Abstract
Metal pollution in aquatic environment of coal mines is of serious concern and requires to be dealt with to maintain sustainable mining practices. The spatio-temporal variation in metal pollution of surface water of Talcher coalfield area were determined by using multivariate statistical techniques and pollution indices. A total of 56 water samples were collected and analyzed for Fe, Zn, Cu, Cd, Pb, Co, Se, As, Hg, Cr, Ni, Mn, and Al in pre-monsoon and monsoon season. Spatial distribution maps were prepared so that the quality of surface water could easily be recognized. High values of Heavy Metal Pollution Index (HPI), Degree of Contamination (Dc), and Heavy Metal Evaluation Index (HEI) were observed for 3%, 6%, 0% samples in pre-monsoon and 1%, 6%, 3% samples in monsoon. Sewage Treatment Plants (STP), Effluent Treatment Plants (ETP), and Mine Discharge Treatment Plants (MDTP) were found to have low to moderate efficiency in treating metals. The HPI of streams and rivers were observed to be higher in pre-monsoon than that of the monsoon season possibly due to dilution effect caused by intense rain in monsoon. The HPI of downstream was noted to be higher than the upstream indicating pollution due to mine effluent discharge. The average concentrations of Cd, Se, As, Ni, and Al in pre-monsoon and Fe, Cd, Se, As, Ni, and Al in monsoon exceeded the permissible drinking water limits set by WHO (WHO, Guidelines for drinking-water quality, World Health Organization, Geneva, 2011) and BIS (BIS (2012) Drinking water specifications 2nd revision. Bureau of Indian standards (IS 10500: 2012). New Delhi. ftp://law.resource.org/in/bis/S06/is.10500.2012.pdf). Analysis of Variance (ANOVA) revealed significant seasonal variation (p < 0.05) of Fe concentration between pre-monsoon and monsoon. Principal Component Analysis (PCA) identified major sources of metal pollution in water such as earth’s crust and the geological formation of the region, coal mining activities, industrial pollution, vehicular emission and coal burning. Cluster analysis (CA) identified 19 moderately polluted sites, 6 highly polluted sites, 3 very highly polluted sites and 1 severely polluted site in and around the Talcher coalfield area. This study is useful for formulating the metal pollution mitigation plan to enhance the water quality of Talcher coalfield area which affect the aquatic organism as well as the human health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mining industry is a major source of non-renewable energy supply in India. India is enriched with Mica, coal, lignite, iron ore, bauxite, manganese, aluminum, etc. among eighty seven different kinds of mineral (Mishra and Das 2017). The biggest usage of energy was in the form of coal and lignite, accounting for approximately 44.1 percent of total consumption in 2017–18. Total Industrial raw coal consumption had been increased from 549.57 MT in 2008–09 to 896.34 MT in 2017–18 in India. (Central Statistics Office 2019). However, coal mining has not only polluted the water but also caused a shortage of water since mining operations are pushed out significant amounts of water from underground pits and sumps of surface mines (Singh et al. 2011). Annually, an average of 86.26 million cubic meter water is drawn from the Brahmani river, its tributaries and distributaries for industrial or mining activity in the Angul–Talcher region of Odisha (Reza and Singh 2010a, b). Mining and industrial processing produce substantial concentrations of heavy metals and affect the biogeochemical cycle (Ali et al. 2019). Heavy metals in water are one of the most serious pollutants due to their excessive accumulation, biomagnification, and toxicity (Ali and Khan 2018; 2019). Although a few metals (such as Cu, Co, Fe, and Mn) are essential for metabolic activities in living organisms in small levels, larger concentrations of these metals can cause health problems (Setia et al. 2020). Trace metals can be toxic even at low concentrations (Sahu and Basti 2021) and can disrupt the food chain of aquatic ecosystem by affecting fish, birds, and mammals, including humans (Briffa 2020). Metal pollutants have detrimental effects such as cellular damage, carcinogenesis and neurotoxicity on terrestrial invertebrates and vertebrates (Monchanin et al. 2021). Water pollution probably causes cancer in sixty–four percent of grey seals, twenty three percent of white-fronted geese, less than 1% of sea otters (Ujvari et al. 2017).
Water bodies near the coal mining area of Jaintia Hills, Meghalaya were observed to be polluted with low pH (between 3 and 5), high conductivity, high sulfate, high iron and calcium concentrations, low DO and high BOD, lower abundance, and species diversity of benthic macroinvertebrate due to acid mine drainage (AMD), leaching of harmful metals, organic loading, and silting (Swer and Singh 2004). AMD is a major source of water pollution in mines area primarily caused by oxidation of sulfides–pyrite, pyrrhotite, and marcasite. AMD is characterized by low pH, high sulfate, iron, aluminum, and other toxic metals (Equeenuddin et al. 2010). Mine water of East Bokaro coalfield was contaminated with a high level of iron (Mahato et al. 2017) and West Bokaro coalfield with high concentrations of iron and aluminum (Tiwari et al. 2015, 2017). Researchers also reported substantial amount of Iron and Nickel in the mine water of Raniganj coalfield (Singh et al. 2010); Iron and manganese in Jharia coalfield (Singh et al. 2012). Surface water in and around the Ib valley coalfield was reported to be polluted with Iron, Cadmium, Selenium, Nickel and Aluminum (Sahoo et al. 2021). Heavy metal pollution index is used to assess the mine water quality (Tiwari et al. 2017; Mahato et al. 2017). Mine water quality plays a very significant role in sustainable environmental practices of coal mines. Studies have been reported on Talcher coalfield regarding groundwater quality (Dhakate and Rao 2010) (Dhakate et al. 2013), surface water quality (Rizwan Reza et al. 2009)(R Reza and Singh 2010a, b), (Sahoo et al. 2016), the social impact of mining (Mishra and Das 2017) and backfilling(Sahoo and Sahu, 2020). However, studies on the spatio-temporal variation in metal pollution of surface water using index approach, multivariate statistical techniques, and treatment efficiency of Sewage Treatment Plants (STP), Effluent Treatment Plants (ETP), and Mine Discharge Treatment Plants (MDTP) are limited. Water quality is a well-known problem of mining industry and to check that, treatment facilities play a significant role. Therefore, the water quality and the efficiency of its treatment facilities are required to be studied for better management of water resources in the Talcher coalfield area. Therefore, the present study is conducted with the following objectives: (1) determine the metal pollution load of water bodies in and around eight open cast mines of Talcher coalfield using index approach; (2) recognize the natural and anthropogenic pollution sources influencing variation in mine water quality by means of principal component analysis (PCA); (3) assess the spatio-temporal variation of pH and metals in surface water across 28 locations and two seasons viz. pre-monsoon and monsoon and their compliance status as per the standards set by World Health Organization-WHO (2011), Bureau of Indian Standard-BIS (2012), United State Environmental Protection Agency-USEPA (2009), Indian Council of Medical Research-ICMR (1975) and Ministry of Environment, Forest and Climate Change-MoEF&CC (2017); (4) evaluate the treatment efficiency of STP, ETP, and MDTP located in Talcher coalfield area.
Study area
Site description
Talcher coalfield is situated in the Angul district of Odisha, India. Talcher coalfield is renowned as India's largest power grade coal reserve with 51.220 BT reserve. Talcher coalfield lies between latitude 20053′ to 21012′N and longitude 840 to 85023′E with an average elevation of 139 m above the mean sea level (MSL) (Central Mine Planning and Design Institute, 2019–2020). The coalfield's strike length in the east–west direction is around 80 km, while its width in the north–south direction is about 26 km (Central Mine Planning and Design Institute, 2019–2020). The coalfield is around 1800 square kilometres in size. The area's climate is dry, arid, and moderate to hot, with temperatures ranging from 7.60 °C to 41.90 °C and humidity levels ranging from 26 to 83 percent. The average annual rainfall is 1361.8 mm and the annual mean wind velocity is 2 km/hr. (Central Mine Planning and Design Institute, 2019–2020). The Angul-Tacher region has eight opencast coal mines, four underground mines besides having several industries such as National Thermal Power Corporation (NTPC) Kaniha, Talcher Thermal Power Station (TTPS), National Aluminum Company (NALCO) Angul, Heavy Water Project Vikrampur, Bhushan Steel and Strips Ltd., Jindal Power and Steel Ltd., and Jindal India Thermal Power Ltd. There are 2595 no. of Micro, Small & Medium Enterprises (MSME) setups in Angul district. Angul–Talcher secured the highest position acquiring the Comprehensive Environmental Pollution Index (CEPI) score of 82.09 used by the Central Pollution Control Board India for assessing the environmental pollution caused by Industrial Clusters. This made the region the most critically polluted area of Odisha (State Pollution Control Board 2020).
Drainage and hydrogeology
The two major rivers of Angul district are the Mahanadi and Brahmani rivers. The Mahanadi river basin encompasses the Athmalik subdivision as well as the southern portion of the Angul subdivision. Brahmani river spreads over the Talcher subdivision which is the study area. The Brahmani River and its major tributaries such as Tikra Jhor, Singhara Jhor, Samakoi, Nandira Jhor, Gambhira, Nigra, Bade Jhor, etc. flow through major parts of the district. Consolidated formation, Semi-consolidated formation and Unconsolidated formation constitute hydrogeology of the district.
Consolidated Formation: Granite, Granite gneiss, Charnockites, Khondalites, Quartzite, Phyllites, Mica schist, and other minerals fall into this category. There is no primary porosity in these rocks. Due to intense weathering and fracturing, secondary porosity developed in the rocks, forming a repository and passage for groundwater movement. At deeper depths, groundwater is found under water table conditions in weathered residuum and in semi-confined to confined conditions.
Semi-Consolidated Formation: It includes the Gondwana formation, which is mostly sandstone and shale and is semi-consolidated. When weathered and fractured, sandstone forms a good aquifer. Groundwater is found under water table conditions in the weathered zone and in the fracture zone under semi-confined to confined conditions.
Unconsolidated Formation: Laterite forms a cap over an older formation and groundwater is found under water table condition. The aquifer is capable of supporting a moderate yield. Alluvium is found along the banks of major rivers and streams but its occurrence is uncommon in pockets. The alluvium promotes a high yield (Central Ground Water Board, SER Bhubaneswar 2016).
Geology
Talcher coalfield constitutes south–eastern part of the lower Gondwana basins within the Mahanadi valley master basin and forms a separate basin surrounded by precambrian rocks. The soil mainly differs from rich loams to gravel detritus of the mountain slopes. The geological succession of the Talcher coalfield (Raja Rao 1982) is presented in.
Table 1.
Materials and methods
Sampling and analytical methodology
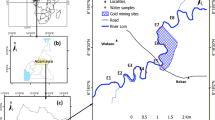
The Talcher coalfield area has been classified into 5 different areas namely Bharatpur, Jagannath, Lingaraj, Hingula, and Kaniha area. Bharatpur area has one opencast project (OCP) namely Bharatpur OCP. Jagannath area has three opencast projects namely Ananta OCP, Bhubaneswari OCP and Jagannath OCP. Lingaraj area has only one opencast project namely Lingaraj OCP. Bangaru nallah is flowing through these mines and finally meets the Brahmani river (Fig. 1). Hingula area has two opencast projects viz., Hingual OCP and Balaram OCP. Singhida jhor and Bangaru nallah flow in this area (Fig. 1). Kaniha area has one opencast project namely Kaniha OCP. Tikira nallah runs in this region. Nadira nallah also flows in the Talcher coalfield area. The sampling locations are depicted in Fig. 1.
In the present work, a total of 56 water samples, of which 28 samples were collected during pre-monsoon (April–May) and 28 samples were collected during monsoon (June–July) period in duplicate for analysis. These samples were collected from the mine sumps, inlet and outlet of ETP, inlet and outlet of STP, inlet and outlet of MDTP of Talcher coalfield area. In addition to that, water samples were also collected from the upstream and downstream (approximately 50 m from the confluence point) of streams (nallahs) and rivers where mine water is discharged to study the pollution status of these water bodies. Details of the Water Sampling locations are given in Supplementary Table S1.
Sampling and analysis of the water samples were performed as per the standard protocols and methodologies prescribed in American Public Health Association (APHA) 2012 and Bureau of Indian Standards (BIS) 1987. Grab Water samples were collected in pre-washed high-density polyethylene (HDPE) bottles of 100 ml capacity from the above-mentioned locations in duplicate and filtered with Millipore filtration unit using filter paper of 0.45 µm pore size. The samples were then preserved with 6 N ultrapure nitric acid to bring the pH down to less than two to avoid metal precipitation (Radojevic and Bashkin 1999). To ensure reliability, appropriate quality assurance procedures, precautions were followed and samples were handled with care to avoid contamination. All the reagents used for analysis were from Merck, Germany and were of analytical grade quality. Before analysis, all glasswares were acid-washed followed by twice with double distilled water and Milli Q water.
Milli Q water was used throughout the experiments. The pH was measured in the field using Systronics pH meter (Model 361) which was calibrated with pH 4.0, 7.0, and 10.0 buffers before analysis. Metals such as Manganese (Mn), Iron (Fe), Zinc (Zn), Copper (Cu), Cadmium (Cd), Lead (Pb), Cobalt (Co), Selenium (Se), Arsenic (As), Mercury (Hg), Chromium (Cr), Manganese (Mn), and Aluminum (Al) were analyzed using an atomic absorption spectrophotometer (PerkinElmer AAnalyst 200). Before the analysis of each element, its three known standards were measured. After every 5 samples, a known standard was determined to maintain analytical precision. For the entire sample, an overall precision expressed as percent relative standard deviation (RSD), was obtained below 5%.
Statistical analysis
The results were statistically processed for Analysis of variance (ANOVA), Correlation Analysis, Principal Component Analysis and Cluster Analysis using IBM SPSS statistic 2019-v26 package. The correlation among various parameters for the entire dataset had been evaluated using Pearson ‘r’ correlation coefficient at 95% and 99% confidence interval level.
Multivariate analytical methods
Factor analysis
Factor analysis is performed on the dataset using the principal component analysis (PCA). PCA is used to extract pollution factors and recognize pollution sources of water quality investigation (Ustaoglu et al. 2020). PCA describes the variance of a huge dataset of inter-correlated variables with a smaller set of independent variables. The eigen values and eigen vectors are extracted from the covariance matrix of the original variables. The principal components are uncorrelated (orthogonal) variables that are produced by the multiplication of the original correlated variables with the eigen vector, which is a set of coefficients (weightings or loadings). For maximizing the variance among the variables under each factor, PCA with varimax rotation of standardized component loadings was used, and PCs with eigen-values > 1 were retained (Wunderlin et al. 2001; Mahato et al. 2017).
Hierarchical cluster analysis
For clustering the locations in the monitoring network based on spatial similarities, hierarchical cluster analysis (HCA) was used. Hierarchical cluster analysis uses an unsupervised pattern recognition methodology to find the internal structure or underlying behavior of any dataset to classify the sites into clusters (Li et al. 2018). It generates higher clusters step by step, starting with the most comparable pair of sites, without making any prior assumptions about the data (Gu et al. 2016). All the sites were grouped in statistically significant clusters at (Dlink/Dmax) × 100 × 60 as per the similarities or differences in water quality parameter values. Ward's approach using Euclidean distance as a measure of similarity and Z-score normalization of the parameters were used. The Euclidean distance is a widely used distance coefficient that typically indicates the similarity of two samples and a distance that can be expressed by the difference in analytical values from both samples (Singh et al. 2004; Zhou et al. 2007).
Pollution indices for assessing metal pollution in water
In this study, Heavy metal pollution index (HPI), Degree of contamination (Dc) and Heavy metal evaluation index (HEI) are some of the significant indices that have been used to evaluate the quality of water.
Heavy metal pollution index (HPI) estimates the combined impact of heavy metals on overall water quality of aquatic system (Kumar et al. 2020). In order to calculate the HPI, a unit weightage (Wi) that is inversely proportionate to the recommended standard (Si) of the associated parameter is taken into account and is calculated as follows (Tiwari et al. 2015):
where \(Q_{i}\) is the ith parameter's sub-index. The ith parameter's unit weightage is \(W_{i}\), and the number of parameters examined is n. \(Q_{i}\) is calculated as:
where \(M_{i}\) is the ith parameter's monitored value, \(L_{i}\) is the ith parameter's ideal value and \(S_{i}\) is the ith parameter's standard value in ppb. The symbol (−) represents the numerical difference between the two values, ignoring the algebraic sign; the absolute value. The HPI rating for drinking water usually has a critical pollution index of 100. Any water with an HPI of greater than 100 is unfit to drink (Prasad and Bose 2001).
The degree of contamination (Dc) usually measures the quality of water (Backman et al. 1997). The Dc value is categorized into three classes: low (Dc < 1), medium (1 < Dc < 3) and high (Dc > 3) (Pobi et al. 2019). The Dc is determined as:
where \({\text{C}}_{{\text{f}}}\) is the contamination factor, \({\text{C}}_{{{\text{Ai}}}}\) is the ith parameter's analytical value, \({\text{C}}_{{{\text{Ni}}}}\) is the ith parameter’s upper permissible limit.
Heavy metal evaluation index (HEI) assesses the water quality with respect to heavy metals. The HEI is determined as (Edet and Offiong 2002):
where Hc is the ith parameter’s analytical concentration.
Hmac is the ith parameter’s maximum permissible concentration.
Pollution Indices and their mean-based reclassification method
The HPI, Dc, and HEI readings for water samples that are below the mean value or have a negative percentage deviation suggest that the water quality is comparatively better. Water quality is classified as "low" when the value is less than 100, "low-medium" when the value is between 100 and the mean value, "medium" when the value is between the mean and two times the mean value, and "high" when the value is more than two times the mean value (Prasad and Bose 2001; Edet and Offiong 2002).
Spatial distribution map
One of the best methods for visualizing spatial differences in water quality is contour mapping. The concentration contour maps of the research area were prepared using Golden Software Surfer 13.0 with kriging interpolation technique. Kriging is an autocorrelation-based statistical model. The distance or direction between the sample points is assumed to show a spatial correlation in this technique, which is used to explain variance in the surface. Kriging normally entails statistical data analysis, a variogram model, creation of a surface and optionally exploration of a variance surface.
Result and discussion
The results of the seasonal (temporal) variations of water quality parameters in surface water of the Talcher coalfield area are provided in Table 2.
pH and metal pollution
pH is a significant chemical as well as biological parameter and its fluctuation in water may influence toxicity of some other compounds (Ustaoglu and Tepe, 2019). The mine water was observed to be acidic to neutral to slightly alkaline in nature. The pH was found in the range of 5.35–8.35 with an average of 7.24 ± 0.75 in pre-monsoon and 3.87–8.28 mg/l with a mean of 7.27 ± 0.95 in monsoon. Most acidic water was found at BRSS (pH-5.35) in pre-monsoon and BRNS (pH-3.87) in monsoon. 14.28% samples in pre-monsoon and 10.71% samples in monsoon were found to be non-compliant to the drinking water standard of 6.5–8.5 prescribed by BIS (2012) and USEPA (2009) and effluent discharge standard of 6.5–9.0 set by MoEF&CC (2017). The presence of pyrite minerals in some coal patches, oxidation of organic molecules, and hydrolysis of heavy metal salts such as ferric chloride, aluminum sulphate, and manganese oxide could be responsible for low pH level (USEPA 2000; Sahoo et al. 2021).
Table 2 may be referred for seasonal (temporal) variations of metals in surface water of the Talcher coalfield area. The descending order of mean metal concentration was Al > Fe > Ni > Se > Zn > Co > Mn > As > Cu > Cd = Cr >
Hg > Pb in pre-monsoon and Fe > Al > Ni > Zn > Se > Co > Cu = Mn > As > Cr > Cd > Hg = Pb in monsoon. Mining activity, coal mine waste effluents, coal and overburden leachates and surface runoff could be the major causes of metal pollution of mine water as earlier study by Sahoo and Sahu (2020) observed that the overburden of Talcher coalfield was characterized by high concentration of Fe and Al; mild concentration of K, Ca, Mg and Na; low content of Zn, Mn, Cr and Ni; and traces of Se, As, Cd and Co. The decreasing order of heavy metals in overburden were found to be Fe > Al > K > Mg > Ca > Na > Zn > Mn > Cr > Cu > Ni > Se > Cd > Co > As > B > Pb. The decreasing order of heavy metals observed in the leachate samples extracted from overburden of Talcher coalfield were recorded as Fe > Mn > Ni > Cu > Zn > Se > Co > Cd > Cr > As (Sahoo and Sahu, 2020). pH also influences solubility of toxic metal in aquatic bodies which can affect the aquatic organism as well as human health (Aydin et al. 2021). Low pH can increase the concentration of iron, aluminum and nickel in Water (USEPA 2000).
Iron was found in the range of 0.002–0.44 mg/l with an average of 0.09 ± 0.08 mg/l in pre-monsoon and 0.02–1.31 mg/l with an average of 0.55 ± 0.45 mg/l in monsoon in Talcher coalfield. Various studies reported iron pollution was predominant in most coalfields of India (Mahato et al. 2017; Tiwari et al. 2017; Singh et al. 2012; Singh et al. 2010; Singh et al. 2017; Equeenuddin et al. 2010). 3.57% samples and 53.57% samples were found to be above the standard of 0.3 mg/l set by ICMR (1975) and BIS (2012) in pre-monsoon and monsoon, respectively. The average Iron content was 3.33 times less than the standard in pre-monsoon and 0.54 times greater than the standard in monsoon. The average Iron content of Talcher coalfield in pre-monsoon was less than East Bokaro, West Bokaro, Jharia, Raniganj, Korba, Mokum coalfields in pre-monsoon, whereas the average iron concentration reported in West Bokaro coalfield and Mokum coalfield was greater than that of Talcher coalfield in monsoon (Table 2). Iron naturally occurs in bedrock, soil, ore, and minerals. Zinc was found in the range of BDL to 0.45 mg/l with an average of 0.05 ± 0.11 mg/l in pre-monsoon and 0.01–0.45 mg/l with an average of 0.16 ± 0.12 mg/l in monsoon. Zinc concentrations at Mokum coalfield were higher than other coalfields mentioned in Table 2 but are under the standard limits. Chief zinc bearing ores are zinc carbonate known as calamine or smithsonite, sphalerite, wurtzite, smithsonite, and hemimorphite. Average zinc concentrations were observed to be well below the standard of 5 mg/l across both seasons. Copper was found in the range of below detection level to 0.059 mg/l with an average of 0.01 ± 0.01 mg/l in pre-monsoon and 0.004–0.06 with an average of 0.02 ± 0.01 mg/l in monsoon. 53.57% and 39.28% samples showed below detection level with respect to copper in pre-monsoon and monsoon seasons. Average copper concentrations were observed to be fairly below the standard of 0.05 mg/l across both seasons. Copper is a micronutrient for the living organism but in excess amount, can be accumulated in the liver. Cu is particularly more toxic to fish at lower pH. The most toxic form of Cu is Cu+. Copper and cadmium concentrations at various coalfields were quite low except for Mokum coalfield. Cadmium is found in the range of 0.001–0.007 mg/l with an average of 0.0043 ± 0.001 mg/l in pre-monsoon and 0.001‒0.007 mg/l with an average of 0.0041 ± 0.001 mg/l in monsoon. The average cadmium concentration was found to be 1.43 and 1.36 times above the standard set by WHO (2011) and BIS (2012) in pre-monsoon and monsoon. The cadmium concentration was recorded non-compliant to standards in 67.86% samples in pre-monsoon and 67.86% samples in monsoon. Cadmium occurs in greenockite (CdS) ore. Coal and overburden also contain cadmium. Cadmium can degenerate bone, dysregulate messenger ribonucleic acid (mRNA) expression, endoplasmic reticulum stress, dysregulation of Ca, Zn, and Fe homeostasis and alter phosphorylation cascades (Wang et al. 2018; Balali-Mood et al. 2021). High concentration of lead (Pb) leads to CNS injury, lungs dysfunction, hematological changes (Anemia), gastrointestinal colic, liver damage, reduce pulmonary function and dysfunction cardiovascular system (Balali-Mood et al. 2021). Lead was not detected in any location in any season in Talcher coalfield. However, lead was reported slightly higher than the standard of 0.01 mg/l in Raniganj coalfields (Singh et al. 2010) and Mokum coalfields (Equeenuddin et al. 2010). Cobalt was found in the range of 0.005 to 0.291 mg/l with an average of 0.04 ± 0.07 mg/l in pre-monsoon and 0.001–0.216 mg/l with an average of 0.03 ± 0.06 mg/l in monsoon. Cobalt was detected in all samples in both pre-monsoon and monsoon. Cobalt is a major byproduct of metallurgy of certain metals such as Cu, Ni, Fe, etc. Its exposure can result in skin irritation, ulcers, etc. Selenium concentration was recorded varied from 0.015 to 0.125 with a mean of 0.06 ± 0.026 mg/l in pre-monsoon and ranged from 0.029–0.088 mg/l with an average of 0.05 ± 0.018 mg/l in monsoon. The average selenium concentration was observed to be 6 and 5 times above the standard set by WHO and BIS in pre-monsoon and monsoon and was highest among all the coalfields (Table 2). The selenium concentration was recorded above the standard limit in all samples in both pre-monsoon and monsoon. Selenium is found in both organic and inorganic forms in coal and overburden. Selenite is a mineral found in oxidized coal. The sulfidic form of selenium is found in pyrite. Selenium in selendic type named clausthalite (PbSe) is also found in coal (Yudovich and Ketris 2005) Arsenic was found in the range of below detection level to 0.081 mg/l with an average of 0.013 ± 0.022 mg/l in pre-monsoon and 0.001–0.073 mg/l with a mean of 0.0165 ± 0.019 mg/l in monsoon. Arsenic was observed in 75% samples in pre-monsoon and 71.43% samples in monsoon. The average arsenic concentration was 0.013 mg/l and 0.016 mg/l in pre-monsoon and monsoon which were 1.3 and 1.6 times the standards and more than the concentrations reported in other coalfields (Table 2). Arsenic concentration was reported below the standards in other coalfileds. 28.57% and 21.42% samples were recorded non-confirming to the standard in pre-monsoon and monsoon. Arsenic in high concentration affects cardiovascular system and liver, damage capillary endothelium, damage skin and hair, harm central nervous system, inhibit Adenosine triphosphate (ATP) formation, discomfort GI tract and alter neurotransmitter (Balali-Mood et al. 2021). Mercury was found in the range of BDL‒0.013 (0.001 ± 0.002) mg/l in pre-monsoon and was not observed at any locations in monsoon. Mercury was present in 28.57% samples in pre-monsoon and was completely remain undetected in monsoon. In pre-monsoon, mercury average was found much below the standard of 0.01 mg/l set by BIS (2012). Mercury in excess causes organ toxicity such as central nervous system injuries, renal dysfunction, gastrointestinal ulceration, hepatotoxicity (Chen et al. 2019; Zhang et al. 2020). Mercury was not measured and reported in other coalfileds except this study that should have been measured. Chromium was found in the range of BDL‒0.03(0.004 ± 0.007) mg/l in pre-monsoon and 0.002‒0.019 (0.006 ± 0.008) mg/l in monsoon. Chromium was detected in 46.42% samples in pre-monsoon and 14.28% samples in monsoon. The average chromium concentration was recorded well below the standards across both seasons. The chromium concentrations at Talcher coalfield were at par with the chromium concentrations of East and West Bokaro coalfield but lower than the Jharia coalfield, Raniganj coalfield, Korba coalfield and Mokum coalfield. Excess chromium (Cr) has been linked to kidney failure, DNA damage, genomic instability, dermatological problems, as well as an increase in lung, throat, bladder, kidney, testicular, bone, and thyroid cancers (Deng et al. 2019; Pavesi and Moreira 2020). Nickel was observed in the range of BDL‒0.513 (0.08 ± 0.148)mg/l in pre-monsoon and 0.006‒0.61 (0.18 ± 0.19) mg/l in monsoon. Main nickel bearing ores are pentlandite, garnierite, and limonite. Ni is present in the form of Ni2 + , NiCOH2, NiS, and NiSO4 in water. The most abundant isotope is nickel-58. Nickel is required for the cells of plants and some microorganisms. Nickel in higher concentrations is become acutely toxic and a potent carcinogen. Its contact can result in a very harmful disease nickel-itch (Abdul 2014). Highest average nickel concentration was observed at Mokum coalfield followed by Talcher coalfield, West Bokaro coalfield, Raniganj coalfield, Jharia coalfield, and East Bokaro coalfield. Manganese was observed in the range of 0.01‒0.05 mg/l in pre-monsoon and 0.01‒0.05 mg/l in monsoon. The average manganese content was 0.022 mg/l and 0.020 mg/l in pre-monsoon and monsoon which were just 2.2 and 2 times higher than the standards set by BIS (2012) and ICMR (1975). 64.29% and 67.86% samples were recorded to be non-conforming to the standard in pre-monsoon and monsoon. Average manganese was found maximum at Mokum coalfiled followed by Korba coalfield, Jharia coalfield, West Bokaro coalfield, Raniganj coalfield, Talcher coalfield, and East Bokaro coalfield. Aluminum was observed in the range of 0.06‒1.1 (0.32 ± 0.21) mg/l in pre-monsoon and 0.08‒1.5 (0.36 ± 0.28) mg/l in monsoon. The average aluminum concentration was 0.32 mg/l which is 10.66 times higher than the standards set by BIS (2012) in pre-monsoon. In monsoon, the average aluminum content was 0.36 mg/l which is 12 times higher than the standards. All samples were recorded above the aluminum standard across both seasons. Coal and overburden contain aluminum and might have contributed to the rise of aluminum levels in mine water. Peak average aluminum concentration was reported at Mokum coalfield followed by Talcher coalfield, West Bokaro coalfield and East Bokaro coalfield (Table 2).
The mineral phases namely quartz (SiO2), kaolinite (Al2O3 2SiO2·2H2O), dickite (Al2Si2O5(OH)4), muscovite (KAl2(AlSi3O10(FOH)2), zinnwaldite ((Li, K, Al, Fe)3(Al, Si)4O10(F, OH)2 and illite ((K,H3O)(Al,Mg,Fe)2 (SiAl)4O10(OH)2(H2O)) are found in the overburden mainly composed of sandstone and shale materials of Kamthi, Barakar, Karharbari as well as Talchir geological formation and also lithology of recent age consists of alluvium (silt, sand, clay, gravel & organic matter) and laterite (contains the iron oxide minerals goethite(HFeO2), lepidocrocite(FeO(OH)) and hematite(Fe2O3)) at Talcher coalfield (Sahoo and Sahu, 2020). Weathering and leaching of such minerals and soil might have caused metal pollution in the mine water of Talcher coalfield.
Statistical analysis results of water quality parameters
The results of ANOVA revealed significant seasonal variation (p < 0.05) with respect to Iron during the entire sampling period, whereas no significant seasonal variation (p > 0.05) was observed in hydro-chemical parameters such as manganese, zinc, copper, cadmium, lead, cobalt, selenium, arsenic, mercury, chromium, manganese, and aluminum. Iron showed a moderate positive correlation with zinc, lead, and chromium. Zinc exhibited a moderate positive correlation with copper, lead, cobalt, arsenic, and nickel. Copper showed a moderate and low positive correlation with lead and selenium. Cadmium displayed a low positive correlation with selenium and arsenic. Cobalt showed a high and moderate positive correlation with nickel and arsenic, respectively. Selenium displayed a moderate positive correlation with mercury. Arsenic exhibited a moderate positive correlation with nickel. Manganese was having a low positive correlation with aluminum (Table 3). This shows the similar sources of contamination of water with metals in the study area. According to Dhaliwal et al. (2021), a significant positive connection among heavy metals indicates a common source and similar behavior in variation. The coal mining activities such as drilling, blasting, excavation, washing, processing, stocking, dumping, etc. are the major reasons for rise of metal concentration in water.
PCA was performed by considering the rotated varimax variables with kaiser normalization on all the water quality parameters (Table 4). Based on Eigenvalues > 1.0, five factors were extracted with the cumulative loadings showing a total variability of 76.91%. Factor 1 with eigenvalue 3.53 accounted for 26.34% of the total variance. Factor 1 showed strong positive loading for zinc, cobalt, arsenic, nickel and moderate negative loading for pH. This may be due to the earth’s crust and the geological formation of the region. Factor 2 with eigenvalue 2.46 accounted for 16.49% of the total variance. Factor 2 showed high positive loading for Iron, Copper, Manganese and may be attributed to coal mining activities. Factor 3 reported 14.39% of the total variance with eigenvalue 1.55. Cadmium, selenium, and mercury all had high positive loadings in Factor 3. Factor 4 accounted for 10.42 percent of the overall variance, with an eigenvalue of 1.39. Aluminum had a substantial positive loading in Factor 4, while selenium had a moderate positive loading. Factor 5 with eigenvalue 1.07 accounted for 9.27% of the total variance. Factor 5 showed strong positive loading for chromium. Factor 3, 4 and 5 could be attributed to industrial pollution, vehicular emission, coal burning, etc.
The dataset was subjected to hierarchical cluster analysis to cluster similar sample sites (spatial variability) across the Talcher coalfield. All 28 locations were classified into four statistically significant clusters at (Dlink/Dmax) × 100 × 60, as shown in a dendrogram (Fig. 2). The clustering method formed groups having similar characteristic features and natural background source types. Cluster 1 (19 Sites viz. JMI, LSPO, BBN, DDOM, BRNK, BDS, LS, AEO, BHSD, HSS, JEO, HDDD, JSS, HDD, JMO, BUS, BNS, KCDP, & HDDU), cluster 2 (6 Sites namely AS, BFD, BRSS, AEI, BRNS, & BRDP), cluster 3 (2 Sites such as ASO & JEI) and cluster 4 (1 site named ASI) represented moderate pollution, high pollution, very high pollution, and severe pollution, respectively.
HPI, Dc, HEI and their mean-based reclassification method
Ananta OCP STP inlet was recorded to have the highest HPI of 862.38 and outlet was observed having second best HPI of 484.08 in pre-monsoon. Further, the highest HPI of 201.17 was observed in Bharatpur OCP east Sump in the monsoon. The highest Dc was noted in the direct discharge of Hingula OCP and Bhushan fly ash dumping site at Jagannath OCP in pre-monsoon and monsoon, respectively. Maximum HEI was recorded at Bharatpur OCP east quarry and Bhushan fly ash dumping site at Jagannath OCP in pre-monsoon as well as monsoon (Table 5). Low and medium degree of pollution was observed in a total of 42.86% and 39.29% of samples in pre-monsoon and 64.29% and 0% of samples in monsoon. 64.29% and 57.12% samples in pre-monsoon, and 67.86% and 64.29% samples in monsoon had Dc and HEI indices below their mean values. Further, a total of 7.14%, 14.28%, 42.86% and 32.14%, 10.71%, 25% of the sample were recorded between the mean and 2 times of mean for HPI, Dc and HEI in pre-monsoon and monsoon, respectively. High values of HPI, Dc and HEI were observed for 3%, 6%, 0% and 1%, 6%, 3% samples in pre-monsoon and monsoon (Table 6). These observations indicated that the mine water was moderately polluted, which could be due to coal mining, industrialization, natural mineralization, etc. Kumar et al (2019) reported that Coal combustion is one of the top anthropogenic source of a number of metals in surface water.
Evaluation of performances of ETP, STP and MDTP based on metal pollution indices
STP: Ananta OCP STP inlet was having highest HPI of 862.38 and the HPI of outlet was 484.08 which showed STP was operated with an HPI removal efficiency of 43.87% in pre-monsoon, however, the degree of pollution was still high in the outlet effluent. As observed during the field visit, this could be due to mixing of mine effluent with sewage water, excessive sludge accumulation at Inlet of STP, irregularities in sludge removal, improper operation and maintenance of primary settling tank, aeration tank, secondary settling tank and filtration unit of STP. In monsoon, HPI of STP inlet and outlet were 98.62 and 91.71 recorded below the standard of 100 and mean of 163.60 implied low degree of pollution. This could be due to dilution of effluent during heavy rain but the STP was operated with a HPI removal efficiency of 7.01% in monsoon.
ETP: The HPI, Dc, and HEI of Ananta OCP EPT inlet were 188.69, 20.71, 32.71 in pre-monsoon and 165.47, 44.50, 56.50 in monsoon. The HPI, Dc, and HEI of Ananta OCP EPT outlet were 103.92, −4.90, 26.73 in pre-monsoon and 126.71, −3.55, 35.97 in monsoon. The HPI of the inlet and outlet effluent was recorded below the mean but above 100 across both seasons and hence had low-medium degree of pollution. With respect to HPI, the plant efficiency was 44.93% in pre-monsoon and 23.42% in monsoon. The low efficiency in monsoon may be due to excessive accumulation of mine effluent in the ETP outlet due to heavy rain as excess water could not be drained outside the ETP immediately due to improper drainage system. The HPI of Jagannath OCP EPT inlet was 385.70 in pre-monsoon and 80.93 in monsoon. The HPI of inlet water was higher than twice of mean and became highly polluted in pre-monsoon. The outlet HPI was 137.42 in pre-monsoon and 67.71 in monsoon which made the outlet effluent to have low-medium degree of pollution in pre-monsoon and low degree of pollution in monsoon. The efficiency of Jagannath OCP ETP was 64.37 in pre-monsoon and 16.34 in monsoon.
MDTP: The HPI, Dc, and HEI of Jagannath OCP MDTP inlet were 88.17, 7.20, 19.20 in pre-monsoon and 84.52, 12.03, 24.03 in monsoon while the outlet was having HPI, Dc and HEI of 75.04, 6.38, 18.38 in pre-monsoon and 60.91, 6.57, 18.57 in monsoon. The metal removal efficiency in terms of HPI was 14.89% in pre-monsoon and 27.93% in monsoon.
Impact of mine effluent on Stream and River
To study the impact of mine discharge on the surrounding stream and river, water samples were collected from Singhada Jhor upstream, Singhada Jhor downstream near Hingula OCP, Banguru Nallah upstream, Banguru Nallah downstream, Bangaru nallah near Balaram OCP and Brahmani river near NTPC and Kaniha OCP. From the analytical results, it was observed that HPI, Dc and HEI of Singhada Jhor upstream were 40.86, −4.18, 7.82 in pre-monsoon and 42.94, −3.73, 8.27 in monsoon. The HPI, Dc and HEI of Singhada Jhor downstream were observed to be higher than the upstream water and recorded as 117.48, 15.69, and 27.69 in pre-monsoon which made it unfit for drinking without proper treatment. The HPI, Dc and HEI of Singhada Jhor downstream were observed to be less than pre-monsoon and noted as 97.94, 9.70 and 21.70 in monsoon. This indicated mine effluent may have deteriorated the Singhada jhor water quality. Furthermore, the HPI of Banguru nallah upstream was found less than its corresponding downstream across both seasons indicating metal pollution due to mixing of mine effluent. The HPI of Bangur nallah near Balaram OCP in pre-monsoon was higher than the monsoon season but remained less than 100, which made it slightly less polluted. All the streams or nallahs are connected with river Brahmani, which is the major source of water in the talcher area. The HPI of river Brahmani was found 99.10 in pre-monsoon and 98.04 in monsoon. The water quality of Brahmani river has been a matter of concern and should be protected from mine effluents as people directly depend upon it for drinking water, agriculture, plantation, etc. The values of indices displayed great seasonality for the streams as well as river and found higher in pre-monsoon than that of monsoon. This may be due to high evaporation rate, intense mining and agriculture activities in pre-monsoon season and dilution effect due to heavy rain in monsoon (Olias et al. 2004; Tiwari et al. 2015). The concentration contour maps for some of the important parameters viz. pH, Fe, Se, Mn, Ni, and Al have been presented in Figs. 3 and 4.
Conclusion
The mine water was acidic to neutral to slightly alkaline in nature. 14.28% samples in pre-monsoon and 10.71% samples in monsoon were found to be non-compliant to the standard of 6.5 to 8.5 prescribed by BIS (2012) and USEPA (2009). The descending order of mean of metal concentration was Al > Fe > Ni > Se > Zn > Co > Mn > As >
Cu > Cd = Cr > Hg > Pb in pre-monsoon and Fe > Al > Ni > Zn > Se > Co > Cu = Mn > As > Cr > Cd > Hg = Pb in monsoon. Iron concentration in 3.57% samples and 53.57% samples were found above the standard in pre-monsoon and monsoon, respectively. Aluminum was observed above the standard in all samples across both seasons and its average concentration was 10.66 and 12 times higher than the standards in pre-monsoon as well as monsoon. The selenium concentration was recorded above the standard limit in all samples in both pre-monsoon and monsoon. Nickle was found above the standards in 64.29% and 67.86% samples. STP, ETP, and MDTP were found to have low to moderate efficiency in treating metals. The HPI of downstream of Singhada Jhor, Bangur Nallah, were noted to be higher than the upstream indicating pollution due to mine effluent discharge. The HPI of river Brahmani was found to be 99.10 in pre-monsoon and 98.04 in monsoon and hence vulnerable to pollution. The HPI of streams and river were observed higher in pre-monsoon than the monsoon season. The average concentrations of Cd, Se, As, Ni, Al in pre-monsoon and Fe, Cd, Se, As, Ni, Al in monsoon exceeded the permissible drinking water limits set by WHO (2011) and BIS (2012). Analysis of Variance (ANOVA) revealed significant seasonal variation (p < 0.05) of Iron concentration between pre-monsoon and monsoon. Five variables with Eigenvalues > 1.0 were found by Principal Component Analysis (PCA) and explained 76.91 percent of the total variance in the entire system. Four different clusters were identified by Cluster analysis (CA). Cluster 1 corresponded to nineteen moderately polluted sites, cluster 2 grouped six highly polluted sites, cluster 3 accounted for two very highly polluted sites and cluster 4 indicated one severely polluted site in Talcher coalfield area. High values of HPI, Dc and HEI were observed just for 3%, 6%, 0% samples in pre-monsoon and 1%, 6%, 3% samples in monsoon indicated high degree of pollution. Further, 7.14%, 14.28%, 42.86% and 32.14%, 10.71%, 25% of the samples were recorded between the mean and 2 times of the mean for HPI, Dc, and HEI in pre-monsoon and monsoon showed moderate degree of pollution. Thus, it can be concluded that surface water of Talcher coalfield area requires appropriate treatment before it may be used for drinking, household use, or even discharge into the land surface. The balanced and sustainable water quality in the area can be achieved by upgrading the current treatment facilities as well as adopting sustainable mining practices with regular monitoring by pollution control boards. More water quality studies on adjacent area and research on groundwater quality which are beyond the scope of the current work are required for better management of the water resources of the entire Talcher area.
References
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington, DC
Abdul AM (2014) Analysis of environmental pollution in Sundarbans. Am J Biomed Life Sci 2(5):98–107
Ali H, Khan E (2018) Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ Chem Lett 16(3):903–917
Ali H, Khan E (2019) Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/websdconcepts and implications for wildlife and human health. Hum Ecol Risk Assess. 25(1353):e1376
Ali H, Khan E, Ilahi, I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem
Aydin H, Ustaoğlu F, Tepe Y, Soylu EN (2021) Assessment of water quality of streams in northeast Turkey by water quality index and multiple statistical methods. Environ Forensics 22(1–2):270–287
BIS (1987) Bureau of Indian standard, Method of sampling and test (IS: 3025), (Physical and Chemical) for water and wastewater
BIS (2012) Drinking water specifications 2nd revision. Bureau of Indian standards (IS 10500: 2012). New Delhi. ftp://law.resource.org/in/bis/S06/is.10500.2012.pdf
Backman B, Bodis D, Lahermo P, Rajpant S, Tarvainen T (1997) Application of ground water contamination index in Finland and Slovakia. Environ Geol 36:55–64
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:643972
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):04691
Censi PA, Spoto SE, Saiano FI, Sprovieri M, Mazzola S, Nardone G, Ottonello D (2006) Heavy metals in coastal water systems. A case study from the northwestern Gulf of Thailand. Chemosphere 64(7):1167–1176
Central Ground Water Board, South Eastern Region Bhubaneswar (2016) Report on national aquifer mapping and management plan, parts of Angul District, Odisha. Ministry of Water Resources, River development and Ganga Rejuvenation government of India 1–64
Central Mine Planning and Design Institute (2019–2020) Annual environmental monitoring report, talcher coalfield, mahanadi coalfield limited 1–402
Central Statistics office (2019) Report on Energy Statistics. Ministry of statistics and programme implementation, Government of India 1–123
Chen R, Xu Y, Xu C, Shu Y, Ma S, Lu C (2019) Associations between mercury exposure and the risk of nonalcoholic fatty liver disease (NAFLD) in US adolescents. Environ Sci Pollut Res 26(30):31384–31391. https://doi.org/10.1007/s11356-019-06224-5
Deng Y, Wang M, Tian T, Lin S, Xu P, Zhou L (2019) The effect of hexavalent chromium on the incidence and mortality of human cancers: a meta-analysis based on published epidemiological cohort studies. Front Oncol 9:24. https://doi.org/10.3389/fonc.2019.00024
Dhakate R, Mahesh J, Sankaran S, Rao VVSG (2013) Multivariate statistical analysis for assessment of groundwater quality in Talcher coalfield area, Odisha. J Geol Soc India 82(4):403–412
Dhakate R, Rao TG (2010) Assessment of groundwater quality in Talcher coalfield area, Orissa. India Intern J Earth Sci Engg 3(1):43–55
Dhaliwal SS, Setia R, Kumar V, Ghosh T, Taneja S, Singh R, Ansari J, Kukal SS, Pateriya B (2021) Assessment of seasonal variations and human health risks due to heavy metals in water, soils and food crops using multi-indices approach. Environ Earth Sci 80(11):1–1
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo Odukpani area, lower cross river Basin (southeastern Nigeria). GeoJournal 57(4):295–304
Equeenuddin SM, Tripathy S, Sahoo PK, Panigrahi MK (2010) Hydrogeochemical characteristics of acid mine drainage and water pollution at makum coalfield. India J Geochem Explor 105(3):75–82
Gu F, Hall P, Miles NJ (2016) Performance evaluation for composites based on recycled polypropylene using principal component analysis and cluster analysis. J Clean Prod 115(343):e353. https://doi.org/10.1016/j.jclepro.2015.12.062
ICMR (1975) Indian Council of Medical Research, Manual of Standards of Quality for Drinking water, Indian Council of Medical Research
Jain CK, Bandhyopadhyay A, Bhadra A (2010) Assessment of groundwater quality for drinking purpose, district Nainital, Uttarakhand, India. Environ Monit Assess 166:663–676
Kumar V, Parihar RD, Sharma A, Bakshi P, Sidhu GP, Bali AS, Karaouzas I, Bhardwaj R, Thukral AK, Gyasi-Agyei Y, Rodrigo-Comino J (2019) Global evaluation of heavy metal content in surface water bodies: a meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 236:124364
Kumar V, Sharma A, Kumar R, Bhardwaj R, Kumar Thukral A, Rodrigo-Comino J (2020) Assessment of heavy metal pollution in three different Indian water bodies by combination of multivariate analysis and water pollution indices. Hum Ecol Risk Assess Int J 26(1):1–6
Li T, Li S, Liang C, Bush RT, Xiong L, Jiang Y (2018) A comparative assessment of Australia’s Lower Lakes water quality under extreme drought and post-drought conditions using multivariate statistical techniques. J Clean Prod 190:1–11. https://doi.org/10.1016/j.jclepro.2018.04.121
Loska K, Wiechuła D, Korus I (2004) Metal contamination of farming soils affected by industry. Environ Int 30(2):159–165
Mahato MK, Singh G, Singh PK, Singh AK, Tiwari AK (2017) Assessment of mine water quality using heavy metal pollution index in a coal mining area of Damodar River Basin, India. Bull Environ Contam Toxicol 99(1):54–61
Mishra N, Das N (2017) Coal mining and local environment: a study in Talcher coalfield of India. Air Soil Water Res 10:1178622117728913
MoEF&CC (2017) Ministry of Environment, Forest and Climate Change, Discharge standard for sewage (effluent), Government of India
Monchanin C, Devaud JM, Barron AB, Lihoreau M (2021) Current permissible levels of metal pollutants harm terrestrial invertebrates. Sci Total Environ 779:146398
Olias M, Nieto JM, Sarmiento AM, Ceron JC, Canovas CR (2004) Seasonal water quality variations in a river affected by acid mine drainage: the Odiel River (South West Spain). Sci Total Environ 333:267–281
Pavesi T, Moreira JC (2020) Mechanisms and individuality in chromium toxicity in humans. J Appl Toxicol 40:1183–1197. https://doi.org/10.1002/jat.3965
Pobi KK, Satpati S, Dutta S, Nayek S, Saha RN, Gupta S (2019) Sources evaluation and ecological risk assessment of heavy metals accumulated within a natural stream of Durgapur industrial zone, India, by using multivariate analysis and pollution indices. Appl Water Sci 9(3):1–6
Prasad B, Bose JM (2001) Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environ Geol 41:183–188
Radojevic M, Bashkin VN (1999) Practical environmental analysis. Royal Chemical Sc Publications, London, pp 154–155
Raja Rao CS (1982) Coalfield of India. Coal resouerces of Tamilnadu, Andhra Pradesh, Orissa, Maharastra. Bull Geol Surv India Ser A 45:1–101
Reza R, Jain MK, Singh G (2009) Impact of mining activities on surface water quality in Angul-Talcher region of Orissa. India Min Eng J 10(11):22–28
Reza R, Singh G (2010a) Heavy metal contamination and its indexing approach for river water. Int J Environ Sci Technol 7(4):785–792
Reza R, Singh G (2010b) Impact of industrial development on surface water resources in Angul region of Orissa. Int J Environ Sci 1(4):514–522
Sahoo M, Mahananda MR, Seth P (2016) Physico-chemical analysis of surface and groundwater around Talcher Coal field. J Geosci Environ Prot 4:26–37
Sahoo BP, Sahu HB (2020) Assessment and characterization of mine waste and fly ash material for effective utilization in opencast coal mines. Int J Recent Technol Eng 9(1):2490–2500. https://doi.org/10.35940/ijrte.A1985.059120
Sahoo BP, Sahu HB, Pradhan DS (2021) Hydrogeochemistry and surface water quality assessment of IB valley coalfield area. India Appl Water Sci 11(9):1–27
Sahu C, Basti S (2021) Trace metal pollution in the environment: a review. Int J Environ Sci Technol 18:211–224
Setia R, Dhaliwal SS, Kumar V, Singh R, Kukal SS, Pateriya B (2020) Impact assessment of metal contamination in surface water of Sutlej River (India) on human health risks. Environ Pollut 1(265):114907
Singh AK, Mahato MK, Neogi B, Mondal GC, Singh TB (2011) Hydrogeochemistry, elemental flux, and quality assessment of mine water in the Pootkee-Balihari mining area, Jharia coalfield. India Mine Water Environ 30(3):197–207
Singh AK, Mahato MK, Neogi B, Singh KK (2010) Quality assessment of mine water in the Raniganj coalfield area. India Mine Water Environ 29(4):248–262
Singh AK, Mahato MK, Neogi B, Tewary BK, Sinha A (2012) Environmental geochemistry and quality assessment of mine water of Jharia coalfield. India Environ Earth Sci 65(1):49–65
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Res 38(18):3980–3992
Singh R, Venkatesh AS, Syed TH, Reddy AG, Kumar M, Kurakalva RM (2017) Assessment of potentially toxic trace elements contamination in groundwater resources of the coal mining area of the Korba Coalfield, Central India. Environ Earth Sci. 76(16):1–7. https://doi.org/10.1007/s12665-017-6899-8
State Pollution Control Board (2020) Action plan for abatement of pollution in Ib Valley. Odisha, India, pp 1–16
Swer S, Singh OP (2004) Status of water quality in coal mining areas of Meghalaya, India. In Proceedings of the national seminar on environmental engineering with special emphasis on mining environment, 19–20
Tiwari AK, De Maio M, Singh PK, Mahato MK (2015) Evaluation of surface water quality by using GIS and a heavy metal pollution index (HPI) model in a coal mining area, India. Bull Environ Contam Toxicol 95(3):304–310
Tiwari AK, Singh PK, Mahato MK (2017) Assessment of metal contamination in the mine water of the West Bokaro Coalfield. India Mine Water Environ 36(4):532–541
Ujvari B, Roche B, Thomas F (eds) (2017) Ecology and evolution of Cancer. Academic Press, Elsevier
USEPA (2000) Drinking water standards and health advisories. United State Environmental Protection Agency, Washington DC
USEPA (2009) National primary drinking water regulations federal register, EPA816-F-09-004. United State Environmental Protection Agency, Washington, DC
Ustaoglu F, Tepe Y (2019) Water quality and sediment contamination assessment of Pazarsuyu Stream, Turkey using multivariate statistical methods and pollution indicators. Int Soil Water Conserv Res 7(1):47–56
Ustaoglu F, Tepe Y, Taş B (2020) Assessment of stream quality and health risk in a subtropical Turkey river system: a combined approach using statistical analysis and water quality index. Ecol Ind 113:105815
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
Wang Y, Mandal AK, Son YO, Pratheeshkumar P, Wise JTF, Wang L (2018) Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol Appl Pharmacol 353:23–30. https://doi.org/10.1016/j.taap.2018.06.003
Wunderlin DA, Dias MP, Amemaria V, Pesce SF, Hued AC, Bistoni MA (2001) Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: suquia river basin Cordoba-Argentina. Water Res 35(12):2881–2894
Yudovich YE, Ketris MP (2005) Selenium in coal: a review. Int J Coal Geol 61(3–4):141–196. https://doi.org/10.1016/j.coal.2004.09.003
Zhang C, Gan C, Ding L, Xiong M, Zhang A, Li P (2020) Maternal inorganic mercury exposure and renal effects in the Wanshan mercury mining area, southwest China. Ecotox Environ Safe 189:109987. https://doi.org/10.1016/j.ecoenv.2019.109987
Zhou F, Liu Y, Guo H (2007) Application of multivariate statistical methods to water quality assessment of the watercourses in North-western New Territories, Hong Kong. Environ Monit Assess 132:1–13. https://doi.org/10.1007/s10661-006-9497
Acknowledgements
The authors thankfully acknowledge the Director, National Institute of Technology, Rourkela, for providing the necessary facilities for this research. The authors thank Prof. Kakoli Karar(Paul), Associate Professor, Department of Civil Engineering, NIT Rourkela; Prof. R. K. Patel, Professor, Department of Chemistry, NIT Rourkela; for their assistance in conducting the research. We also appreciate the support provided by the Talcher coalfield authorities in carrying out the study.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sahoo, B.P., Sahu, H.B. Assessment of metal pollution in surface water using pollution indices and multivariate statistics: a case study of Talcher coalfield area, India. Appl Water Sci 12, 223 (2022). https://doi.org/10.1007/s13201-022-01743-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01743-y