Abstract
The continuous generation of waste resulting from the industrial activities of humans has significantly been on the rise, especially liquid wastes emanating from cassava processing mills, which is a major cause for concern in developing countries. This study focused on the preparation of H3PO4 activated periwinkle carbon (APSC) and use in the removal of cyanide in cassava wastewater. The influence of variables such as pH, adsorbent dosage, contact time, and different cyanide concentrations was investigated in batch procedures. Results from the batch studies reveal a strong pH-dependent adsorption process with optimum cyanide removal occurring at pH 8. An equilibrium time of 80 min and adsorbent dosage of 3.0 g gave the highest percentage of cyanide adsorbed at 83.93%. The Pseudo-first-order, Pseudo-second-order, and Elovich kinetic models were used for the analysis of experimental data while equilibrium data analysis using Langmuir, Freundlich, and Redlich–Peterson was carried out to determine the best-fit isotherm model. The Coefficient of determination (r2), Sum of square error (SSE), and Chi-square (\(\chi^{2}\)) were used to estimate the error deviations between the predicted and the experimental models using nonlinear regression analysis to determine models that best explain the adsorption process. Kinetic data fitted well to the Elovich and Pseudo-second-order kinetic model which implies chemisorption as the dominant adsorption process. The Redlich–Peterson and Langmuir model best describes the adsorption process suggesting mono-layer adsorption with the monolayer adsorption capacity of APSC found to be 2.856 mg.g−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cassava (Manihot esculenta Crantz) an edible starchy root crop, is grown in different parts of Sub-Saharan Africa, Central and South America, and Southeastern Asia. It is a staple food source consumed by close to 500 million people (Wade et al. 2002). The cultivation and processing of cassava most especially in Africa are encouraged in rural areas to provide food security for the rapidly growing population as well as income to farmers and foreign exchange-earners for regional governments (Okunade and Adekalu 2013; Eletta et al. 2016). Nigeria accounts for 19% of total cassava production worldwide at 45 million tonnes per annum making it the world’s leading producer of cassava (Obueh and Odesiri-Eruteyan 2016). In Nigeria cassava is processed into a variety of products such as garri and fufu which are produced from the fermentation of cassava and are rich sources of calories high in starch content. The numerous uses of cassava industrially include its use as a binder, paper production and plywood, monosodium glutamate, and sweeteners used in cooking, ethanol as well as animal feed (Okunade and Adekalu 2013; Onyenwoke and Simonyan 2014).
Unit operations such as washing, cutting, soaking, and fermentation carried out in cassava processing usually generate lots of liquid and solid wastes (Lawal et al. 2019). These activities lead to the discharge of large quantities of cyanogenic glucoside and other by-products such as hydrogen cyanide and cyanohydrin. These by-products are toxic and a cause for concern as they adversely affect soil chemistry, soil organisms, and plants when disposed of indiscriminately on land. Also, the disposal of cassava wastewater effluent into water bodies is known to affect aquatic life negatively with 50% cases of the mortality rate in fishes exposed to cassava wastewater effluent with cyanide concentrations as low as 0.95 mg.L−1 in stagnant water bodies (Wade et al. 2002).
The chemical compound cyanide consists of an atom of carbon triple bonded to one nitrogen atom. Despite its toxicity, it is found in different life forms such as certain bacteria, algae, and fungi, insects like centipedes, beetles, and certain species of butterflies. It also occurs naturally in foods such as cashew nuts, lima beans, and certain root tubers such as cassava and coco-yam (Dash et al. 2008, 2009). The concentration of cyanide per 100 g of plant tissue increases can be as high as 104 mg (Kuyucak and Akcil 2013). Although, this may depend on various factors such as; the plant’s age, the cassava cultivar, harvest period, etc. (Oliveira et al. 2001). The presence of both complex and simple forms of cyanide, each exhibiting varying levels of stability and toxicity presents a problem with detrimental effects on the environment and health.
Several researchers have studied the use of micro-organisms such as certain bacteria (biotreatment) for cyanide degradation in cassava wastewater (Kaewkannetra et al. 2009). Also, chemical degradation making use of alkalies such as sodium hydroxide and caustic hydrogen peroxide has been used to treat effluent from cassava processing industries which convert the main pollutant into cyanate (Ugwu and Agunwamba 2012; Omotosho and Amori 2015). These methods, however, are plagued by certain setbacks which include requiring specialized skills to fully implement along with cost implications. However, adsorption continues to be widely used as a result of its numerous applications in removing pollutants, ease, and relatively cheaper cost (Eletta et al. 2016).
Periwinkle shells are hard outer casings usually 1.3–2.5 cm long (Bello and Ahmad 2011), with color variations ranging from dark brown to black and small protrusions usually discarded after consuming the tiny small greenish-blue marine sea snails which they house. The discarded shells are commonly found all over the Niger-Delta area in the Southern parts of Nigeria (Obot et al. 2015) where they have been utilized as concrete aggregates for construction in local communities and landfills (Etuk et al. 2012; Ettu et al. 2013). However, large quantities of these shells are still disposed of as waste littered in dumpsites and markets which become an environmental nuisance especially from the accumulation of large deposits over long periods. Activated carbon produced from periwinkle shells through acid activation has been reported to alter and enhance quite considerably adsorbent properties such as the pore distribution, adsorbent surface area, and functional groups (Babayemi et al. 2017). The use of phosphate-containing compounds such as phosphoric acid (H3PO4) in wastewater treatment and adsorbent modifications has proven to be quite effective in enhancing the adsorptive properties of carbon-based adsorbents (Naeem et al. 2019). Also, the availability of the precursor periwinkle shells at little or no cost, along with its reported use as activated carbon in the treatment of wastewater is considered environmentally sustainable as the degradation of the environment is reduced (Owabor and Iyaomolere 2013; Nworie et al. 2020), hence making it a suitable material for use in eliminating organic chemicals and heavy metals.
Research on the adsorption of cyanide found in the effluents from cassava processing is quite limited, and there is the nuisance of discarded periwinkle shells littered all over the environment; therefore, this study was focused on evaluating the treatment by adsorption of cyanide present in cassava wastewater making use of acid-activated periwinkle shell carbon. The functional groups present in the prepared activated were investigated using the Fourier Transform Infrared Spectroscopy (FTIR) analysis to get some insight into how these groups could inherently affect the adsorption process. The influence of adsorption variables such as pH of the aqueous solution, contact time, adsorbent dosage, and initial cyanide concentration in the adsorption process was evaluated to determine optimum adsorption conditions. The experimental data was systematically studied and fitted to various kinetic and isotherm models.
Materials and methods
Materials
The periwinkle shell waste used in this study was sourced from a dumpsite in Uyo, Nigeria. The bitter cassava variety used in the study (TMS98/0581) was sourced from the Research Institute of Root Crops in Umudike, Nigeria. Chemicals used were analytical grade and products of Sigma-Aldrich, Germany, with no required need for additional purification.
Pre-treatment of periwinkle shell
The periwinkle shells were soaked in hot water for 2 h to remove any remnant flesh of periwinkle along with dirt and thereafter washed thoroughly using distilled water. The shells were subsequently sundried for 48 h. The carbonization of the sundried shells was done in a Muffle furnace set at a temperature of 600 °C for 2 h in a limited supply of oxygen and at the end of completion time allowed to cool down to room temperature. A hammer mill was used to pulverize the shells into powder and sieved to the required particle size using a Tyler sieve of mesh size 100 µm to maintain adsorbent uniformity.
Acid activation of periwinkle shell
The acid impregnation of the carbonized powdered periwinkle shell using H3PO4 was done following a slightly modified procedure to the method outlined by Verla et al (2012). A 200 g carbonized sample was immersed in 200 ml of H3PO4 of 0.5 Molar concentration for 24 h. This is to allow sufficient time for the surface pores to be properly activated. The sample was then heated using a burner to form a paste that was subsequently introduced into a Muffle furnace pre-set at a temperature of 700 °C and maintained for 4 h. The activated periwinkle shell carbon was allowed to cool down to ambient temperature and rinsed properly with distilled water until an almost neutral pH was observed. Drying of the wet activated sample was carried out in an oven at 100 °C for 12 h, then subsequently placed in a desiccator to cool down.
Characterization of adsorbent (Activated periwinkle shell carbon)
The activated periwinkle shell carbon was characterized for the presence of surface functional groups using Fourier transform infra-red spectrophotometer (Fisher Thermoscientific, USA) at adsorption spectral range of 500–4000 cm−1. The activated periwinkle shell carbon was subjected to proximate analysis to determine physical properties such as ash content, moisture content, and pH using standard ASTM procedures as outlined in previous work (Nnanna and Etuk. 2019). The method outlined by Gumus and Okpeku (2015) was used to determine the bulk density of the activated sample. The specific surface area was determined using the Sears method (Nwabanne and Igbokwe 2012).
Preparation and characterization of cassava wastewater sample
The cassava was peeled, washed, crushed to a pulp, and allowed to soak in water overnight which is the normal practice in cassava processing. The cassava pulp was then squeezed and sieved out leaving the remaining filtrate added to the water used to wash the peeled tubers. Subsequently, the cassava wastewater sample was stored in an opaque container and refrigerated in the laboratory to slow down microbial activity. The cyanide content of the cassava wastewater was analyzed using the alkaline picrate method as described by Eke-emezie and Etuk (2019) with slight modifications. 2 ml of the sample cassava wastewater was mixed with 2 ml of alkaline picrate solution. The solution was incubated in a water bath for 10 min at 60 °C. The absorbance of the resulting deep orange color solution formed was measured at 490 nm using a U-V Spectrophotometer (Jenway 73). Cyanide concentration was extrapolated from an already prepared potassium cyanide curve used for reference. The pH of the simulated cassava mill wastewater was determined using a digital pH meter (Model 3510 Jenway).
Adsorption experiment conditions
The adsorption experiments were conducted in batches using 500 ml Erlenmeyer flasks; 50 ml volume of cassava wastewater sample was placed in the flasks at room temperature of 25 °C and agitation was achieved using an orbital shaker set at 150 rpm rotation speed. The variables in the batch adsorption experiments; activated periwinkle shell carbon (2–4 g/l), pH (2–12), contact time (20–120 min), and initial cyanide concentration (40–120 mg/l) were investigated. Adjustments were made to pH by using 0.1 M of HCL and NaOH. Initial cyanide concentrations were prepared from a stock solution of potassium cyanide by dilution. To study individual parameters, other parameters were kept constant while the specific parameter under investigation was adjusted accordingly. The residual cyanide concentration in the filtered solution at equilibrium was determined using a U-V Spectrophotometer at 490 nm. Readings were taken in duplicates and the results were recorded.
Experimental analysis
The adsorption capacity is calculated using Eq. 1:
Here, qe is the adsorption capacity per unit mass of activated periwinkle shell in milligram per gram (mg.g−1), Co and Ce are the initial and final concentration of cyanide, respectively, in milligrams per liter (mg.L−1), the volume of the cassava wastewater V is in milliliters (ml) and M is the quantity of activated periwinkle shell adsorbent used in each experiment in grams (g).
The removal efficiency usually expressed as percentage is given by Eq. 2:
Kinetic analysis of the adsorption experiments was conducted at two initial potassium cyanide concentrations (80, and 100 mg.L−1) using an adsorbent dose of 3.0 g. At different time intervals sampling was carried out to determine the equilibrium concentration of cyanide.
Kinetic modeling
The analysis of adsorption kinetics helps in selecting optimum conditions for subsequent use in a full-scale batch study where the parameters provide information for design and modeling. The Pseudo-first-order, Pseudo-second-order, and Elovich models were applied to determine kinetic data from the experiment for cyanide adsorption. The nonlinear expression of the pseudo-first-order kinetic model is presented in Eq. 3:
The pseudo-second-order kinetic model shown in its nonlinear form in Eq. 4:
where, k1(min−1) and k2 (g.mg−1.min−1) are the pseudo-first-order and pseudo-second-order adsorption rate constant, respectively, qe (mg.g−1) and qt (mg.g−1) is the quantity of cyanide adsorbed on the APSC adsorbent at equilibrium and at time “t” (min), respectively, t is the time (min).
The empirical form of the Elovich kinetic model is shown in Eq. 5
where the parameter β is related to the activation energy and extent of surface coverage for chemisorption (g.mg−1) and α is the initial sorption rate (mg.g−1.min−1).
Adsorption isotherms
Three adsorption isotherm models were used to analyze the adsorption of cyanide onto the activated periwinkle shell carbon. The models used for adsorption data analysis in this study include the Langmuir, Freundlich, and Redlich–Peterson models. For decades adsorption isotherm models have been used to give insights into adsorption mechanisms revealing the effects of the relationship between the adsorbates and adsorbent. The Langmuir equation is expressed as Eq. 6 (Lach 2019):
where qe (mg.g−1) is the amount of cyanide adsorbed at equilibrium, qm (mg.g−1) is the maximum adsorption capacity, Ce (mg.L−1) is the equilibrium cyanide concentration in solution.
The dimensionless parameter known as the separation factor can be used to further validate the favorability of a Langmuir type process as is expressed in Eq. 7 (Langmuir 1918):
Here KL is the Langmuir isotherm constant, Co is the adsorbate initial concentration. The RL values are an indication of the favorability of the adsorption process and shape of the isotherm shape with values greater than 1 signifying the adsorption process is poor.
The Freundlich isotherm can be expressed in Eq. 8 (Hameed 2009) as:
where KF (mg.g−1) is the Freundlich constant, 1/n is the constant related to adsorption intensity.
The Redlich–Peterson isotherm model is a hybrid between the Langmuir and Freundlich models that incorporates three parameters in the empirical isotherm equation which can be utilized either for homogeneous or heterogeneous surfaces. The Redlich–Peterson isotherm is expressed in Eq. 8 (Foo and Hameed 2010) as:
where KR (L.mg−1) and αR (L.mg−1) are Redlich–Peterson constants, g is the exponent lying between 0 and 1.
Nonlinear regression analysis
The model parameters for the kinetic and isotherm models were determined using nonlinear regression analysis. Contrary to the usual linearization of model equations, nonlinear regression involves the maximization or minimization of error distribution between the experimental data and predicted model based on a convergence criterion which ultimately gives results with greater accuracy for model parameters (Can 2014). The nonlinear regression analysis was carried out to determine model parameters by minimizing the error function using the Solver add-in function in the Microsoft Excel spreadsheet. An in-depth explanation of designing and using Excel spreadsheet for nonlinear regression analysis is outlined from previous work (Brown 2001).
Error function analysis
Error functions are generally used to determine the deviation of the experimental data values from the predicted data. To further validate the best fit kinetic and adsorption isotherm models three error functions were used namely; the sum of squared errors (SSE), chi-square errors (\(\chi\) 2), and the coefficient of determination (r2) expressed in Eqs. 10, 11, and 12, respectively.
where, \(q_{e.\exp }\)(mg.g) is the equilibrium capacity obtained from the experiment, \(q_{e.cal}\)(mg.g) is the equilibrium capacity obtained from the model, \(\overline{{q_{e.\exp } }}\) is the mean of \(q_{e.\exp }\) values. The best fit models will have values of r2 close to 1 and the lowest values of \(\chi^{2}\) and SSE.
Results and discussion
Cassava wastewater
Properties of the simulated cassava wastewater are presented in Table 1. The pH value of the wastewater sample is seen to be acidic and within the expected range of 3.8–5.7 from previous studies (Kaewkannetra et al. 2009). A reason for the acidity could be as a result of the presence of prussic acid (HCN) in the wastewater. Cyanide concentration at 80 mg.L−1 is significantly high, probably due to the bitter variety of cassava specifically used for this study. The acidic nature and high cyanide concentration exceed recommended limits required by WHO for the discharge of effluent wastewater-bearing cyanide into natural water bodies.
Characterization of acid-activated periwinkle shell carbon
The proximate analysis of the produced activated periwinkle shell carbon is reported in Table 2. As seen in Table 2, a low ash content of < 15% indicates that the adsorbent produced is porous given that adsorbent strength and adsorptive capacity can be significantly hindered by high ash content (Sabino et al. 2016). The low moisture content of 5.2% suggests a carefully prepared and properly handled activated carbon. The moisture content in no way affects the adsorptive capacity of the adsorbent although, an extremely high moisture content demands the additional use of more carbon weight during treatment processes. The pH of the activated periwinkle shell is seen to be within acceptable limits of pH 6–8 for use in the treatment of wastewater.
FTIR analysis of activated periwinkle shell carbon
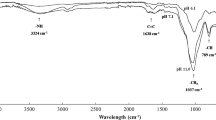
The change in surface functional groups and structure of the activated periwinkle shell carbon determined through FTIR analysis are presented in supplementary materials (Table SM1). The presence of oxygen functional groups generally influences the surface properties of an adsorbent (Babayemi et al. 2017). Broadband absorption peak seen at 3353.93 cm−1 indicates an O–H stretch for alcohol and phenol due to water adsorption which reveals the presence of free hydroxyl groups (Bello and Ahmad 2011). The peak at 2942.42 cm−1 indicates the alkane stretch of C-H (Bello and Ahmad 2011). Peaks prominent at 1740.00 cm−1 and 1642.74 cm−1 are assigned to the C=O stretch of esters, ketones, and amides indicating the presence of carbonyl groups (Nworie et al. 2020). Silanol (Organosilicon) groups with Si–O bonds are indicated by the wave numbers 930.91 cm−1 and 872.64 cm−1. The broadband at 1150 cm−1 and 1078.69 cm−1 is indicative of the C-O stretch of alcohols and C–O–C bond assigned to ethers. Peaks located at 765.44 cm−1 and 710.65 cm−1, respectively, indicate the C-H out-of-plane bend of aromatic compounds which are commonly found on the surface of several activated carbons (Nworie et al. 2020; Islam et al. 2016). The visible presence of polar functional groups (carboxylic acid, primary amines, amide, hydroxyl, and phenolic groups) has been reported to enhance quite considerably the adsorptive capacity of periwinkle shell (Okuo. 2006). Other notable absorption peaks are summarized in supplementary materials (Table SM1).
The influence of PH on the removal of cyanide
The influence of pH as it affects the adsorption process of cyanide in cassava wastewater is shown in Fig. 1. The surface charges present on adsorbent surfaces are known to be influenced by the pH of the solution hence could have a significant effect on the adsorption process. Also, the pH of the solution can have a significant influence on the ionization of the contaminants present in the solution (Naveen et al. 2016). The percentage of cyanide removed at lower pH was observed to be low with 43.28% removed under acidic conditions. It is observed that as the pH of the cassava wastewater was increased the amount of cyanide removed also increased with the highest cyanide removal seen at pH 8 which remained relatively the same at pH 10. The percentage of cyanide removed at this pH was 76.66%. This increase in the percentage of removal is a result of the deprotonation of the adsorbent surface thereby making the surface charge of the activated periwinkle shell likely to be negative at that pH (Badmus and Audu 2009). At lower pH, the cyanide in the wastewater exists predominantly as HCN which may explain the partial adsorption of cyanide occurring at acidic pH. However, as the solution pH is increased, the amount of cyanide increases considerably where HCN in its molecular form dissociates to CN− + H+ which is its ionic form. Cyanide in this form is nucleophilic, and when in contact with the negatively charged adsorbent surface binds to anionic functional groups such as Carbonyl, Carboxylic, and Phenols present as seen from the FTIR analysis of the adsorbent, therefore improving the adsorption process (Naveen et al. 2016). The predominant adsorption mechanism as the surface of the adsorbent is negatively charged is likely to be ion exchange. Similar trends comparable to the present study have been reported by other researchers of increased rates of cyanide adsorption at pH of 9–11 (Nwoko et al. 2016; Agarwal et al. 2013; Stavropoulos et al. 2013). Therefore, the optimum pH used for subsequent adsorption experiments was carried out at pH 8.
Adsorbent dosage effect on removal of cyanide
The removal efficiency of APSC in the adsorption of cyanide using different adsorbent dosages is presented in Fig. 2. Results reveal a constant rise in removal efficiency of cyanide as the adsorbent doses increased from 2.0 to 3.0 g due to the availability of fresh active sites where maximum removal efficiency was at 83.91% for 3.0 g. However, above 3.0 g the removal efficiency of APSC remained constant or decreased marginally. One reasonable explanation for this noticeable trend could be due to the overlap of active sites for adsorption present at higher APSC adsorbent doses hence limiting the adsorption of more cyanide even at higher dosages (Asgari et al. 2012). Therefore, 3.0 g was used subsequently for further adsorption experiments because higher dosages may not be economical.
The influence of contact time on removal of cyanide
The effect of contact time investigated on the adsorption process is shown in Fig. 3. It is observed that the equilibrium time for the adsorption of cyanide was 80 min with the percentage of cyanide adsorbed at this time at 83.93%. Rapid adsorption of cyanide was observed within the first 20 min to when equilibrium was reached at 80 min. This noticeable fast rate of adsorption is possible as a result of numerous available active sites present on the surface of the APSC during the early stages of the adsorption process. This, and the large concentration of cyanide present at the start of the experiment, could be responsible for the rapid adsorption rate. Also, the agitation of the samples provided the energy required for the mass transfer of the cyanide present in the solution to the active sites present on the surface of the APSC. Adsorption performance of APSC after 80 min remained almost constant with the percentage of cyanide removed remaining at 83.95% and 83.96% for 100 min and 120 min, respectively. A likely cause of this could be the exhaustion and saturation of available binding sites at equilibrium time indicating monolayer sorption of cyanide hence the inability of APSC in adsorbing more cyanide. Comparable trends such as those observed have been reported in similar studies (Eletta et al. 2016; Asgari et al. 2012). The equilibrium time of 80 min was used for subsequent experiments.
The influence of initial cyanide concentration on the removal of cyanide
The influence of initial cyanide concentration as a variable is presented in Fig. 4. The percentage of cyanide removed was seen to be considerably high as the concentration of cyanide varied from 40 to 120 mg.L−1 where 89.13% was removed at 40 mg.L−1. Higher concentrations of cyanide in solution revealed a decrease in the amount of cyanide removed at equilibrium with 80.99% of cyanide removed at 120 mg.L−1. The trend agrees with similar findings and could likely result from limited available active sites for the fixed adsorbent dosage used and the saturation of the adsorbent surface (Eletta et al. 2016; Nwoko et al. 2016). This is an indication of the ability of APSC to be quite effective in the adsorption of high concentrations of cyanide within normal ranges found in cassava mill wastewater.
Sorption kinetics
The calculated constants of the three kinetic models derived using nonlinear regression along with the error functions are presented in Table 3. Graphical time profiles of the experimental and predicted nonlinear kinetic models at cyanide concentrations of 80 mg.L−1 and 100 mg.L−1, respectively, are presented in Figs. 5 and 6.
From the sorption kinetic data in Table 3 the Elovich model is seen to have the highest value of the coefficient of determination at 0.996 and 0.997 for 80 mg.L−1 and 100 mg.L−1, respectively. The values of error functions SSE and \(\chi^{2}\) at the different concentrations on further analysis are seen to be the lowest making it the kinetic model that best fits the uptake of cyanide onto APSC. Coefficient of determination (r2) values for concentrations of cyanide at 80 mg.L−1 and 100 mg.L−1 for the pseudo-second-order kinetic model were higher compared to the values for the pseudo-first-order kinetic model with lower values of SSE and \(\chi^{2}\). The coefficient of determination for the pseudo-first-order and pseudo-second-order kinetic models was 0.989 and 0.995, respectively. Moreover, results show the values of the calculated adsorption capacity q.cal (1.14600, 1.40310 mg.L−1) are closer to the experimental adsorbed capacity q.exp (1.1180, 1.36083 mg.L−1) for the pseudo-second-order kinetic model at 80 mg.L−1 and 100 mg.L−1, respectively, which shows a good agreement. The coefficient of determination for the pseudo-first-order kinetic model was seen to show a good correlation at r2 > 0.98. Despite the high coefficient of determination, there seems to be a considerable deviation between the experimental adsorbed capacities q.exp at equilibrium with the calculated adsorbed capacities q.cal. The higher r2 values and lower values for error functions for the pseudo-second-order kinetic model when compared to the pseudo-first-order kinetic model error functions reveal the dominance of the pseudo-second-order kinetic model over the pseudo-first-order kinetic model. Hence best fits the kinetic data. This is an indication that the retention of cyanide during the adsorption process takes place on the surface of the APSC by electrostatic interactions which require two binding sites. Results also reveal that the coefficient of determination (r2) for the pseudo-first-order kinetic model is quite high which could indicate the possibility of physisorption and chemisorption occurring simultaneously on the surface of the adsorbent. In this case, the adsorbate molecules may be adsorbed over a chemisorbed layer (Jasper et al. 2020).
Adsorption isotherms
From the results of the derived parameters presented in Table 4 the maximum monolayer adsorption capacity from the Langmuir equation is calculated as 2.856 mg.g−1. The RL value is seen as 0.19248 which is an indication of the adsorption process of cyanide being favorable since it lies between 0 and 1 (Foo and Hameed 2010). The Freundlich isotherm model constant n was calculated as 1.693. The constant n is an indication of a strong adsorption bond between the adsorbent and adsorbate which favors the adsorption process. When the value of n is greater than 1, the adsorbent is considered appropriate for the adsorption of cyanide with values of n less than 1 suggesting reduced adsorption capacity (Foo and Hameed 2010). The value of n, in this case, reveals that APSC favored the adsorption of cyanide. The value of KF which denotes the adsorption intensity is seen as 0.269. The hybrid Redlich–Peterson isotherm model reveals the value of “g” as 0.919. The convergence of g toward unity suggests that cyanide adsorption onto APSC approaches the Langmuir isotherm model rather than the Freundlich isotherm model (Foo and Hameed 2010). Comparing the data of cyanide adsorption for the two-parameter isotherm models from Table 4, the Langmuir isotherm model showed a better fit based on a higher value of r2 and lower error functions when compared to the Freundlich isotherm model. The Langmuir model is seen to have error values of SSE and \(\chi^{2}\) as 0.00095 and 0.00016, respectively, with r2 of 0.998 in comparison to 0.00103 and 0.00018 for the Freundlich isotherm with r2 of 0.992. It is equally important to note that the high value for the coefficient of determination (r2) for the Freundlich isotherm shows a good agreement but not a perfect one. This is confirmed by the constants n and KF indicating the adsorption of cyanide is favorable. This could be an indication that the adsorption of cyanide adheres to the Langmuir isotherm at higher cyanide concentrations and the Freundlich isotherm when concentrations are low. This could result from both physisorption and chemisorption involved in the adsorption process as confirmed from the kinetic models. The data from cyanide adsorption is seen to fit the Redlich-Peterson isotherm model with a higher value of r2 and lower error functions than the two-parameter models. The convergence of g toward unity suggests that cyanide adsorption onto APSC approaches the Langmuir isotherm model rather than the Freundlich isotherm model (Foo and Hameed 2010). Figure 7 shows the fitting curves of the isotherms with the experimental data where the Redlich–Peterson isotherm is seen to coincide with the Langmuir model. This suggests that the Redlich–Peterson model tends toward the Langmuir model, as confirmed by the constant “g” approaching unity value. From the table, the best-fit adsorption isotherm indicates that the Redlich–Peterson > Langmuir > Freundlich model is in the following order.
Conclusion
In the present study, acid-activated periwinkle shell carbon was successfully prepared and subsequently used for the adsorption of cyanide present in simulated cassava wastewater. The potential utilization of APSC as an effective adsorbent for cyanide removal in cassava wastewater is revealed from both physicochemical and FTIR analysis. The presence of surface functional such as Carbonyl, Carboxylic, and Phenols from FTIR analysis on the acid-treated adsorbent enhanced the adsorption process. Optimum adsorption of cyanide is seen to be strongly influenced by the pH of the wastewater with 76.6% of cyanide removed at a pH of 8. The optimal adsorbent dosage of APSC for cyanide removal was at 3.0 g.L−1. Removal of cyanide changed from 80.99 to 89.13% as initial cyanide concentration varied from 120 to 40 mg.L−1 at an equilibrium time of 80 min. The kinetics investigations carried out show that the Elovich and Pseudo-second-order models are the best fit in explaining the adsorption process with strong indications of the process being chemisorption. Equilibrium isotherm analysis reveals that the Redlich–Peterson model provides the best fit with the model tending toward the Langmuir isotherm suggesting mono-layer adsorption on the adsorbent surface. From the final results obtained, acid-activated periwinkle shell can effectively be used for the treatment of cassava mill wastewater and hence reduce the concentrations of cyanide present in the effluent.
References
Agarwal B, Balomajumder C, Thakur PK (2013) Simultaneous co-adsorptive removal of phenol and cyanide from binary solution using granular activated carbon, Chemical. Eng J 228:655–664
Asgari G, Bahman R, Abdol Motaleb SM, Jamal MAP (2012) The investigation of kinetic and isotherm of cyanide adsorption onto bone charcoal. Int Proc Chem, Biolog Environ Eng 43(25):119–122
Babayemi AK, Onukwuli OD (2017) Equilibrium studies and optimization of phosphate adsorption from synthetic effluent using acid modified bio-sorbent. Am J Eng Appl Sci 10(4):980–991
Badmus MAO, Audu TOK (2009) Periwinkle shell: based granular activated carbon for treatment of chemical oxygen demand (COD) in industrial wastewater. Can J Chem Eng 87:69–77
Brown AM (2001) A step-by-step guide to non-linear regression analysis of experimental data using a microsoft excel spreadsheet. Comput Methods Programs Biomed 65:191–200
Can M (2014) Studies of the kinetics for rhodium adsorption onto gallic acid derived polymer: the application of non-linear regression analysis. Acta Physica Polonica 127(4):1309–1310
Dash RR, Balomajumder C, Kumar A (2009) Removal of cyanide from water and wastewater using granular activated carbon. Chem Eng J 146:408–413
Dash RR, Gaur A, Balomajumuder C (2008) Cyanide in industrial wastewater and its removal: A review on biotreatment. J Hazard Mater 163:1–11
Eke-emezie NC, Etuk BR (2019) Cyanide adsorption from cassava wastewater onto calcined periwinkle shell. J Degrade Min Land Manage 7(1):1929–1934
Eletta OAA, Ajayi OA, Ogunleye OO, Akpan IC (2016) Adsorption of cyanide from aqueous solution using calcined eggshells. equilibrium and optimization studies. J Environ Chem Eng 4:1367–1375
Ettu LO, Ibearugbulem OM, Ezeh JC, Anya UC (2013) A Reinvestigation of the prospects of using periwinkle shell as partial replacement for granite in concrete. Int J Eng Sci Invent 2(3):54–59
Etuk BR, Etuk IF, Asuquo LO (2012) Feasibility of using sea shells ash as admixtures for concrete. J Environ Sci Eng 1:121–127
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. https://doi.org/10.1016/j.cej.2009.09.013
Gumus RH, Okpeku I (2015) Production of activated carbon and characterization from snail shell waste (Helix pomatia). Adv Chem Eng Sci 5:51–61
Hameed BH (2009) Grass waste: a novel sorbent for the removal of basic dye from aqueous solution. J Hazard Mater 166:233–238. https://doi.org/10.1016/j.jhazmat.2008.11.019
Islam MS, Ang BC, Gharehkhani S, Afifi ABM (2016) Adsorption capability of activated carbon synthesized from coconut shell. Carbon Lett 20:1–9. https://doi.org/10.5714/CL.2016.20.001
Jasper EE, Ajibola VO, Onwuka JC (2020) Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl Water Sci 10:132. https://doi.org/10.1007/s13201-020-01218-y
Kaewkannetra P, Imai T, Garcia-Garcia FJ, Chiu TY (2009) Cyanide removal from cassava mill wastewater using Azotobactor vinelandii TISTR 1094 with mixed microorganisms in activated sludge treatment system. J Hazard Mater 172:224–228
Kuyucak N, Akcil A (2013) Cyanide and removal options from effluent in gold mining and metallurgical processes. Miner Eng 50–51:13–29
Lach J (2019) Adsorption of chloramphenicol on commercial and modified activated carbons. Water 11:1141. https://doi.org/10.3390/w11061141
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lawal NS, Babalola AA, Makinde IO (2019) Application of activated carbon impregnated composite ceramic filters in cassava mill effluent treatment: prospects and limitations. J Degrade Min Land Manage 6(2):1579–1586. https://doi.org/10.15243/jdmlm.2019.062.1579
Naeem MA, Imran M, Amjad M, Abbas G, Tahir M, Murtaza B, Zakir A, Shahid M, Bulgariu L, Ahmad I (2019) Batch and column scale removal of cadmium from water using raw and acid activated wheat straw biochar. Water 11(7):1438
Naveen D, Chandrajit B, Prasenjit M (2016) Comparative investigation on the removal of cyanide from aqueous solution using two different bio adsorbents. Water Res Indus 15:28–40. https://doi.org/10.1016/j.wri.2016.06.002
Nwabanne JT, Igbokwe PK (2012) Application of response surface methodology for preparation of activated carbon from Palmyra palm nut. NewYork Sci J 5(9):18–25
Nwoko CIA, Mbonu PI, Okoji JN (2016) A comparative study of adsorption of cyanide onto dried peels of plantain and banana. Int J Rec Innov Trends Comput Commun 4(7):190–196
Nworie CE, Onukwuli DO, Ekebafe LO, Ezeugo JO (2020) Preparation and characterization of periwinkle shell activated carbon. Nigerian Res J Chem Sci 8(1):244–254
Obot MU, Yawas DS, Aku SY (2015) Development of an abrasive material using periwinkle shells. J King Saud Uni Eng Sci. https://doi.org/10.1016/j.jksues.2015.10.008
Obueh HO, Odesiri-Eruteyan E (2016) A Study on the effects of cassava processing wastes on the soil environment of a local cassava mill. J Pollution Eff & Control 4(4):177. https://doi.org/10.4176/2375-4397.1000177
Okunade DA, Adekalu KO (2013) Physico-chemical analysis of contaminated water resources due to cassava wastewater effluent disposal. Eur Int J Sci Technol 2(6):75–83
Okuo JM (2006) Removal of heavy metal ions by blended periwinkle shells. J Appl Sci 6:567–571. https://doi.org/10.3923/jas.2006.567.571
Oliveira MA, Reis EM, Nozaki J (2001) Biokenetic parameters investigation for biological treatment of cassava mill effluents. Water Air Soil Pollut 126(3–4):307–319
Olugbenga Solomon Bello and Mohd Azmier Ahmad (2011) Removal of remazol brilliant violet-5R dye using periwinkle shells. Chem Ecol 27(5):481–492. https://doi.org/10.1080/02757540.2011.600696
Omotosho O, Amori A (2015) Caustic hydrogen peroxide treatment of effluent from cassava processing industry: prospects and limitations. Int J Eng Technol Innov 5(2):121–131
Onyenwoke CA, Simonyan KJ (2014) Cassava post-harvest processing and storage in Nigeria: a review. Afr J Agric Res 9(53):3853–3863
Owabor CN, Iyaomolere AI (2013) Evaluation of the influence of salt treatment on the structure of Pyrolyzed Periwinkle. J Appl Sci & Environ Manag 17(2):321–327. https://doi.org/10.4314/jasem.v17i2.15
Sabino D, Giusy L, Mariangela G, Michele N (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40
Stavropoulos GG, Skodras GS, Papadimitriou KG (2013) Effect of solution chemistry on cyanide adsorption in activated carbon. J Appl Thermal Eng 74:183–184
Ugwu EI, Agunwamba JC (2012) Detoxification of cassava wastewater by alkali degradation. J Res Environ Sci Toxicol 1(7):161–167
Verla AW, M Horsefall (jnr), EN Verla, A I Spiff, O A Ekpete (2012) Preparation and characterization of activated carbon from fluted pumpkin Telfairia Occidentalis Hook.F seed shell. Asian J Nat Appl Sci 1(3): 39–46.
Wade JW, Omoregie E, Ezenwaka I (2002) Toxicity of cassava (manihot esculenta crantz) effluent on the nile tilapia, oreochromis niloticus (l) under laboratory conditions. J Aquat Sci 17(2):89–94
Funding
This particular research did not receive any external funding from the public, private and non-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eke-emezie, N., Etuk, B.R., Akpan, O.P. et al. Cyanide removal from cassava wastewater onto H3PO4 activated periwinkle shell carbon. Appl Water Sci 12, 157 (2022). https://doi.org/10.1007/s13201-022-01679-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01679-3