Abstract
Groundwater is a vital natural resource in the Kathua region of the Union Territory of Jammu and Kashmir, Northern India, where it is used for domestic, irrigation, and industrial purposes. The main purpose of this study was to assess the hydrochemistry of the groundwater and to determine its suitability for drinking, irrigation, and industrial uses in the Kathua region. In this study, 75 groundwater samples were collected and analyzed for the physicochemical parameters such as electrical conductivity (EC), total dissolved solids , pH, and various cations and anions. The analyzed data were computed for designing groundwater quality index to know the suitability for drinking purposes. The EC, sodium percentage, permeability index, and magnesium hazard were assessed to evaluate groundwater suitability for irrigation. Further, the corrosivity ratio was assessed to find the groundwater quality criteria for industrial purposes. The comprehensive results obtained from the water quality index indicate that almost all groundwater samples are suitable for drinking. The ionic abundance is in the order of Ca2+ > Na+ > Mg2+ > K+ for cations, and HCO3− > SO42− > Cl− > NO3− for anions, respectively. The Piper diagram shows that hydrochemistry of the groundwater is dominated by alkaline earth metals (Ca2+, Mg2+) and weak acids (HCO3−). According to the Gibbs diagram, the chemistry of groundwater is mainly controlled by the rock–water interaction process, indicating that most of the groundwater samples of the area are of bicarbonate type. The EC results classify the groundwater as excellent to good; the sodium percentage also indicates that the water is fit for irrigation. According to the Wilcox and USSLS diagrams, and permeability index, a majority of samples are suitable for irrigation with a few exceptions. The magnesium hazard depicts that there are few samples (19%), which are unsuitable for irrigation. According to the corrosivity ratio, 65 samples are safe for industrial use while the remaining 10 samples are considered to be unsafe. Thus, it is found that most of the groundwater in the area can be used for drinking, irrigation, and industrial purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is an invaluable natural resource, which is essential for the survival of life on the planet Earth. It has a significant impact on the socioeconomic, industrial, and agricultural developments of society (Bouslah et al. 2017). Water is useful to keep all ecosystems (terrestrial, aquatic, and human) and environmental conditions healthy, alive, and sustainable (Adimalla and Venkatayogi 2018; Adimalla and Taloor 2020). It occurs as surface water and groundwater. Groundwater is estimated to meet nearly 40% of the global needs of water for food production and 30% for drinking (Amiri et al. 2021; Chowdhury et al. 2021). Water demand has increased due to its use for a variety of purposes such as urbanization, agriculture, industry, and improving living standards. Currently, an increasing trend in groundwater utilization has been observed due to several reasons such as climate change, incessant population growth, and inadequate precipitation (Li et al. 2013; IPCC Report 2021; Verma 2021). It has been found that groundwater is at an alarming stage of water crisis for cities, towns, and rural habitations in almost all parts of the world (Jasrotia et al. 2019; Adimalla and Taloor 2020; Amiri et al. 2021). Nearly one-third of the world’s population uses groundwater for drinking purposes (UNEP 1999). Around 50% of water demands in urban and nearly 85% in rural regions are being fulfilled by groundwater (World Bank 2010). Globally, 65% of groundwater is used for drinking, 20% for agriculture, and 15% for industry and mining purposes (Saeid et al. 2018). The study of groundwater quality is helpful in determining the source rocks and minerals that interact with aquifers, groundwater level fluctuations, and recharge as well as discharge of groundwater (Adimalla 2020; Reddy et al. 2020). Recently, the geographic information system (GIS) has become a vibrant tool in hydrogeological studies especially in groundwater quality modeling, due to its ability to store, analyze, manipulate, and visualize spatial data (Rao and Latha 2019). Many studies have been conducted to evaluate the groundwater quality using GIS (Singh et al. 2017; Jasrotia et al. 2019; Taloor et al. 2020, 2021; Ram et al. 2021).
India is one of the largest populated and agriculture dependent countries of the world. As a result, it needs a huge amount of water for various purposes. As groundwater is easily available even during the summer months, therefore, its demands and needs are growing exponentially (Taloor et al. 2020, 2021). Thus, groundwater crises can be seen in many parts of India, with varying scales and intensities depending on the different intervals of a year (Kumar et al. 2005, 2021; Jain et al. 2007; Rodell et al. 2009; Singh et al. 2017; Jasrotia et al. 2019; Taloor et al. 2020). In recent times, the increasing population along with other activities notably, agriculture, industrialization, and urbanization have augmented the demand of water to such an extent that it has adversely affected its quality and quantity (Howard and Bartram 2003; Taloor et al. 2020; Verma 2021). Rapid population growth, an ever-increasing economy, and various anthropogenic activities not only increased the demand for groundwater, but also polluted and degraded its quality (Chowdhury et al. 2021; Wang and Li 2021; Zhao et al. 2021). Groundwater quality has been rapidly deteriorating in various parts of the country, particularly in states of Punjab, Haryana, Rajasthan, Gujarat, Madhya Pradesh, Odisha, West Bengal, Karnataka, Andhra Pradesh, and Telangana, which in turn, making it unsuitable for various uses (Bajaj et al. 2011; Hundal 2011; MacDonald et al. 2016; Lapworth et al. 2017; Singh et al. 2017; Mukherjee et al. 2019; Haque et al. 2020; Khan et al. 2020). As a consequence, the deteriorated groundwater causes many diseases such as fluorosis, arsenicosis, haemochromatosis, bronchitis, cancer, and many other chronic diseases in various parts of the country (e.g., Mukherjee et al. 2019).
It is critical to investigate the groundwater quality of any region to determine its suitability for drinking, agricultural, and industrial purposes (Todd 1976; Tatawat and Chandel 2008). In India, detailed investigations of groundwater quality have been conducted in various parts of the country (Singh et al. 2008; Afroza et al. 2009; Dar et al. 2011; Vahab et al. 2015; Li et al. 2016; Disli 2017; Adimalla and Venkatayogi 2018; Adimalla et al. 2018, 2022; Patil et al. 2020; Karunanidhi et al. 2021). According to Faten et al. (2016), the groundwater quality deteriorates due to natural processes like rock–water interaction, evaporation, and anthropogenic influences such as industrial pollution, excess use of fertilizers, and human waste. Various researchers in the Union Territory (UT) of Jammu and Kashmir, Northern India, have been working on the groundwater, spring water, and river water quality in various parts of the UT and found that the water is polluted naturally and anthropogenically in some areas, particularly in Jammu, Doda, and Srinagar regions (Dar et al. 2011; Jeelani et al. 2014; Yaseen et al. 2015; Chandan 2017; Haq et al. 2017; Lone et al. 2020; Murtaza et al. 2020; Dar et al. 2020; Taloor et al. 2020, 2021). Furthermore, deteriorated groundwater quality has been reported in the Kathua District’s neighboring regions, which is posing a serious threat to the local population’s health, economic development, and social prosperity (Kanwar and Khanna 2014). While examining the literature, it is found that the Kathua region of the Jammu and Kashmir is largely neglected, and a few studies have been carried out in the evaluation of groundwater resources of the region (Pir 2020; Taloor et al. 2020). Currently, the groundwater demand has increased in the study area for various purposes such as domestic, agricultural, livestock and industrial (Pir 2020; Taloor et al. 2020). The primary objectives of the present study are to: (1) evaluate the overall quality and understand the hydrogeochemical characteristics; (2) know the hydrogeochemical facies and evolution of groundwater using Piper and Gibbs diagrams; (3) develop groundwater quality indices for drinking purposes by considering Groundwater Quality Index (GWQI) method; and (4) examine the groundwater quality for irrigation, and industrial purposes using various indicators such as Wilcox diagram, United State Soil Laboratory Staff (USSLS) diagram, permeability index (PI), magnesium hazard (MH), and corrosivity ratio (CR). The results obtained from this study would prove useful for the policy makers and planners to design suitable plans and scientific techniques for sustainable development and management of groundwater in the study area.
Study area
The study area is situated in the foothill zone of the Himalayan mountains chain, and part of the Indo-Gangetic plains in the Kathua region of the UT of Jammu and Kashmir, India (Fig. 1). It lies between the latitude 32° 16′ to 32° 55′ N and longitude 75° 06′ to 75° 54′ E. The climate varies with altitude, ranging from subtropical to moist temperate. The winter temperature ranges from 0.9 to 21 °C and summer temperature from 27 to 47 °C, respectively (Jasrotia and Kumar 2014; DDC Report 2019; Pir 2020). The average rainfall is about 1116 mm. There are numerous ephemerals and small perennial streams that originate from the northern mountainous region and flow toward the south-western direction. The Ravi is the perennial river and its tributaries such as Ujh, Tarnah, and Bein, and some seasonal streams (locally known as khad) flow in the area. It has been observed that structure and lithology play a vital role in the evolution of different types of drainage system in the area (Pir 2020; Taloor et al. 2020). The hydrogeological conditions are complex due to highly dissected hills, topographical barriers, hydrological boundaries, and varied geological units (CGWB 1997, 2013; Kanwar and Khanna 2014).
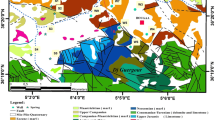
Geological map of the study area showing the location of the groundwater samples. Inset is the map of India showing study area, highlighted by red color (redrawn and modified after Karunakaran and Ranga Rao 1976)
Geological setting
During the Early Cenozoic, some 55–35 million years ago, the Tethys Ocean closed due to the collision of the Indian plate with the Eurasian plate, forming the Himalayan Mountain range (Gansser 1964; Najman 2006; Valdiya 2016; Verma et al. 2016; An et al. 2021). Subsequently, the sedimentations of the Subathu, Murree, and Siwalik basins took place in the foreland basin situated toward the south of the rising Himalaya, and later, these basins got uplifted, folded, tilted, and faulted due to post-collisional Himalayan orogenic movements (Najman and Garzanti 2000; Kumar et al. 2003; Valdiya 2016; Shah 2018; Prashanth et al. 2021, 2022). The rocks and sediments of the Siwalik Group and the Kandi and Sirowal formations dominantly cover the study area.
Stratigraphically, the Siwalik Group is divided into three subgroups: Lower, Middle, and Upper. The Lower Siwalik Subgroup is composed red mudstone, fine- to medium-grained sandstone, and green sandstone of the Middle Miocene age (Nanda 2015; Fig. 1). The overlying Middle Siwalik Subgroup is made of medium to coarse-grained sandstone and subordinate gray brown mudstone of the Upper Miocene age (Karunakaran and Ranga Rao 1976; Nanda 2015). The topmost Upper Siwalik Subgroup comprises conglomerate, coarse-grained sandstone, and pink gray mudstone of Pliocene to middle Pleistocene age (Ranga Rao et al. 1988; Jasrotia et al. 2019). A large portion of the study area is covered by the rocks of the Upper Siwalik Subgroup. The Upper Siwalik Subgroup of the Jammu region is subdivided into the three formations such as Parmandal Sandstone, Nagrota Silt, and Boulder Conglomerates (Agarwal et al. 1993; Taloor et al. 2020). The study area is represented by two significant geological formations such as Kandi and Sirowal, which are spread over the southwestern most part of the study area. The Kandi Formation comprises boulders, cobbles, pebbles, and coarse sands. The Sirowal Formation includes fine to coarse sands with clay and silt.
Material and methods
For the present study, a total of 75 groundwater samples were collected using a random sampling technique in October 2020 during the post-monsoon period from various tube wells and dug wells of the study area. Water samples were collected in 2-L polythene bottles, and before sample collection, these bottles were pre-washed, then soaked with 1:1 diluted HCl solution, and further, washed twice with distilled water. The bottles were filled with water samples and subsequently sealed with double plastic caps to avoid evaporation. The in situ parameters such as temperature, pH, electrical conductivity (EC), and total dissolved solids (TDS) were measured in the field at the time of sample collection by portable digital meters such as pH, EC, and TDS meter. Further, samples were examined in the laboratory for water quality metrics and chemical parameters using standard methods as suggested by the American Public Health Association (APHA 1998), and the Manual of Pollution Control Board, New Delhi (MPCB 1997). The total hardness (TH), calcium (Ca2+), and magnesium (Mg2+) were determined by the titrimetric method by following the standard ethylene diaminetriacetic acid (ETDA) method, sodium (Na+), and potassium (K+) by flame photometer and bicarbonate (HCO3−), and chloride (Cl−) by titration method. The fluoride ion concentration was obtained with an atomic absorption photometer. Sulfate and nitrate ion concentration was estimated by the gravimetric method and using a flame photometer. All the values were represented in milligram per liter (mg/L). The analyzed hydrochemical data (Table 1) were plotted on Piper and Gibbs diagrams to understand hydrogeochemical facies, hydrochemistry, and water types, and processes that control the geochemistry of water, respectively (Piper 1944; Schoeller 1967). The salinity and sodium hazard diagrams were also prepared for determining the suitability of groundwater for irrigation uses.
To determine the reliability of the water quality analysis for major cations and anions, the ion balance error (IBE) or charge balance error (CBE) technique was performed (Freeze and Cherry 1979). It is proposed that the IBE should be less than ± 5% to consider an analysis is valid, and if it is greater than ± 5%, results are not to be acceptable and required to investigate the reason of error (Freeze and Cherry 1979; Hounslow 1995; Naidu et al. 2021). The error percentage in cations and anions ion balance was calculated by using the following equation (Hem 1991; Freeze and Cherry 1979; Ansari and Umar 2019; Naidu et al. 2021):
All the groundwater samples in the current study area are within the limits of 5%, indicating a valid water quality analysis. Table 1 demonstrates the data for the analyzed physicochemical parameters of the samples, and Table 2 shows a statistical summary of the data.
GIS has emerged as a significant tool for performing numerous spatial operations that are useful for various decision-making processes over the last few decades (Burrough et al. 1998; Jasrotia et al. 2019; Taloor et al. 2020). GIS-based outputs relating to hydrochemical data of groundwater in the form of spatial analysis/distribution have been extensively used all around the globe for making groundwater assessment, development, and management (Singh et al. 2017; Khan et al. 2020; Taloor et al. 2020). In the present study, sample location data of all the groundwater samples obtained by the global positioning system (GPS) was mapped in ARC GIS 10.4 environment. The spatial analysis had been performed to generate various distribution maps of groundwater quality index (GWQI), electricity conductivity (EC), sodium percentage (Na%), sodium adsorption ratio (SAR), magnesium hazard (MH), and corrosivity ratio (CR) by spatial interpolation technique using inverse distance weighted (IDW) method (Latha and Rao 2012; Rao and Latha 2019; Adimalla and Taloor 2020).
Result and discussion
Groundwater chemistry
To determine groundwater suitability for drinking, evocative statistics are computed and compared with the WHO (2011, 2017) and BIS (2012) drinking water quality standards (Table 2). The pH value ranges from 6.45 to 7.73, with a mean value of 6.95 indicating a slightly acidic to alkaline nature of groundwater. The EC value of groundwater samples ranges from 80 to 1500 μS/cm, with a mean of 498 μS/cm. The higher EC value indicates high salinity and mineral content in groundwater with low runoff, high infiltration, and discharges water type (Subba Rao et al. 2012; Ravikumar and Somashekar 2017). Conversely, the low EC value is generally associated with high elevated topography, high runoff, low infiltration and recharge water type, and low salt enrichment. Moreover, the groundwater can be classified as: type I, if the concentration of salts is low (< 1500 μS/cm), type II, if the salts show medium enrichment (1500 and 3000 μS/cm), and type III, if the salts enrichment is high (> 3000 μS/cm) (Subba Rao et al. 2012). Almost all groundwater samples of the study area fall under the type I (i.e., water with low salt enrichment) except one sample, which falls under type II (i.e., medium salt enrichment). The TDS in water comprises all inorganic salts that demonstrate the water salinity and its suitability for human consumption (WHO 2011, 2017). The TDS in groundwater samples ranges from 51.46 to 964.80 mg/L, with a mean value of 317.97 mg/L (Table 2). As a result, almost all of the samples are below the acceptable limit (500 mg/L), except two samples that are above the desirable limits, but within the permissible limits. It is commonly assumed that if 99% of samples fall within the acceptable limit and only 1% falls outside the permissible limit, the groundwater can be considered desirable for drinking and irrigation purposes (Davis and Dewiest 1966; Sawyer and McCarty 1967). The total hardness (TH) in groundwater water is caused by the presence of calcium and magnesium and other metal ions (Razowska-Jaworek 2014). Jain et al. (2010) stated that intake of higher concentrations of water with TH (> 300 mg/L) may cause health issues like kidney problems. Hence, hard water is unsuitable for domestic purposes. According to Sawyer and McCarty (1967) classification, 11 samples fall under the very hard class and other samples fall under the hard water category of the TH (Table 3). Therefore, it is found that groundwater in most parts of the study area is suitable for drinking, irrigation, and agriculture purposes.
Cation chemistry
The concentration of calcium (Ca2+) cation in groundwater is an important component of groundwater chemistry because it aids in the growth of bones and teeth. The Ca2+ concentration in the analyzed samples shows that it varies from 9.0 to 140 mg/L, with an average of 66 mg/L (Table 2). Its comparison with WHO (2017) and BIS (2012) standards indicates that Ca2+ concentration is within the desirable limit. The calcium-bearing minerals (e.g., plagioclase, amphibole, and pyroxene) and rocks (e.g., limestone, dolomite, and shale) commonly make groundwater enriched with calcium. Additionally, the presence of carbon dioxide in the soil zone and ion exchange are other sources from which calcium comes into the groundwater (Hem 1991; Subba Rao 2018). Similarly, in the study area, most of these minerals and rocks serve as a source of calcium to the groundwater. Commonly, magnesium occurs in the natural water in association with calcium and it may also be derived from geogenic (seawater, ferro-magnesium minerals, and ion exchange) or anthropogenic (e.g., mining activities, and industrial wastage) sources. Its concentration in the study area is found between 0.84 and 283.08 mg/L, with an average of 25.86 mg/L. Its comparison with WHO (2017) and BIS (2012) indicates that the groundwater is found to be above the permissible limit (Table 2). The Na+ concentration varies from 1.59 to 238.27 mg/L, with an average of 31.40 mg/L, and its comparison with WHO (2017) and BIS (2012) standards clearly shows that the Na+ concentration is above the permissible limit (Table 2). Zhang et al. (2019) reported that high Na+ concentration in groundwater is possibly due to cation exchange and persistent evaporation. The K+ concentration varies from 0.68 to 392.70 mg/L, with an average of 11.13 mg/L. It is found that K+ concentration in majority of the samples fall within the permissible limits, and in a very few samples, it is found to be above the permissible limits prescribed by the WHO (2017) and BIS (2012) standards (Table 2). It was found that the order of abundance of cations was Ca2+ > Na+ > Mg2+ > K+ in the study area.
Anion chemistry
Bicarbonate (HCO3−) is the most important chemical component that occurs in natural water. Generally, bicarbonate is derived from weathering of the silicate rocks, but also comes from primary carbonate and calcareous rocks (Chandan 2017), and helps to produce alkaline nature to the groundwater (Ram et al. 2021). Its concentration in the area ranges from 123 to 1500 mg/L, with an average of 287 mg/L, which falls well within the permissible limit (WHO 2011, 2017). The chloride (Cl−) is an important anion found in the groundwater, and it may be derived from various sources like leaching, weathering of different minerals, and also from some anthropogenic sources. Its high concentration in groundwater gives a salty taste and effect on human health by contributing to kidney stones (Mohamed et al. 2019). In the study area, Cl− concentration varies from 4.99 to 173.90 mg/L, with an average of 26.60 mg/L, which indicates that the groundwater falls within the permissible limits and is suitable for consumption (BIS 2012; WHO 2017). Sulfate (SO42−) can be derived from geogenic as well as anthropogenic sources, and its high concentration makes groundwater unsuitable for use. The geogenic source of sulfate is resulting from carbonate sedimentary rocks rich in gypsum mineral (Magesh et al. 2013). Its concentration varies from 0 to 297.07 mg/L, with an average of 48.74 mg/L, indicating that they fall within the permissible limit according to prescribed standards (BIS 2012; WHO 2017; Table 2). The results show the dominance of anions as HCO3− > SO42− > Cl− > NO3− in the study area.
The iron is one of the most important natural trace elements associated with fluoride minerals and is responsible for the high concentration of fluoride ions in the groundwater (Handa 1975; Wenzel and Blum 1992; Subramani et al. 2005; Adimalla and Venkatayogi 2017). The higher concentration of fluoride is mainly due to the dissolution of fluoride in groundwater derived from fluoride-bearing minerals and controlled by several factors such as source rocks, depth of wells, residential period, and favorable environments for the upward rise of deep-seated groundwater (Adimalla et al. 2020). The fluoride concentration in the groundwater samples varies from 0 to 2 mg/L, with an average of 0.17 mg/L, indicating that most of the samples of the study area fall within the permissible limit, except two samples that fall above the permissible limit (WHO 2011, 2017; BIS 2012). The moderately high fluoride concentration found in the two samples is probably due to the geogenic processes such as leaching, weathering, and ion exchange. Billings et al. (2004) viewed that consuming fluoride-rich groundwater (> 1.50 mg/L) usually causes dental fluorosis. Nowadays, nitrate (NO3−) concentration is considered a major groundwater pollutant especially in those areas where intensive agriculture activities, industrialization, and increasing population growth were noticed. The source of nitrate may be either geogenic or anthropogenic. The agriculture run-off with the use of different fertilizers, leakage from pipes, and septic tanks in human settlement zones are the main anthropogenic sources of nitrate in the groundwater (Dolma et al. 2015; Zhang et al. 2019; Adimalla and Qian 2019). The high concentration of nitrate in groundwater causes health problems that lead to Methaemoglobinemia (Blue baby) disease, gastric problems, and cancer (Comly 1945; Gilly et al. 1984; Subramani et al. 2005; Adimalla et al. 2020). In the study area, nitrate concentration varies from 0 to 100 mg/L, with an average of 13.28 mg/L, and a majority of the samples fall within the desirable limit, except three samples, which fall above the desirable limit (WHO 2011, 2017; BIS 2012; Table 2).
Groundwater quality index
Groundwater quality index (GWQI) is used to measure the overall qualitative nature of water and to fix spatial boundaries between various zones of the GWQI (Yadav et al. 2010; Adimalla and Qian 2019; Verma et al. 2020). The computation of the groundwater quality data for drinking purposes was carried out and compared with the recommended drinking water standard of BIS (2012) for the calculation of the GWQI. Twelve important physicochemical parameters, namely pH, TDS, TH, Ca2+, Na+, K+, Mg2+, SO42−, HCO3−, Cl−, NO3−, and F−, were considered for calculating GWQI for the current study (Table 4).
The following steps were used to determine GWQI:
-
Step 1 Each physicochemical parameter has been assigned a weight (wi), which is ranging from 2 to 5, and is based on inferences drawn from earlier studies (Singh et al. 2017; Taloor et al. 2020; Verma et al. 2020; Naidu et al. 2021). An assigned weight of 2 shows the least significant parameter, whereas 5 indicates a highly significant parameter (Table 3).
-
Step 2 The relative weight (Wi) for all the twelve parameters, whose assigned weight ranges from 2 to 5, was calculated by using the following equation:
$$Wi = \frac{{{\text{w}}i}}{{\mathop \sum \nolimits_{i = 1}^{n} wi}}$$(2)where “wi” is the weight and “Wi” is the relative weight of each parameter, and “n” is the number of parameters as shown in Table 4.
-
Step 3 Based on BIS (2012) standards, a quality rating scale (qi) for each parameter was computed by using the following equation:
$$qi = \frac{Ci}{{Si}} \times 100$$(3)where “\(Ci\)” is the concentration of each chemical parameter of each groundwater sample in milligram per liter (mg/L) and “\(Si\)” is the Indian standard water guidelines (BIS 2012) for each chemical parameter.
-
Step 4 The groundwater quality subindex (SIi) for each chemical parameter was calculated by using the following equation:
$${\text{SI}}_{i} = {\text{ W}}_{i} q_{i}$$(4)where “SIi” is the groundwater quality subindex of the ith parameter, “Wi” is the relative weight of each parameter, and “qi” is the quality rating scale based on the concentration of ith parameters.
Finally, the whole GWQI was calculated by using the following equation:
where “Sli” is a subindex of ith parameters and “n” is the number of the parameters.
The groundwater samples (n = 75) and their GWQI values are shown in Table 5. The calculated GWQI values range from 30.28 to 318.01, with an average value of 59.90. The GWQI was classified into five types: excellent water type, if it ranges less than 50; good water type, if it ranges between 50 and 100; poor water type, if it ranges from 100 to 200; very poor water type, if it ranges between 200 and 300, and water as unsuitable for drinking, if the GWQI is greater than 300 (Adimalla et al. 2020; Verma et al. 2020). In the study area, at one location namely, Sukrala (sample no. 60) had yielded a GWQI value of 318.01, which indicates that its water is not suitable for drinking (Table 5). The spatial map of GWQI was prepared to depict the spatial distribution of various zones of GWQI (Fig. 2).
Correlation analysis
The Pearson’s correlation analysis was performed to know the interrelationship between the water quality parameters. For correlation analysis, thirteen parameters were computed for the correlation matrices and the correlation coefficient (r) is presented in Table 6. If the “r” value is between + 1 and –1, it shows a perfect linear relationship (Meena et al. 2016; Lakshmi et al. 2021). If the “r” value lies between ± 0.8 to ± 1.0, it shows a strong correlation. If the “r” value is ± 0.5 to ± 0.8, it shows a moderate relationship. If the “r” value is ± 0.0 to ± 0.5, in this case, a weaker relationship occurs between water quality parameters. Further, as shown in Table 6, a strong positive correlation exists between EC and TDS (0.984); TH (0.871); Mg2+ and HCO3− (0.883); TDS and TH (0.870); HCO3− (0.840); and TH and Mg2+ (0.833), showing a major impact on the quality of groundwater compared to other ions and physical parameters. This strong positive correlation shows the influence of weathering process, mineral precipitation, mineral dissolution, and rock–water interaction on the groundwater. Likewise, Subramani et al. (2005) observed that a strong correlation between TH and Mg2+ indicates an enriched carbon dissolution mechanism in a rock–water interface.
Hydrogeochemical facies
The term hydrogeochemical facies refers to the chemical character of groundwater solutions found in hydrogeochemical systems (Back 1966). Establishing facies are useful not only for understanding the similarities and relationships among different ions present in an aquifer’s groundwater, but also for understanding the influence of chemical processes operating in a lithological framework between minerals and groundwater (Back 1966). Numerous techniques, such as graphical and statistical analysis, are used to interpret groundwater hydrogeochemistry, but Piper and Gibbs diagrams are commonly used to establish hydrogeochemical facies (Back 1966; Adimalla et al. 2020; Verma et al. 2020).
Piper diagram depicts hydrogeochemical characteristics of ions (anions and cations) in an aquifer system (Piper 1944, 1953). In this diagram, the cations (Ca2+, Mg2+, Na+, K+) and anions (Cl−, HCO3−, SO42−) concentration were plotted to know the overall geochemical character and water quality. This diagram is represented by six fields: Ca2+-HCO3− type, Na+-Cl− type, Ca2+-Mg2+-Cl− type, Ca2+-Na+-HCO3− type, Ca2+-Cl− type, and Na+-HCO3− type (Piper 1944, 1953). In the present study, the plotted sample data show that water samples fall in the four fields, among which 90% of samples fall in the Ca2+-Mg2+-HCO3− facies, and the rest of the samples fall in other facies (Fig. 3). The Ca2+-Mg2+-HCO3− water type shows that the Ca2+ and Mg2+ are major cations and the HCO3− is the major anion. Thus, the results illustrate that the hydrochemistry of the groundwater is dominated by alkaline earth metals (Ca2+, Mg2+) and weak acids (HCO3−).
Gibbs diagram is used to elucidate the processes and mechanisms controlling the water chemistry and to understand the relationships between water chemistry and aquifer lithologies (Gibbs 1970). Further, Gibbs (1970) argued that atmospheric precipitation, rock weathering, and evaporation-crystallization processes largely control the global groundwater chemistry. Thus, Gibbs’s diagram contains three fields such as precipitation dominance, rock dominance, and evaporation dominance for finding the mechanism controlling water chemistry. In Gibbs diagram, ratio I (Na+ + K+ /Na+ + K+ + Ca2+) for cations and ratio II (Cl−/(Cl− + HCO3−) for anions are shown, where the concentration of ions was represented in meq/L. The ratio I represents the ratio of major cations, and ratio II shows that the ratio of major anions of the water samples was plotted against the relative values of the TDS to determine the mechanism controlling the composition of the groundwater (Fig. 4).
The majority of samples are found in the rock dominance or rock–water interaction dominance zone indicating that the groundwater samples are of bicarbonate type (Zhang et al. 2020). This type of dominance commonly occurs in the hard-rock terrains having high temperature and low rainfall where slow water infiltration rapidly increases the ionic concentration in the groundwater (Subba Rao et al. 2012; Adimalla et al. 2018; Chowdhury et al. 2021). Furthermore, Rao and Latha (2019) observed that the rock–water interaction zone is dominated by rock weathering, secondary carbonate mineral dissolution, precipitation, and the process of ion exchange between water and clay-rich minerals. The scatter plot between HCO3− + SO42−, and Ca2+ + Mg2+ values shows that rock–water interaction is typically associated with carbonate weathering because most of the samples fall within the carbonate weathering region (Fig. 5). It may be noted that some samples are falling in the silicate weathering region (Fig. 5). Finally, it was inferred that rock dominance is the most effective controlling mechanism for knowing the hydrochemistry of the study area, which comprises various geological units.
Irrigation groundwater quality
Groundwater suitability for irrigation purpose depends on the concentration of dissolved ions present in it. Excessive ion concentration can affect the soil, plant growth, and agriculture productivity (Wilcox 1955; Jasrotia et al. 2019; Mandal et al. 2019; Xu et al. 2019; Snousy et al. 2021). Groundwater is the main source of water for irrigation in the study area, and recently increased agriculture activities as well as excessive use of chemical fertilizer, pesticides, and livestock waste have a negative impact on groundwater quality (e.g., CGWB 2013; Jasrotia et al. 2018). Apart from groundwater, surface water resources have also been utilized in some parts of the study area for agricultural purposes. These regions are mostly located along rivers or streams fed by melting water from different Himalayan glaciers while the remaining areas use groundwater as a main source of water for irrigation purpose (CGWB 1997). To assess the groundwater suitability for irrigation purpose, the important groundwater quality parameters: electrical conductivity, sodium percentage, sodium adsorption ratio, permeability index, and magnesium hazard, were analyzed.
Electrical conductivity and sodium percentage
Electrical conductivity (EC) measures the concentration of dissolved salts in groundwater and signifies salinity hazard to crops. Groundwater with high salinity is unsuitable for plants and poses a salinity hazard. Based on EC values, Ayers and Westcott (1985) classified groundwater into three categories: excellent (EC less than 700 µS/cm), good (EC ranges between 700 and 3000 µS/cm), and fair (EC more than 3000 µS/cm). Water with a low EC has a significant impact on crop productivity. In the study area, a small variation in the EC was observed with minimum and maximum values of 80 µS/cm and 1500 µS/cm, respectively, with an average value of 498 µS/cm. According to Ayers and Westcott (1985) classification, all samples fall under the excellent to good category, and hence, groundwater is suitable for the growth of crops.
The sodium content of groundwater can be used to determine its quality for irrigation (Wilcox 1955). As sodium reacts with soil and reduces its permeability and texture, a high sodium percentage in water causes soil degradation (Karanth 1987; Naidu 2021). The sodium percentage was calculated by using the following equation:
All cation concentrations are expressed in meq/L.
In the study area, the Na+% values fall between 4 and 88%, with a mean of 19%. According to BIS (2012), the water with Na+% up to 60% is recommended fit for irrigation and above it is considered as unsafe. There are only three samples that have yielded Na+% more than 60%.
Wilcox diagram
Wilcox (1955) classified the water for irrigation purposes based on EC and Na+%. He plotted values of EC and Na+% on a diagram, popularly known as Wilcox diagram and classified the water into five types as: class I: excellent to good, class II: good to permissible, class III: permissible to doubtful, class IV: doubtful to unsuitable, and class V: unsuitable. This diagram indicates that water quality decreases with the increase of EC and Na+% concentrations (Wilcox 1955). The values of EC and Na+% were plotted on the Wilcox diagram (Fig. 6). The Wilcox plot depicts that 98% of samples fall under the excellent to good category and one sample falls under the good to permissible limit (Fig. 6).
United state soil laboratory staff diagram
The United State Soil Laboratory Staff (USSLS) diagram given by Richard (1954) shows 16 zones of water suitability for irrigation purposes. The presence of a higher concentration of sodium in water affects the soil characteristics and reduces its permeability (Adimalla et al. 2018). The most significant parameters like sodium and salinity hazard favor water usability for agricultural purposes (Table 7). The salinity hazard (C) is classified into 4 subzones: very low salinity (C1: 250 μS/cm), medium salinity (C2: 250 to 750 μS/cm), high salinity (C3: 750 to 2250 μS/cm), and very high salinity (C4: above 2250 μS/cm). Similarly, the sodium hazard is classified into 4 subzones: low sodium hazard (S1: < 10), medium sodium hazard (S2: 10–18), high sodium hazard (S3:18–26), and very high sodium hazard (S4: > 26). In the study area, the majority of the samples fall under the C1 S1 category of the USSLS diagram, indicating low conductivity and very low sodium hazard, specifying that the groundwater of the study area is suitable for irrigation (Fig. 7). For USSLS diagram, the sodium adsorption ratio (SAR) was calculated by using the following equation:
All ion concentrations are expressed in meq/L.
Permeability index
The long-term use of irrigation water affects soil permeability. Therefore, values of the permeability index (PI) were used to classify the quality of groundwater for irrigation. The PI was calculated based on the following equation (Doneen 1964):
where all cation and anion concentrations are expressed in meq/L.
The groundwater was classified into three classes based on the PI: class I, class II, and class III. The groundwaters of class I and II contain 75% or more permeability and are considered suitable for irrigation, whereas the groundwater of class III holds a maximum of 25% permeability and is regarded as unsuitable for irrigation (Doneen 1964). According to Rao and Latha (2019), the presence of an excess concentration of ions such as Na+, Ca2+, Mg2+, and HCO3− can reduce the permeability and affect the overall soil composition. The majority of samples are classified as class I and II, indicating that groundwater is suitable for irrigation (Fig. 8).
Magnesium hazard
Magnesium hazard (MH) is used to evaluate groundwater suitability for irrigation by determining the concentration of Ca2+ over Mg2+ (Ragunath 1987). Calcium and magnesium are essential nutrients for crops and commonly occur in the soil. Their high value in water increases the pH of the soil (Joshi et al. 2009). Excess quantity of Mg2+ ions in irrigation water has adverse impacts on soil quality (makes soil alkaline) and also reduces crop production (Snousy et al. 2021). The magnesium adsorption ratio (MAR) was calculated by using the following equation (Szabolcs and Darab 1964):
All values of ions are expressed in meq/L.
Groundwaters with a MAR value of 50 or less are classified as suitable for irrigation and those groundwaters with a value of more than 50 are considered unsuitable and regarded as risky for irrigation because they can pose severe impacts on crop yields (Szabolcs and Darab 1964; Paliwal 1972). In the present study, 81% (61 samples) of groundwater samples had a MAR value less than 50 and were found to be suitable for irrigation. The remaining 19% (14 samples) had yielded MAR values more than 50 and were considered to be unsuitable for irrigation. The spatial distribution of EC, SAR, MH, and Na+% of the study area, is shown in Fig. 9.
Industrial groundwater quality
The groundwater utilization for industrial purposes requires a distinct water quality. As a result, every industry has its own set of water quality requirements. According to the AWWA (1971) standard, the groundwater quality in the study area appears suitable for industrial uses. The industrial sector of the study area has seen a tremendous growth in the last two decades as a result of the establishment of numerous new industries (DES Report 2017; DDC Report 2019). Accordingly, an attempt was made to know the groundwater quality for industrial uses. Poor groundwater quality can promote incrustation (formation of calcareous deposits on the metal surface) and corrosion (an electrochemical action in which metal transform into oxide/rust) activities, both of which can have serious adverse impacts on industries, particularly where metals are used (Subba Rao 2018).
Corrosivity ratio (CR) deals with the susceptibility of groundwater to corrosion and is used to determine whether water is safe to transport through metallic pipes. It is shown as the ratio of alkaline earth metals to saline salts in groundwater and is calculated by using the following equation (Ryner 1944; Raman 1985; Jasrotia et al. 2019):
where all the ion concentrations are expressed in meq/L.
The effects of corrosivity on metallic pipes had been thoroughly investigated. Groundwater with a CR less than one is considered safe for its transport in any kind of metallic pipes, and water with a CR more than one is considered to be unsafe (Ryner 1944; Raman 1985; Jasrotia et al. 2019). Corrosion affects the hydraulic capacity of metallic pipes (Rao and Latha 2019). The calculated values of groundwater samples show that 65 samples have a CR less than one and are thus, safe for their transport through pipes, whereas 10 samples have a CR of more than one, and are, therefore, considered to be unsafe. The spatial distribution of CR is shown in Fig. 10.
The general water quality criteria of Anon (1986) for industrial purposes are followed to determine the groundwater suitability for industrial uses because it deals with both incrustation and corrosion (Table 8). According to this standard, incrustation can occur if groundwater yields HCO3− and SO42− more than 400 mg/L and 100 mg/L, respectively. Corrosion can occur if the pH of the groundwater is less than 7 with TDS more than 1000 mg/L or the Cl− more than 500 mg/L. The results obtained show that groundwater quality causes incrustation in 21.3% of the samples, out of which 12% of samples develop incrustation due to high HCO3−, and 9.3% of the samples due to SO42,− whereas 53% of the samples develop corrosion due to less pH (Table 8).
Conclusion
The present study was conducted in the Kathua region of Jammu and Kashmir, Northern India, where the majority of people rely on groundwater for a variety of purposes (domestic, irrigation, and industrial). The obtained hydrochemical results were compared with the WHO (2017) and BIS (2012) water guidelines. It is found that groundwater is soft to hard, excellent to good, and alkaline in nature. The Ca+ is the dominant cation and HCO3− is the dominant anion in groundwater. In the order of abundance, Ca2+ > Na+ > Mg2+ > K+ are the dominant cations, and HCO3− > SO42− > Cl− > NO3− are the dominant anions. The higher HCO3− concentration and less pH (< 8.8) indicate that the chemical weathering had been operating in the area. According to the WHO (2017) and BIS (2012) water guidelines, the groundwater falls within the permissible limits and is good for drinking and irrigation purposes. Groundwater quality index shows that the groundwater is suitable for drinking except at one location. The Piper diagram reveals that nearly 90% of samples fall in the Ca2+-Mg2+-HCO3− facies and groundwater is alkaline in nature and is good for drinking. The Gibbs diagram indicates that the hydrochemistry of the study area is largely influenced by rock–water interaction and varied lithologies are a dominant factor that controls its water composition.
The electrical conductivity and sodium percentage indicate that the groundwater is suitable for irrigation, but three samples have a sodium percentage of more than 60%, and are not suitable for crop growth. According to the Wilcox and USSLS diagrams, the majority of groundwater is suitable for irrigation. The MAR indicates that only groundwater of 14 samples (19%) is unsuitable for irrigation. The corrosivity ratio implies that groundwater of 65 samples is safe to transport through metallic pipes, and for the remaining 10 samples is considered unsafe for industrial purposes. Groundwater, on the other hand, can cause incrustation and corrosion in some parts of the area. Finally, it is found that the groundwater in most parts of the study area is suitable for drinking, irrigation and industrial uses.
References
Adimalla N (2020) Controlling factors and mechanism of groundwater quality variation in semiarid region of South India: an approach of water quality index (WQI) and health risk assessment (HRA). Environ Geochem Health 42(6):1725–1752
Adimalla N, Qian H (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, South India. Ecotoxicol Environ Saf 176:153–161. https://doi.org/10.1016/j.ecoenv.2019.03.066
Adimalla N, Taloor AK (2020) Hydrogeochemical investigation of groundwater quality in the hard rock terrain of South India using geographic information system (GIS) and groundwater quality index (GWQI) techniques. Groundw Sustain Dev 10:100288. https://doi.org/10.1016/j.gsd.2019.100288
Adimalla N, Venkatayogi S (2017) Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State, South India. Environ Earth Sci 76:45. https://doi.org/10.1007/s12665-016-6362-2
Adimalla N, Venkatayogi S (2018) Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl Water Sci 8:44. https://doi.org/10.1007/s13201-018-0682-1
Adimalla N, Dhakate R, Kasarla A, Taloor AJ (2020) Appraisal of groundwater quality for drinking and irrigation purposes in central Telangana, India. Groundw Sustain Dev 10:100334. https://doi.org/10.1016/j.gsd.2020.100334
Adimalla N, Manne R, Zhang Y, Xu P, Qian H (2022) Evaluation of groundwater quality and its suitability for drinking purposes in semi-arid region of Southern India: an application GIS. Geocarto Int. https://doi.org/10.1080/10106049.2022.2040603
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process 5:363–383. https://doi.org/10.1007/s40710-018-0297-4
Afroza R, Mazumdar QH, Choudhary SJ, Kazi MAI, Ahsa N, Al-Mansur MA (2009) Hydrochemistry and origin of salinity in groundwater in the parts of the Lower Tista floodplains, northwest Bangladesh. J Geol Soc India 74(2):223–232
Agarwal RP, Nanda AC, Prasad DN, Dey BK (1993) Geology and biostratigraphy of the Upper Siwalik of Samba area, Jammu Foothills. Him Geol 4(2):227–236
Amiri V, Bhattacharya P, Nakhaei M (2021) The hydrogeochemical evaluation of groundwater resources and their suitability for agricultural and industrial uses in an arid area of Iran. Groundw Sustain Dev 12:100527. https://doi.org/10.1016/j.gsd.2020.100527
An W, Xiumian Hu, Garzanti E, Wang J-G, Liu Q (2021) New precise dating of the India‐Asia collision in the Tibetan Himalaya at 61 Ma. Geophys Res Lett. https://doi.org/10.1029/2020GL090641
Anon F (1986) Groundwater and wells. Johnson Screens St. Paul, Minnesota
Ansari JA, Umar R (2019) Evaluation of hydrogeochemical characteristics and groundwater quality in the quaternary aquifers of Unnao District, Uttar Pradesh, India. Hydro Res 1:36–47
APHA (1998) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association and Water Environmental Federation
AWWA (1971) Water quality and treatment- a handbook of public water supplies. McGraw-Hill, New York
Ayers RS, Westcott DW (1985) Water quality for agriculture (No. 29). Food and Agriculture Organization of the United Nations, Rome
Back W (1966) Hydrochemical facies and groundwater flow patterns in northern part of Atlantic Coastal Plain. US Geol Surv Prof Pap 498:1–42
Bajaj M, Eiche E, Neumann T, Winter J, Gallert C (2011) Hazardous concentrations of selenium in soil and groundwater in North-West India. J Hazard Mater 189(3):640–646
Billings RJ, Berkowitz RJ, Watson G (2004) Teeth. Pediatrics 113:1120–1127
BIS (2012) Indian standard: drinking water-specification. Manak Bhavan, Bahadur Shah Zafar Marg, New Delhi, India. http://cgwb.gov.in/Documents/WQ-standards.pdf
Bouslah S, Lakhdar D, Larbi H (2017) Water quality index assessment of Koudiat Medouar reservoir, northeast Algeria using weighted arithmetic index method. J Water Land Dev 35:221–228
Burrough PA, Mcdonnel RA, Lloyd CD (1998) Principles of geographical information systems. Oxford University Press, Oxford
CGWB (1997) Groundwater water quality studies in Jammu and Kathua districts (J&K). Roorkee: National Institute of Hydrology, India. https://www.indiawaterportal.org/sites/default/files/iwp2/Groundwater_quality_studies_ in_Jammu_and_Kathua_districts__J_K__NIH_1996_97.pdf
CGWB (2013) Brochure of Kathua District, Jammu & Kashmir State. North-Western Himalayan Region, Jammu, Central Ground Water Board, New Delhi http://cgwb.gov.in/District_Profile/JandK/kathua.pdf
Chandan R (2017) Groundwater quality deterioration due to unplanned industrialization: a case study of district Samba, J&K, India. J Emerg Technol Innov Res 4(12):266–271
Chowdhury P, Mukhopadhyay BP, Nayak S, Bera A (2021) Hydro-chemical characterization of groundwater and evaluation of health risk assessment for fluoride contamination areas in the eastern blocks of Purulia district, India. Environ Dev Sustain. https://doi.org/10.1007/s10668-021-01911-1
Comly HH (1945) Cyanosis in infants caused by nitrates in well water. J Am Med Assoc 129(2):112–116
Dar IA, Sankar K, Dar MA (2011) Spatial assessment of groundwater quality in Mamundiyar basin, Tamil Nadu, India. Environ Monit Assess 178:437–447
Dar T, Rai N, Bhat A (2020) Delineation of potential groundwater recharge zones using analytical hierarchy process (AHP). Geol Ecol Lands 5(4):292–307
Davis SN, Dewiest RJM (1966) Hydrogeology. John Wiley and Sons, New York
DDC Report (2019) District environment plan for Kathua District. District Development Commissioner Kathua, Government of Union Territory of Jammu and Kashmir. https://Cdn.S3waas.Gov.In/S3eb163727917cbba1eea208541a643e74/Uploads/2019/12/2019122011.Pdf
DES Report (2017) Economic Survey 2017. Directorate of Economics and Statistics J&K, Govt. of Jammu and Kashmir. http://ecostatjk.nic.in/Economic%20Survey%202017.pdf
Dişli E (2017) Hydrochemical characteristics of surface and groundwater and suitability for drinking and agricultural use in the Upper Tigris River basin, Diyarbakır–Batman, Turkey. Environ Earth Sci. https://doi.org/10.1007/s12665-017-6820-5
Dolma K, Rishi MS, Herojeet R (2015) Baseline study of drinking water quality- a case of Leh town, Ladakh (J&K), India. Hydrol Curr Res 6(1):2–6
Doneen LD (1964) Water quality for agriculture, California. Department of Irrigation, University of California, California, USA
Faten H, Azouzi R, Charef A, Bedir M (2016) Assessment of groundwater quality for irrigation and drinking purposes and identification of hydrogeochemical mechanisms evolution in Northeastern Tunisia. Environ Earth Sci 75:746. https://doi.org/10.1007/s12665-016-5441-8
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, Englewood Cliffs, USA, New Jersey
Gansser A (1964) Geology of Himalaya. Wiley-Interscience, London
Gibbs RJ (1970) Mechanisms controlling worlds water chemistry. Sci 170:1088–1090
Gilly G, Corrao G, Favilli S (1984) Concentrations of nitrates in drinking water and incidence of gastric carcinomas: first descriptive study of the Piemonate region, Italy. Sci Total Environ 34:35–37
Handa BK (1975) Geochemistry and genesis of fluoride containing ground waters in India. Groundwater 13(3):275–281
Haq S, Ara S, Bhatti AA, Jalal F, Bhat MS (2017) Determination of water quality of surface water resources of central Kashmir, Jammu and Kashmir, India. J Pharmacogn Phytochem 6(4):2034–2039
Haque S, Kannaujiya S, Taloor AK, Keshri D, Bhunia RK, Ray PKC, Chauhan P (2020) Identification of groundwater resource zone in the active tectonic region of Himalaya through earth observatory techniques. Groundw Sustain Dev 10:100337. https://doi.org/10.1016/j.gsd.2020.100337
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water. Scientific Publishers, Jodhpur
Hounslow AW (1995) Water quality data: analysis and interpretation. CRC Press, Boca Raton
Howard G, Bartram J (2003) Domestic water quantity, service level and health. WHO Press, Geneva
Hundal HS (2011) Geochemistry and assessment of hydrogeochemical processes in groundwater in the southern part of Bathinda district of Punjab, northwest India. Environ Earth Sci 64(7):1823–1833
IPCC Report (2021) Climate change 2021: the physical science basis. https://www.ipcc.ch/report/sixth-assessment-report-working-group-i/
Jain SK, Agarwal PK, Singh VP (2007) Hydrology and water resources of India. Geological Society of India, Bangalore
Jain CK, Bandyopadhyay A, Bhadra A (2010) Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India. Environ Monit Assess 166:663–676
Jasrotia AS, Kumar A (2014) Estimation of replenishable groundwater resources and their status of utilization in Jammu Himalaya, J&K, India. Eur Water 48:17–27
Jasrotia AS, Taloor AK, Andotra U, Bhagat BD (2018) Geoinformatics based groundwater quality assessment for domestic and irrigation uses of the Western Doon valley, Uttarakhand, India. Groundw Sustain Dev 6:200–212
Jasrotia AS, Taloor AK, Andotra U, Kumar R (2019) Monitoring and assessment of groundwater quality and its suitability for domestic and agricultural use in the Cenozoic rocks of Jammu Himalaya, India: a geospatial technology based approach. Groundw Sustain Dev 8:554–566
Jeelani GH, Shah RA, Hussain A (2014) Hydrogeochemical assessment of groundwater in Kashmir valley, India. J Earth Syst Sci 123(5):1031–1043
Joshi DM, Kumar A, Agrawal N (2009) Assessment of the irrigation water quality of river Ganga in Haridwar District. Rasayan J Chem 2(2):285–292
Kanwar P, Khanna P (2014) Appraisal of ground water quality for irrigation in outer plains of Kathua District, J&K, India. Int J Geol Earth Environ Sci 4(3):74–80
Karanth KR (1987) Groundwater assessment, development and management. Tata McGraw-Hill Publishing, New Delhi
Karunakaran C, Ranga Rao A (1976) Status of exploration for hydrocarbons in the Himalayan region: contributions to stratigraphy and structure. Geol Surv India Misc Publ 41:1–66
Karunanidhi D, Aravinthasamy P, Subramani T, Muthusankar G (2021) Revealing drinking water quality issues and possible health risks based on water quality index (WQI) method in the Shanmuganadhi River basin of South India. Environ Geochem Health 43(2):931–948
Khan A, Govil H, Taloor AK, Kumar G (2020) Identification of artificial groundwater recharge sites in parts of Yamuna river basin India based on remote sensing and geographical information system. Groundw Sustain Dev 10:100415. https://doi.org/10.1016/j.gsd.2020.100415
Kumar R, Ghosh SK, Mazari RK, Sangode SJ (2003) Tectonic impact on the fluvial deposits of Plio-Pleistocene Himalayan foreland basin, India. Sed Geol 158(3):209–234
Kumar R, Singh RD, Sharma KD (2005) Water resources of India. Curr Sci 89(5):794–811
Kumar J, Biswas B, Verghese S (2021) Assessment of groundwater quality for drinking and irrigation purpose using geospatial and statistical techniques in a semi-arid region of Rajasthan, India. J Geol Soc India 97:416–427
Lakshmi RV, Raja V, Sekar CP, Neelakantan MA (2021) Evaluation of groundwater quality in Virudhunagar Taluk, Tamil Nadu, India by using statistical methods and GIS technique. J Geol Soc India 97(5):527–538
Lapworth DJ, Krishan G, MacDonald AM, Rao MS (2017) Groundwater quality in the alluvial aquifer system of northwest India: new evidence of the extent of anthropogenic and geogenic contamination. Sci Total Environ 599:1433–1444
Latha PS, Rao KN (2012) An integrated approach to assess the quality of groundwater in a coastal aquifer of Andhra Pradesh, India. Environ Earth Sci 66:2143–2169
Li P, Wu J, Qian H (2013) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69(7):2211–2225
Li P, Wu J, Qian H (2016) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab J Geosci 9(1):15. https://doi.org/10.1007/s12517-015-2059-1
Lone SA, Jeelani G, Mukherjee A, Coomar P (2020) Geogenic groundwater arsenic in high altitude bedrock aquifers of upper Indus river basin (UIRB) Ladakh. Appl Geochem 113:104497. https://doi.org/10.1016/j.apgeochem.2019.104497
MacDonald AM, Bonsor HC, Ahmed KM, Burgess WG, Basharat M, Calow RC, Dixit A, Foster SSD, Gopal K, Lapworth DJ, Lark RM (2016) Groundwater quality and depletion in the Indo-Gangetic Basin mapped from in situ observations. Nat Geosci 9(10):762–766
Magesh NS, Krishnakumar S, Chandrasekar N, Soundranayagam JP (2013) Groundwater quality assessment using WQI and GIS techniques, Dindigula district, Tamil Nadu, India. Arab J Geosci 6:4179–4189
Mandal J, Golui D, Raj A, Ganguly P (2019) Risk assessment of arsenic in wheat and maize grown in organic matter amended soils of Indo-Gangetic plain of Bihar, India. Soil Sediment Contam 28(8):757–772
Meena PL, Jain PK, Meena KS (2016) Assessment of groundwater quality and its suitability for drinking and domestic uses by using WQI and statistical analysis in river basin area in Jahzpur Tehsil, Bhilwara District (Rajasthan, India). Int J Curr Microbiol App Sci 5:415–423
Mohamed AK, Liu D, Song K, Mohamed MA, Aldaw E, Elubid BA (2019) Hydrochemical analysis and fuzzy logic method for evaluation of groundwater quality in the North Chengdu Plain, China. Int J Environ Res Public Health 16(302):1–21. https://doi.org/10.3390/ijerph16030302
MPCB (1997) Manual for water testing kit, Delhi. Central Pollution Control Board, New Delhi, India
Mukherjee A, Duttagupta S, Chattopadhyay S, Bhanja SN, Bhattacharya A, Chakraborty S, Sarkar S, Ghosh T, Bhattacharya J, Sahu S (2019) Impact of sanitation and socio-economy on groundwater fecal pollution and human health towards achieving sustainable development goals across India from ground-observations and satellite-derived nightlight. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-019-50875-w
Murtaza KO, Romhoo SA, Rashid I, Shah W (2020) Geospatial assessment of groundwater quality in Udhampur District, Jammu and Kashmir. Proc Natl Acad Sci India Phys Sci 90(5):883–897
Naidu S, Gupta G, Singh R, Tahama K, Erram VC (2021) Hydrogeochemical processes regulating the groundwater quality and its suitability for drinking and irrigation purpose in parts of coastal Sindhudurg District, Maharashtra. J Geol Soc India 97:173–185
Najman Y (2006) The detrital record of orogenesis: a review of approaches and techniques used in the Himalayan sedimentary basins. Earth Sci Rev 74(1–2):1–72
Najman Y, Garzanti E (2000) Reconstructing early Himalayan tectonic evolution and paleogeography from tertiary foreland basin sedimentary rocks, northern India. GSA Bull 112(3):435–449
Nanda AC (2015) Siwalik mammalian faunas of the Himalayan foothills: with reference to biochronology, linkages and migration. Dehradun: Wadia Institute of Himalayan Geology India. Monograph 2:1–341
Paliwal KV (1972) Irrigation with saline water, New Delhi. Monogram no. 2, Indian Agricultural Research Institute, New Delhi, India
Patil VBB, Pinto SM, Govindaraju T, Hebbalu VS, Bhat V, Kannanur LN (2020) Multivariate statistics and water quality index (WQI) approach for geochemical assessment of groundwater quality—a case study of Kanavi Halla Sub-Basin, Belagavi, India. Environ Geochem Health 42(9):2667–2684
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–928
Piper AM (1953) A graphic procedure in the geochemical interpretation of water analysis. Groundwater Note 12, US Geol Surv, Washington DC, USA
Pir RA (2020) Ground Water Year Book 2018–19 Jammu and Kashmir. Central Ground Water Board, North Western Himalayan Region, Jammu. http://cgwb.gov.in/Regions/NWHR/Reports/GWYB%202018-19.pdf
Prashanth M, Kumar A, Dhar S, Verma O, Sharma S (2021) Morphometric characterization and prioritization of sub-watersheds for assessing soil erosion susceptibility in the Dehar watershed (Himachal Himalaya), Northern India. Him Geol 42(2):345–358
Prashanth M, Kumar A, Dhar S, Verma O, Gogoi K (2022) Hypsometric analysis for determining erosion proneness of Dehar watershed, Himachal Himalaya, North India. J Geosci Res 7(1):86–94
Ragunath HM (1987) Ground water. Wiley Eastern Ltd, New Delhi
Ram A, Tiwari SK, Pandey HK, Chaurasia, AK, Singh S, Singh YV (2021) Groundwater quality assessment using water quality index (WQI) under GIS framework. Appl Water Sci. https://doi.org/10.1007/s13201-021-01376-7
Raman V (1985) Impact of corrosion in the conveyance and distribution of water. J Indian Water Works Assn 11:115–121
Ranga Rao A, Agarwal RP, Sharma UN, Bhalla MS, Nanda AC (1988) Magnetic polarity stratigraphy and vertebrate palaeontology of the Upper Siwalik Subgroup of Jammu Hills, India. J Geol Soc India 31:361–385
Rao KN, Latha PS (2019) Groundwater quality assessment using water quality index with a special focus on vulnerable tribal region of Eastern Ghats hard rock terrain, southern India. Arab J Geosci 12:267. https://doi.org/10.1007/s12517-019-4440-y
Ravikumar P, Somashekar RK (2017) Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Appl Water Sci 7(2):745–755
Razowska-Jaworek L (2014) Calcium and magnesium in groundwater: occurrence and significance for human health. CRC Press, London
Reddy YS, Sunitha V, Suvarna B (2020) Groundwater quality evaluation using GIS and water quality index in and around inactive mines, Southwestern parts of Cuddapah basin, Andhra Pradesh, South India. Hydro Res 3:146–157
Richard LA (1954) Diagnosis and improvement of saline alkali soils. Agriculture handbook 60. US Department of Agriculture, Washington DC, USA
Rodell M, Velicogna I, Famiglietti JS (2009) Satellite based estimates of groundwater depletion in India. Nat 460(7258):999–1002
Ryner JW (1944) A new index for determining amount of calcium carbonate scale formed by water. J Am Wat Assoc 36:472–486
Saeid S, Chizari M, Sadighi H, Bijani M (2018) Assessment of agricultural groundwater uses in Iran: a cultural environmental bias. Hydrogeol J 26(1):285–295
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers. McGraw Hill, New York
Schoeller H (1967) Geochemistry of groundwater: an international guide for research and practice. UNESCO, Paris
Shah SK (2018) Historical geology of India. Scientific Publishers, Jodhpur
Singh AK, Mondal GC, Kumar S, Singh TB, Tiwari BK, Sinha A (2008) Major ion chemistry, weathering processes and waste water quality assessment in upper catchment of Damodar river basin, India. Environ Geol 54(4):745–758
Singh AK, Jasrotia AS, Taloor AK, Kotlia BS, Kumar V, Roy S, Ray PKC, Singh KK, Singh AK, Sharma AK (2017) Estimation of quantitative measures of total water storage variation from GRACE and GLDAS-NOAH satellites using geospatial technology. Quat Int 444:191–200
Snousy MG, Wu J, Su F, Abdelhalim A, Ismail E (2021) Groundwater quality and its regulating geochemical processes in Assiut Province, Egypt. Expo Health. https://doi.org/10.1007/s12403-021-00445-1
Subba Rao N (2018) Groundwater quality from a part of Prakasam District, Andhra Pradesh, India. Appl Water Sci. https://doi.org/10.1007/s13201-018-0665-2
Subba Rao N, Surya Rao P, Venktram Reddy G, Nagamani M, Vidyasagar G, Satyanarayana NLVV (2012) Chemical characteristics of groundwater and assessment of groundwater quality in Varaha river basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 184:5189–5214
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47(8):1099–1110
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content on soils. In: Szabolics I (eds) Proceedings of the 8th international congress soil science sodics soils. Res Inst Soil Sci Agric Chem Hungarian Acad Sci, ISSS Trans II, pp 802–812
Taloor AK, Pir RA, Adimalla N, Ali S, Manhas DS, Roy S, Singh AK (2020) Spring water quality and discharge assessment in the Basantar watershed of Jammu Himalaya using geographic information system (GIS) and water quality index (WQI). Groundw Sustain Dev 10:100364. https://doi.org/10.1016/j.gsd.2020.100364
Taloor AK, Kotlia BS, Kumar K (2021) Water, cryosphere, and climate change in the Himalayas: a geospatial approach. Springer, Switzerland
Tatawat RK, Chandel CS (2008) A hydrochemical profile for assessing the groundwater quality of Jaipur City. Environ Monit Assess 143(1):337–343
Todd DK (1976) Groundwater hydrology. Wiley, New York
UNEP (1999) Global environment outlook 2000. Earthscan Publications Ltd, London
Vahab A, Sohrabi N, Dadgar MA (2015) Evaluation of groundwater chemistry and its suitability for drinking and agricultural uses in the Lenjanat plain, central Iran. Environ Earth Sci 74:6163–6176
Verma O (2021) Climate change and its impacts with special reference to India. In: Taloor AK, Kotlia BS, Kumar K (eds) Water, Cryosphere, and Climate Change in the Himalayas: a geospatial approach. Springer, Cham, pp 39–55. https://doi.org/10.1007/978-3-030-67932-3_3
Valdiya KS (2016) The making of India: Geodynamic Evolution. Springer, Switzerland
Verma O, Khosla A, Goin FJ, Kaur J (2016) Historical biogeography of the Late Cretaceous vertebrates of India: comparison of geophysical and paleontological data. New Mexico Mus Nat Hist Sci Bull 71:317–330
Verma P, Singh PK, Sinha RR, Tiwari AK (2020) Assessment of groundwater quality status by using water quality index (WQI) and geographic information system (GIS) approaches: a case study of the Bokaro district, India. Appl Water Sci. https://doi.org/10.1007/s13201-019-1088-4
Wang Y, Li P (2021) Appraisal of shallow groundwater quality with human health risk assessment in different seasons in rural areas of the Guanzhong Plain (China). Environ Res. https://doi.org/10.1016/j.envres.2021.112210
Wenzel WW, Blum WE (1992) Fluorine speciation and mobility in F-contaminated soils. Soil Sci 153(5):357–364
WHO (2011) Guidelines for Drinking-Water Quality. WHO Press, World Health Organization, Geneva
WHO (2017) Guidelines for Drinking-Water Quality: fourth edition incorporating the first addendum. World Health Organization, Geneva
Wilcox LV (1955) Classification and Use of Irrigation Waters. DC Circular USDA, Washington
World Bank (2010) Deep wells and prudence: towards pragmatic action for addressing groundwater overexploitation in India. The international bank for reconstruction and development. The World Bank USA, Washington DC
Xu P, Feng W, Qian H, Zhang Q (2019) Hydrogeochemical characterization and irrigation quality assessment of shallow groundwater in the Central-Western Guanzhong Basin, China. Int J Environ Res Public Health 16(9):1492. https://doi.org/10.3390/ijerph16091492
Yadav AK, Khan P, Sharma SK (2010) Water quality index assessment of groundwater in Todaraisingh Tehsil of Rajasthan state, India-a greener approach. E J Chem 7(s1):S428–S432. https://doi.org/10.1155/2010/419432
Yaseen ZM, El-Shafie A, Jaafar O, Afan HA, Sayl KN (2015) Artificial intelligence-based models for stream-flow forecasting: 2000–2015. J Hydrol 530:829–844
Zhang Q, Xu P, Qian H (2019) Assessment of groundwater quality and human health risk (HHR) evaluation of nitrate in the Central-Western Guanzhong Basin, China. Int J Environ Res Public Health 16(21):4246. https://doi.org/10.3390/ijerph16214246
Zhang Q, Xu P, Qian H (2020) Groundwater quality assessment using improved water quality index (WQI) and human health risk (HHR) evaluation in a semi-arid region of northwest China. Expo Health 12:487–500
Zhao H, Song F, Su F, Shen Y, Li P (2021) Removal of cadmium from contaminated groundwater using a novel silicon/aluminum nanomaterial: an experimental study. Arch Environ Contam Toxicol 80(1):234–247
Acknowledgements
The authors are thankful to the Director, School of Sciences, Indira Gandhi National Open University, New Delhi, for providing the necessary support to conduct this work. Thanks also go to the Panjab University, Chandigarh, for helping to carry out the analysis of water quality. One of the authors BK would like to thank Indira Gandhi National Open University for providing financial support to carry out this research work under the research fellowship no. IG/RU/IGNOU-RF/2019/6656. The authors are grateful to the Editor-in-Chief and anonymous reviewers for their critical review, valuable suggestions, and comments that greatly improved the quality of the manuscript.
Funding
Indira Gandhi National Open University, New Delhi, India.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors have participated in this research, and there is no conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kouser, B., Bala, A., Verma, O. et al. Hydrochemistry for the assessment of groundwater quality in the Kathua region, Jammu and Kashmir, India. Appl Water Sci 12, 143 (2022). https://doi.org/10.1007/s13201-022-01673-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01673-9