy metals likely originated from automobile and electrical appliances wastes at the dumpsite area (see Fig. 7). Principal component 2 can also be attributed to dumpsite activities and substances. Biplots of components, before and after Varimax rotation, as shown in Fig. 12, strongly suggest that the heavy metals identified in the water sources originated from the dumpsite.
Pearson correlation
The Pearson correlation proximity matrix is presented in Table 5. It shows that a very strong positive relationship exists between Mn+ and Fe3+ (with correlation coefficient, R2 = 0.991), Mn+ and As+ (R2 = 0.978), Mn+ and Pb+ (R2 = 0.932), Fe3+ and As+ (R2 = 0.968), Fe3+ and Pb+ (R2 = 0.932), and As+ and Pb+ (R2 = 0.944). This statistical relationship confirms the earlier observation in the results of the principal component analysis that Mn+, Fe3+, As + , and Pb+ in the tested samples have a direct positive relationship.
Analysis of variance
The results of a single-factor ANOVA test are presented in Table 6. It is shown in the table that the calculated factor (Fcalc) is consistently higher than the standardized factor (Fcrit), at a 95% confidence interval. This is an indication that was a significant differences in datasets between and within all the tested sample sources. Distance from the source of the heavy metal contaminants, biological influence, the origin of the natural water bodies, physical and chemical processes, topography and hydrologic flow pattern and directions have been suggested by Onyekwelu and Aghamelu (2019) as being responsible for variation in the datasets of the organic compounds in natural water sources from this area.
Agglomerative hierarchical cluster analysis of variance
Table 7 shows the results of the agglomerative hierarchical cluster analysis, while Figs. 13 and 14 present the obtained levels bar chart and dendrogram, respectively. As shown in both the table and figures, three different data clusters are identified. Cluster 1 consists of sample nos. 1, 2, 3, with sample no. 1 (from leachate) as its central object, whereas cluster 2 comprises samples nos. 4 and 5, with sample no. 4 (from groundwater) as its central object, and cluster 3 contains sample nos. 6, 7, 8, 9, 10, 11, and 12, with sample no. 10 (from a surface water) as its central object. The heavy metal test results in this study had earlier revealed that samples from leachates have the higher concentrations of heavy metals among the tested samples. As the zone of influence expands, the concentration of heavy metals decreases, hence, buttressing the fact that leachates from the dumpsite are directly associated with heavy metal contamination of domestic water sources in the area.
Human health risk assessment
Environmental and exposure assessment
The results of various health risk assessment indices are presented in Table 8. Table 9 provides the classification chart for the various indices utilized (Wagh et al. 2018; Ukah et al. 2019). The contour plots that show zones of influence of these health risk factors are presented in Fig. 15. The HEI values varied from 44.429 to 946.905 with a mean of 371.490, the HPI values ranged from 10.254 to 166.325 with a mean of 9.329, and the CI values ranged from 9.571 to 678.619 with a mean of 223.323. Based on the classification chart (Table 2). The HEI divides water into three categories: low contamination (< 400), medium contamination (400–800), and high contamination (> 800) based on the water quality classification table for the indices used.
The HPI classifies water into two categories: safe (< 20) and critical (> 20), whereas the CI categorizes water as low, medium, or high contamination, based on CI values of 40, 40–80, and > 80, respectively (Wagh et al. 2018; Ukah et al. 2019). Based on the obtained results, the HEI show that 58.33% of the sampled water is of low contamination, 25% is of medium contamination, and 16.67% is of high quality. For the HPI, 41.67% are of safe quality, and 58.33% are of critical quality. However, the CI indicates that 41.67% are low contamination and 58.33% are high contamination. Based on this assessment, a greater percentage of the water samples are moderate to highly polluted. Thus, they have tendency to pose a threat to human health.
Non-carcinogenic health risk assessment
For both adults and children in the area, the median values from the HQ assessment revealed that heavy metals have an impact on chronic health change in the order Pb > Ni > Cd > Mn > Fe > As > Cr. Pb and Ni are the two most important contaminants for both populations, according to HQ effects; nevertheless, Mn has the least impact on both populations. The adult population's HI values ranged from 0.168 to 6.679 with a mean of 2.264, while the children's HI values ranged from 0.390 to 15.552 with a mean of 5.272, as shown in Table 8. According to earlier studies by the US-EPA (1999) and Su et al. (2017), HI values of more than one (HI > 1) indicate that the non-carcinogenic health risk is beyond the permissible limit, while HI 1 indicates that it is below the allowable threshold. As presented in Table 9, the non-carcinogenic risk is classed as negligible, low risk, medium risk, and high risk based on HI values (Ukah et al. 2019). According to the findings, 16.67% of the samples are negligible, 33.33% are a low hazard, 25% are a medium hazard, and 25% are a high hazard in the adult population. However, it was discovered that 16.66% of children have a low hazard, whereas 83.34% have a medium or high hazard. From the result of the analysis, children in the area are significantly more exposed to non-carcinogenic chronic health risks than the adult population.
Carcinogenic health risk assessment
The cancer risk (CR) for adults and children population for analysed carcinogenic metals Cd, Cr, As, Pb and Ni in this study was calculated and is presented in Table 10. An acceptable range for cancer risk is within ≤ 1 × 10−6 to 1 × 10−4 (US-EPA 1999; Rahman et al. 2018; Ukah et al. 2019). Based on the obtained results, 66.67% of the total samples have high Cd and Pb cancer risk for adults and children, and 75% and 16.67% have high Cr and Ni cancer risk, respectively, for both populations. However, 83.33% of all samples are of negligible risk for adults, while 41.67% are of high risk for the children population due to As contamination. The health risks, however, decrease in distance away from the dumpsite, as shown in Fig. 15.
Conclusions
From the study, it is evident that leachates yielding heavy metals from the Ugwuaji dumpsite have marginal impact on the natural water sources in the Enugu metropolis. The distribution of the heavy in the studied water samples revealed that both the quality of groundwater and surface water sources are impacted to different degrees. The results showed that all the analysed heavy metals including Mn, Fe3+, Cd, As, Pb, Ni, and Cr were present in both surface and groundwater within the area, with Mn+, As+, and Pb+ having their concentrations higher than the maximum permissible limits for water consumption, given by the World Health Organization. Probable sources of these heavy metals include automobile parts, engine oils and lubricants, as well as electrical and electronic appliances disposed of at the dumpsite.
Findings from health assessment indices in this study indicates negligible risks associated with natural water sources from the area when ingested untreated. Diseases such as cancers, diarrhoea, typhoid, cholera, respiratory diseases, Wilson's disease, dermatitis and other forms of metabolic disorders are attendant risks associated with the intake of such untreated water, with children being at more risk than adults. Although the water sources are shown to be contaminated by heavy metals, they might be used after treatment, especially for other domestic purposes such as laundry and cleaning. The intensity of the treatment required would increase towards the dumpsite.
The concerns of heavy metal contamination' transit duration, flow rate, and migration paths from the dumpsite to natural water sources should be investigated further. It would also be necessary to examine the medical records of the city's residents. When the suggested studies are conducted, the flow pattern data and attenuation potential model of the formation developed, combined with medical information, will aid in identifying the dumpsite leachate contamination's real impact zone and the percentage of the metropolis's population at danger. Due to technical constraints such as the lack of relevant data or sufficient equipment, as well as the refusal of some medical authorities in the region to release vital and confidential medical records, the current study was unable to carry out this task.s
Data availability
The authors declare that all the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
Abd El-Salam MM, Abu-Zuid GI (2015) Impact of landfill leachate on the groundwater quality: a case study in Egypt. J Adv Res 6(4):579–586. https://doi.org/10.1016/j.jare.2014.-02.003
Adamu CI, Nganje TN, Edet A (2015) Heavy metal contamination and health risk assessment associated with abandoned barite mines in cross river state, southeastern Nigeria. Environ Nanotech Monitor Manage 3:10–21. https://doi.org/10.1016/j.enmm.2014.11.001
Afolayan OS, Ogundele FO, Omotayo A (2012) Comparative analysis of the effect of closed and operational landfills in groundwater quality in solous, lagos. Nigeria J Appl Tech Environ San 2(1):67–76
Aghamelu OP, Ezeh HN, Obasi AI (2013) Groundwater exploitation in the abakaliki metropolis (southeastern Nigeria); issues and challenges. Afr J Environ Sci Tech 7(11):1018–1027
Aghamelu OP, Amah JI, Nwatalari AN (2022) An investigation of the 2013 emergence of an ephemeral pool like surface water body in the nachi area of southeastern Nigeria. Arabian J Geosci. https://doi.org/10.1007/s12517-021-09125-5
Aleke CG, Okogbue CO, Aghamelu OP, Nnaji NJ (2016) Hydrogeological potential and qualitative assessment of groundwater from the ajali sandstone at ninth mile area, southeastern Nigeria. Environ Earth Sci. https://doi.org/10.1007/s12665-015-4843-3
Annapoorna H, Janardhana MR (2015) Assessment of groundwater quality for drinking purpose in rural areas surrounding a defunct copper mine. Aqua Procedia 4:685–692
American Public Health Association (APHA, 2005, 2012). In: Standard methods for the examination of water and wastewater. 22nd ed. American Public Health Association and American Water Works Association, Colorado
Barzegar R, Asghari A, Moghaddam AA, Adamowski HN (2018) Assessing the potential origins and human health risks of trace elements in groundwater: a case study in the Khoy plain Iran. Environ Geochem Health 41(2):981–1002. https://doi.org/10.1007/s10653-018-0194-9
Beaven RP, Walker AN (1997) Evaluation of the total pollution load of MSW, Proceedings Sardinia ’97 6th International Landfill Symposium. CISA, Cagliari, pp 57–71
Brown MJ, Margolis SB (2012) Lead in drinking water and human blood lead levels in the United States. MWWR Supplements 61(4):1–9
Coker AO, Achi CG, Sridhar MKC, Donnet CJ (2016) Solid waste management practices at a private institution of higher learning in Nigeria. Procedia Environ Sci 35:28–39. https://doi.org/10.1016/j.-proenv.2016.07.003
Egboka BCE (1993) The raging war, flood, erosion and gully ravage-Anambra State. A Publ Anam State Go Nigeria, Awka
Engelmann PD, dos Santos VHJM, Barbieri CB, Augustin AH, Ketzer JMM, Rodrigues LF (2018) Environmental monitoring of landfill area through the application of carbon stable isotopes, chemical parameters and multivariate analysis. Waste Manage 76:591–605
Esakku S, Palanivelu K, Joseph K (2003) Assessment of heavy metals in a municipal solid waste dumpsite. Workshop Sustain Landfill Manage, Chennai, pp 139–145
Fatta D, Papadopoulos A, Loizidou M (1999) A study on the landfill leachate and its impact on the groundwater quality of the greater area. Env Geochem Health 21:175–190
Hoffmann L, Christiansen K, Petersen C (1991) Lead and cadmium contamination of compostable waste derived from source-separated household waste. International conference on heavy metals in the environment. CEP Consultants. Edinburgh. 2: 264–267
Itumoh EJ, Aghamelu OP, Izuagie T (2015) Influence of mining and agricultural activities on the quality of groundwater from some rural areas of southeastern Nigeria. Global NEST J 17(2):406–417
Jenn F, Kofahl C, Muller M, Radschniski J, Voigt HJ (2007) Interpretation of geological, hydrogeological, and geochemical results. In: Knodel K, Lange G, Voigt HJ (eds) Environmental geology: handbook of field methods and case studies. Springer, Berlin, p 818
Li P, Li X, Meng X, Li M, Zhang Y (2016) Appraising groundwater quality and health risks from contamination in a semiarid region of northwest China. Expo Health 8(3):361–379. https://doi.org/10.1007/s12403-016-0205-y
Lim HS, Lee JS, Chon HT, Sager M (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned songcheon Au–Ag mine in Korea. J Geochem Expl 96:223–230
Mgbenu CN, Egbueri JC (2019) The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Appl Water Sci. https://doi.org/10.1007/s13201-019-0900-5
Mor S, Ravindra K, Dahiya RP, Chandra A (2006) Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Env Monitor Assess 118:435–456. https://doi.org/10.1007/S10661-006-1505-7
Nagajyoti PC, Lee KD, Sreekanth TV (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Naveedullah M, Hashmi Z, Yu C, Shen H, Duan D, Shen C, Lou L, Chen Y (2014) Concentrations and human health risk assessment of selected heavy metals in surface water of the siling reservoir watershed in zhejiang province. China Pol J Env Stu 23(3):801–811
Nazarpour A, Watts MJ, Madhani A, Elahi S (2019) Source, spatial distribution and pollution assessment of pb, zn, cu, and pb, isotopes in urban soils of ahvaz city, a semi-arid metropolis in southwest Iran. Sci Reports 9:5349. https://doi.org/10.1038/s41598-019-41787-w
Nigerian Industrial Standard (NIS) (2007) Nigerian standard for drinking water quality. Stand Org Nigeria, Abuja 554:13–14
Nriagu JO (1989) A global assessment of natural sources of atmospheric trace metals. Nature 338:47–49
Nweke OM, Aghamelu OP, Obasi IA (2013) Hydrogeochemical analysis and quality evaluation of groundwater from Onicha-Uburu, southeastern Nigeria for irrigation purposes. Afr J Env Sci Tech 7(5):222–228
Nwigwe N (2008) Problems and prospects of refuse disposal in Nigerian urban centers. Int J Nat Appl Sci. https://doi.org/10.4314/ijonas.v4i4.49880
Obasi PN, Aghamelu OP, Akudinobi BEB (2013) Determination of hydraulic conductivity of sandstones of ajali formation in uturu area (southeastern Nigeria) using grain size analysis. J Nat Sci Res 3(3):49–54
Okogbue CO, Omonona OV, Aghamelu OP (2012) Qualitative assessment of groundwater from egbe-mopa basement complex, northcentral Nigeria. Env Earth Sci 67(4):1069–1083
Olobaniyi SB, Owoyemi FB (2006) Characterization by factor analysis of the chemical facies of groundwater in the deltaic plain sands aquifer of Warri, western Niger delta. Nigeria African J Sci Technol 7(1):73–81
Omaka ON, Aghamelu OP, Ike-Amadi CA, Ofoezie RC (2017) Assessment of the quality of groundwater from different parts of southeastern Nigeria for potable use. Env Earth Sci 76(9):1–24. https://doi.org/10.1007/s12665-017-6680-z
Onwe IM, Akudinobi BEB, Aghamelu OP (2016) Estimating the hydraulic conductivity of the ajali sandstone in the udi area of South Eastern Nigeria from pumping test and grain size based empirical analysis. J Hydrogeol Hydrol Eng 5(3):1–7
Onyekwelu IL, Aghamelu OP (2019) Impact of organic contaminants from dumpsite leachates on natural water sources in the enugu metropolis, southeastern Nigeria. Environ Monitor Assess 191(9):1–20. https://doi.org/10.1007/s10661-019-7719-2
Onyekuru SO, Nwankwor GI, Akaolisa CZ (2010) Chemical characteristics of groundwater systems in the southern Anambra Basin, Nigeria. J Appl Sci Res 6:2164–2172
Onyekwelu IL (2016) Assessment of contaminants in natural waters by leachates from Ugwuaji dumpsite. Dissertation, Enugu State University of Science and Technology, Agbani
Pawari MJ, Gawande S (2015) Groundwater pollution and its consequence. Int J Eng Res Gen Sci 3(4):773–776
Plant JA, Raiswell R (1983) Principles of environmental geochemistry. In: Thornton I (ed) Applied Environmental Geochemistry. Academic Press, London, pp 1–39
Plant JA, Smith B, Williams J (2000) Environmental geochemistry at global scale. J Geol Soc London 157:837–849
Rahman M, Islam M, Bodrud-Doza, et al (2018) Spatio-temporal assessment of groundwater quality and human health risk: a case study in Gopalganj Bangladesh. Expos Health 10(3):167–188. https://doi.org/10.1007/s12403-017-0253-y
Schock MR (1989) Understanding corrosion control strategies for lead. J Amer Water Works Assoc 81(7):88–100. https://doi.org/10.1002/j.1551-8833.1989.tb03244-x
Schock MR (1990) Causes of temporal variability of lead in domestic plumbing systems. Env Monit Assess 15:59
Su H, Kang W, Xu Y, Wang J (2017) Evaluation of groundwater quality and health risks from contamination in the north edge of the Loess Plateau, Yulin City. Northwest China Env Eartn Sci 76:467. https://doi.org/10.1002/s12665-017-6781-8
Taiwo AM, Michael JO, Gbadebo OFO (2020) Pollution and health risk assessment of road dust from Osogbo metropolis, Osun state, southwestern Nigeria. Human Ecol Risk Assess 26(5):1254–1269. https://doi.org/10.1080/10807039.2018.1563478
Ukah BU, Egbueri JC, Unigwe CO, Ubido OE (2019) Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area, Lagos, Nigeria. Int J Energy Water Resour 3:291–303. https://doi.org/10.1007/s42108-019-00039-3
US-EPA (US Environmental Protection Agency) (1997). Exposure factors handbook, 1: General factors Washington, DC: US Environmental Protection Agency, Office of Research and Development
US-EPA (US Environmental Protection Agency (1999) A risk assessment multi-way exposure spreadsheet calculation tool. The United States Environmental Protection Agency, Washington DC
Wagh VM, Panaskar DB, Mukate SV, Gaikwad S, Muley A, Varade AM (2018) Health risk assessment of heavy metal contamination in groundwater of kadava river basin, Nashik, India. Model Earth Syst Env 4:969–980. https://doi.org/10.1007/s40808-018-0496-z
World Health Organization (WHO) (1993) Guidelines for drinking water quality, 2nd edn. World Health Organization, Geneva
World Health Organization (WHO) (2005) International health regulations, 3rd edn. World Health Organization, Geneva
World Health Organization (WHO) (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
World Health Organization (WHO) (2003) Background document for development of WHO guidelines for drinking water quality. World Health Organization, Geneva, WHO/SDE/WSH/03.04/08
Yang M, Fei Y, Ju Y, LiH MZ (2012) Health risk assessment of groundwater pollution-a case study of typical city in north China plain. J Earth Sci. https://doi.org/10.1007/s12583-012-0260-7
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Env Earth Sci 77(7):273. https://doi.org/10.1007/s12665-018-7456-9
Acknowledgements
Mr. Chinanu Unigwe of the Department of Geology and Geophysics, Alex Ekwueme Federal University, Ndufu-Alike is appreciated for assisting in the human health risk evaluation of the dataset. Dr. Emmanuel Aniwetalu of the Department of Applied Geophysics and Mr. Ogonna Emenaha of the Department of Geological Sciences, both of the Nnamdi Azikiwe University, Awka are acknowledged for their assistance during the drafting of the initial manuscript. The anonymous reviewers are appreciated for their useful comments that helped to improve the quality of the revised manuscript.
Funding
This research was privately funded by the authors. No fund was received from any agency or institution.
Author information
Authors and Affiliations
Contributions
DCO and ILO conceptualized and designed the study, as well as carried out material preparation and data collection, while ILO and OPA conducted the statistical data analysis and health risk evaluations. The first draft of the manuscript was written by ILO and OPA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest or any competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ozoko, D.C., Onyekwelu, I.L. & Aghamelu, O.P. Multivariate and health risks analysis of heavy metals in natural water sources around Enugu dumpsite, southeastern Nigeria. Appl Water Sci 12, 224 (2022). https://doi.org/10.1007/s13201-022-01746-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01746-9