Abstract
An attempt to evaluate potential hazard for quality of manufactured construction elements and concrete in case of use of leachate as technological water was made in this paper. This may lead to negative impact on strength parameters of the product made in the analysed company. Raw industrial leachate must not be discharged into sewerage system due to increased concentrations of some mineral components; it must be initially purified. Selected elements of multiple regression module and principal components analysis were used to describe the above phenomena. The model we have developed uses selected significant parameters of pollutants contained in leachate from a process industrial waste heap. The model takes into account leachate conductivity and sulphate ions, in which increased lead compounds concentration was determined. The model was verified during its use. The variables used in the model explain the significant percentage of variability noted for the dependent variable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manufacturing of construction materials, in particular concrete, is associated with generation of solid waste and wastewater. Washing of concrete mixers and units generates wastewater polluting water and soil. Concrete washing water features high pH fluctuating between 11 and 12 due to high limestone content in the solution. This water contains dissolved solids such as hydroxides and sulphates from cement, chlorides from application of calcium chloride admixture, oils and greases from plant and machinery as well as small amounts of other chemicals associated with Portland cement hydration and derivates from chemical admixtures. Many publications contain descriptions of use of concrete wash water for making mortar and concrete (Meena and Luhar 2019; Vaičiukynienė et al. 2021; Taghizadeh et al. 2021; Varshney et al. 2021). However, such materials must comply with the quality standards. The standards had been established for identification of particular concrete quality parameters such as strength, flexibility, hardness and workability. It has been proved that concrete wash water featured increased alkalinity and solids content going beyond the limits of popular standard ASTMC94, which caused that manufactured concrete was more porous. This limits the interest in application of technological water recycling mostly in the case of use of various admixtures in concrete production ASTM C94 (Asadollahfardi et al. 2015; Meena and Luhar 2019).
Leachates generated during construction materials production fed into technological water make an additional source of pollution of technical concrete and prefabricated elements (Bonaccorsi et al. 2004; Chen et al. 2009; Dai et al. 2009). Leachates fed into the manufactured product feature increased concentration of mineral components such as sulphates, carbonates, chlorides, nitrates and compounds of such heavy metals as chromium, lead, nickel, copper, cadmium and zinc (Kurdowski 2010; Szymański et al. 2018). Examples of such waste and leachate management were found in one of the companies manufacturing construction materials (Magistri and D’Arcangelo 2008).
In the EU countries, the Landfill Directive, the Waste Framework Directive 2008/98/EC, the Urban Wastewater Treatment Regulations Council Directive 99/31/EC and the Water Framework Directive 2000/60/EC belong to the main European regulations concerning waste landfilling and leachates management. They put an obligation on the member states to regularly monitor leachate condition. Despite implementation of the said regulations, the scope of the parameters to be monitored has not been strictly defined. It has been only stated that the analysed substances should be selected based on the composition of the landfilled waste (Brennan et al. 2016, 2017; Wdowczyk and Szymańska-Pulikowska 2020; Ida and Eva 2021).
In all member states, the regulations concerning the monitoring of wastewater discharge exist on the national level; subsequently, they are being supplemented or defined at the regional or local level. For example, in Germany, Austria, Romania, Ukraine and Czech Republic testing of heave metals content is monitored only if there occurs a strong impact of industrial waste that is channelled via a sewerage system to any municipal wastewater treatment plant. Slovakia, Hungary, Romania and Moldova keep monitoring heavy metals content in a more regular way within the self-monitoring procedure. In Ireland, the parameters to be analysed in landfill leachates are determined individually in the waste disposal licence. Their scope depends on the type and composition of the landfilled waste (Naveen et al. 2017; Wdowczyk and Szymańska-Pulikowska 2020; Ida and Eva 2021).
In the USA, the Environmental Protection Agency regulates wastewater discharge and treatment based on the Clean Water Act (CWA). The National Pollution Discharge Elimination System (NPDES) issues licences for all discharges and wastewater treatment plants as well as sets the norms regarding quality of wastewater discharged to surface water and to municipal wastewater treatment plants. In China, detailed regulations regarding heavy metals content in wastewater and drinking water are also set at the national level. In China, limits of the admissible heavy metals content have been defined by the Chinese Ministry of Health (CMH) and The National Standard of China, in Kenya—by the National Environment Management Authority (NEMA) and Kenya Bureau of Standards (Kinuthia et al. 2020). In Canada, the standards and regulations pertaining to the collection, protection, treatment and elimination of leachates are very strict; they also put emphasis on regular monitoring of surface and ground waters nearby any landfill location (Naveen et al. 2017).
According to Polish law, the concentration of the said metals must not exceed the admissible values in leachates discharged also to the aquatic environment or ground as well as to sewerage systems (Regulation 2006; Regulation 2019). Discharge of leachates featuring higher concentration of those metals than pure replenishing water may catalyse generation of new crystallographic structures in the manufactured material having consequently negative impact on strength parameters of the material (Baetzner 2002; Nocuń-Wczelik and Łój 2006; Czarnecki et al. 2015; Nalet and Nonat 2016). This has also impact on processes of interaction between particular parameters/components contained in the generated leachate that is reused (Król 2012; Czarnecki et al. 2015) in replenishment water. Leachates may also have negative impact on the natural environment unless subjected to a pre-treatment before discharging to a sewerage system, in particular to the ground or aquatic environment (Magistri and D’Arcangelo 2008; Król 2012; Czarnecki et al. 2015).
This paper illustrates an effort to assess potential hazards for product (structural elements) quality and the phenomena that occur in leachates from industrial waste dump. The objective of the research work was to determine statistically the significant impact of particular components (independent variables) on concentration of one of toxic lead compounds in tested leachate. Selected elements of mathematical statistics in the form of the multiple regression module and main components analysis were used to describe those phenomena (Stanisz 2001).
Tested material and research methodology
Materials and scope of research
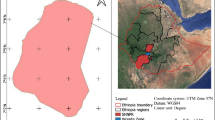
The leachate quality tests performed at the production unit site allowed to determine basic physicochemical indicators as well as heavy metals and alkaline leachates sampled for testing (Figs.1, 2) including:
-
Nearby a sewer sump in A1 and A2 locations,
-
From existing leachate pond near the construction waste heap at A3 and A4 locations.
Locations A1 and A2 are specific for leachate originating from the washing facility of the concrete production unit, whereas A3 and A4 are located at leachate pond nearby the waste heap. The tests were performed during 2014–
2018 period. Five (5) test series were effected in total.
Analytical methodology and test results
Particular determinations were performed in accordance with Regulation of the Minister of Maritime Economy and Inland Navigation of 12 July 2019 Journal of Laws 2019 item 1311, Regulation of the Minister of Construction Sector of 14 July 2006 Journal of Laws 2006 No 136, item 964 (Regulation 2006; Regulation 2019) as well as methods described in the literature (Kowal and Świderska-Bróż 1996).
Particular parameters of leachates (technological water) were being determined using mainly standard research methods in accordance with Polish standards (Table 1).
The content of total organic carbon (TOC) were determined using the Vario Max CN macroanalyser. Samples of leachates for testing were mineralised using a mixture of acids (65% HNO3, 70% HClO4 and 30% H2O2) using microwave energy (Milestone 1200 Mega apparatus). The contents of heavy metals and alkali metals were determined using FAAS technique (iCE 3500Z Thermo Scientific SOLAAR) (Czerniak and Poszyler-Adamska 2006; Szymański et al. 2018).
Statistical analysis
To evaluate tested pollutants, components occurring in particular samples of leachate from concrete production STATISTICA—multiple regression module—were used (Stanisz 2001). To describe correlations between particular variables, an estimator of the correlation coefficient for tested features, as r correlation, was used. For this purpose, Fischer’s test (F) verifying the hypothesis that the multiple correlation coefficient is significantly different from “0” was useful; this means that at least one model structural parameter (without free term) is different from zero.
A number of notations that are specific for the analysis of results originating from statistical method research were used in the presented paper (Stanisz 2001) and they are: R2—a multiple determination coefficient measuring which proportion of variation of the dependent variable is explained by the regression model calculated from the equation: R2 = 1- [Rest SS/ Total SS], where Rest SS is a sum of squares error and Total SS is a total sum of squares. The multiple correlation coefficient is a root of R2. Corrected R2 is interpreted just like R2. The corrected R2 coefficient takes into account a variable number of the degrees of freedom for Total SS and Rest SS.
The estimation standard error Se provides information about the average quantity of empirical deviations of the dependent variable from the model-derived value, defined by formula:
where the estimation standard error pertains to: yi—independent variable values. \(\hat{y}_{i}\)—model-derived independent variable value. ei = yi—\(\hat{y}_{i}\) rests. Se = estimation standard error.
-
The statistics F (Fischer’s test) is used to verify the significance of the entire model.
-
The critical level of significance (p) is a probability of making an error consisting in refusal of the true null hypothesis based on the observed sample values. Low values of the significance level (usually α < 0.05) indicate low risk whilst the decision to reject the null hypothesis is made.
-
The correlation is a measure of association between two or more variables. The correlation of two variables can be expressed numerically using the linear correlation coefficient (r). The linear correlation coefficient takes values from − 1.00 to + 1.00 interval. The – 1.00 value represents strong negative correlation whereas + 1.00 value represents strong positive correlation. Value r = 0.00 indicates absence of the correlation.
Results and discussion
Results of physicochemical analysis
The scope of the said research work is illustrated in Tables 2 and 3. Table 2 comprises test results for basic physicochemical indicators, whereas Table 3 comprises test results for heavy and alkali metals.
Tests performed on leachates from construction waste heap and technological devices of the concrete production plant indicated increased contents of some heavy metals including chromium, lead and iron compounds. However, no mercury compounds were found therein. Zinc, cadmium, nickel and manganese occurred in trace concentrations. Calcium, and to a much lesser extent magnesium, dominated in the alkali metals group. The said leachates were also used for the preparation of technological water. Some volume of the leachates was being discharged to the combined sewerage system. Environmental protection services had been indicating unlawful management of the leachates (Regulation 2006; Regulation 2019).
The research results indicate environmental protection problems as well as improper use of leachate for production of concrete and concrete products originating from that company. As it appears from Tables 2 and 3, such excessive values (average daily values) pertained, in accordance with the above-mentioned regulations, to: pH—sample No 3 (12.60), suspended solids—sample No 3 (8600 mg/dm3), BOD5—sample No 1 (596 mgO2/dm3), CODCr—sample No 1 (3980 mg/O2dm3), TOC—sample No 1 (1492 mgC/dm3), sulphates—sample No 4 (676.1 mgSO4/dm3) and calcium—Table 3, sample No 4 (407.53 mgCa/dm3).
The test results presented in Table 2 indicate that leachate samples taken for testing at point A1 (Fig. 1) were exposed to direct contact with the ground and vegetal material. Therefore, organic substances containing humic compounds could migrate to the leachates. This has been indicated by increased loads of BOD5, CODCr and TOC. It can be reckoned that humic compounds formed metal–organic combinations with, for example, iron and magnesium. This may explain their high correlations. Occurrence of calcium compounds favoured combinations with sulphate ions—Fig. 5 (correlation coefficient r = 0.986). Peculiar character of copper behaviour may also be interpreted as formation of this metal complexes with organic compounds. At the same time, the increase in the BOD5 load has shown negative correlation with Cu and positive correlation with magnesium (r = 0.997; Tab.3) and iron (r = 0.990; Fig. 8.) ions. This does not mean that all heavy and alkali metals made similar combinations.
Thus, such leachate must not be drained into waters or ground nor used again as technological water for concrete production (Regulation 2019). Due to occurrence of relatively high amounts of chromium and lead compounds in the leachate, it must not be used as a technological water component. Increased concentrations of heavy metals were also noted in leachate sampling locations A1 and A4, which can originate from higher volume of sewage sludge occurring there. Consequently, production of construction elements using technological water containing such leachate may cause occurrence of unfavourable phenomena in produced materials; therefore, there is a deterioration of their strength parameters (Czerniak and Poszyler-Adamska 2006; Gineys 2011; Król 2012).
Statistical analysis of laboratory test results
Table 4 shows selected significant linear correlation coefficients. It was noted that there occurred a clear positive correlation of lead with: sulphates (r = 0.99), chlorides (r = 0.95), total dissolved solids (r = 0.88) and electrolytic conductivity (r = 0.96). High correlation was noted for chromium and total solids (r = 0.90) as well as total suspended solids (r = 0.93) (Table 4). Also significant negative values of the coefficient of correlation between iron and pH reaction (r = − 0.99), total dissolved solids (r = − 0.89) and positive with total nitrogen (r = 0.97) were found. High correlation of iron with BOD5 (r = 0.99), CODCr (r = 0.98) and TOC (r = 0.98) was noted. Strong correlations between total suspended solids and chromium (r = 0.92) were also noted—this suggests that chromium compounds are sorbed, to a major extent, by suspended solid particles. High correlation between lead compounds and sulphate VI ions (r = 0.96) indicates a probability of generation of sparingly soluble PbSO4.
The determination coefficient calculated for lead and sulphate compounds is R2 = 0.997. This association defines which part of variability of the dependent feature can be interpreted using the developed model (Tab. 5). Examples of statistical analysis results are illustrated in relevant graphs (Figs. 3, 4, 5, 6, 7, 8). Particular correlations shown in those figures illustrate categorised graphs of dispersion along with regression line. Histograms of the variable distribution, at frequency of occurrence of given parameter, were also depicted here. For example, Fig. 3 shows that concentration of lead in leachate varies from approx. 0.005 to 0.048 mg Pb/dm3, which partly correlates with sulphate VI ions indicator within the limits from 41.2 to 679.1 mgSO4/ dm3. Strong correlations for remaining components of the leachate are illustrated in subsequent graphs presented in Figs. 4, 5, 6, 7, 8.
Calcium compounds in examined technological water the main element of alkaline components in leachate maintained at the production plant. Occurrence of calcium compounds in cement, therefore, in leachate containing mineral additives such as volatile silica ashes or granular blast furnace slag, containing hydrated calcium silicates, may lead to a synthesis of amorphous or semi-crystalline structures (Baetzner 2002; Batchelor 2006; Kudrowski 2010). It is believed that such structures composed of calcium ions and [SiO4]4− tetrahedrons are then significantly developed. In practical terms, with the increase in the CaO content in solution, the increase in the Ca/Si ratio in the solid phase can be observed (Kurdowski 2010). It appears from the research work performed on leachates that this environment may also contain calcium compounds in CaSO4 form as well as lead compounds in sparingly soluble PbSO4 form, which was confirmed by very high correlation of SO42− with Ca2+ and Pb2+ ions (r = ~ 0.99).
Particular graphs shown in Figs. 3, 4, 5, 6, 7, 8 are supplemented with histograms categorised with relations to particular groups. Those phenomena were interpreted by the multiple regression module made for parameters of the pollutants occurring in tested leachate originating from technical concrete and construction elements production.
This resulted in development of a model taking into account selected, significant parameters of pollutants contained in leachate from the industrial waste heap based on the example of electrolytic conductivity and sulphate ions, in which high concentration of lead compounds was found. Using this model, its verification was carried out.
Multiple regression module
Multiple regression, used as a tool in evaluation of mutual relationships between particular components of leachate originating from concrete and construction elements production, allows for Fischer’s test (F) to be used.
Lead is a toxic element; its content in cement and leachates is significant—it can migrate to any aquatic environment. High correlation between high Pb content and conductivity as well as sulphates (VI) content allowed for model development (Tab. 4). As a result, a model taking into account some selected significant parameters of pollutants contained in leachate originating from construction elements production, was developed. The said model takes into account leachate electrolytic conductivity and concentration of sulphate ions in this medium. Using this model, its verification was carried out.
The use of statistical analysis for elaboration of test results allowed for finding of significant relationships between particular pollutants, which allowed for the definition of: determination coefficient, corrected determination coefficient, F (Fischer’s) coefficient, critical significance level p and estimation standard error. Particular variables in the model explain the significant percentage of the dependent variable (Tab. 5). The model significance was additionally demonstrated based on the analysis of correlations between Pb measured values and theoretical values originating from Eq. (1). In the case of application of the regression analysis to evaluation of mutual relationships between particular leachate components several, and even a dozen or so, factors can be considered—then, the multiple regression comes into play. The factors that were selected in our case were the solution electrolytic conductivity and lead compounds concentration. This allowed for development of a model describing mutual relationships between those components. In the given case, the highest partial correlation coefficients of lead and sulphate ions compounds was 0.995, whereas for the electrolytic conductivity: 0.959. Those variables independently explain approximately 91% of the dependent variable variances after exclusion of impact of other independent variables.
The adopted model, based on the correlation coefficient values, can be written in the following form:
Fischer’s test (F)
Table 6. contains basic parameters of statistical evaluation including: the multiple correlation coefficient, determination coefficient, corrected determination coefficient, F coefficient, p critical significance level and estimation standard error. F statistic was used to check relevance of the entire model, where:
F (F-statistic)—statistic of the test verifying the hypothesis that the multiple correlation coefficient is significantly different from “0”, which means that at least one of the model structural parameters (without free term) is different from zero.
p (p-value)—critical significance level of the test verifying the hypothesis that the multiple correlation coefficient is significantly different from “0”.
Performed Fischer’s test F indicated the significance level p < 0.05; therefore, it required that at least one coefficient of Eq. (1): β0, β1 or β2 should differ from zero. Estimated values of those coefficients in given case amount to b0 = 0.001291, b1 = − 0.000002 and b2 = 0.000053 respectively. Therefore, there are two cases when p < 0.05. Based on these calculations, Eq. (2) was formulated as a model describing values of the correlation coefficients; it can be written in the following form:
Null hypothesis: H0: b0 = 0, b1 = 0, b2 = 0 (none independent variable has significant impact on the dependent variable).
Alternative hypothesis: H1: There is at least one bi coefficient different from zero.
For the assumed significance level α = 0.05, the null hypothesis p = 0.015634 < α = 0.05 can be rejected.
Conclusion: there is an independent variable, which has a significant impact on the dependent variable.
It appears from the above considerations that using statistical methods for given set of data, significant impact of the independent variable on the dependent variable can be confirmed.
Principal components analysis
Apart from use of descriptive statistics for data analysis, it was also possible to make graphs of 2 W type variables of factor loadings. In this particular case, two variables (for X and Y axes) were selected; then, a two-dimensional dispersion graph was created. It is possible to obtain cumulative eigenvalues and cumulative variance percentage. Part of the variance was isolated here by particular components. Those relationships are illustrated in Fig. 9. It can be noted that the component showing the highest eigenvalue explains 50.61% of total variance. The second one is 39.73% of total variance. Therefore, two first components giving two-dimensional space explaining in total more than 90% variances were considered as significant. Such situation allowed for further conduct of the analysis solely based on two factor loadings. The newly created space, to which original points (variables and objects) were projected, made possible creation of proper data structure allowing to obtain the most significant information.
Figure 9 shows an illustration of statistical analysis results for leachate from production of construction materials, in the form of loadings of variable factors in the so-called unit circle. To do this, an analysis taking into account a correlation matrix was performed. The internal area of the unit circle represents admissible factor loadings. This means that the closer to the circle is located the drawn variable, the better is its representation by graph factors. The majority of examined parameters of leachate mineral pollutants (Fig. 9) (without heavy metals) is located in the circle. In many cases, those correlations are very strong. An example of correlation are associations of nitrogen (without ammonium nitrogen) with BOD5, CODCr, TOC, suspended matter, total solids and significantly weaker between total phosphorus and sulphates, chlorides, electrolytic conductivity and pH. Sets of those variables show positive correlation. Such correlation could not be observed in the case of heavy and alkali metals, which originated, partly, from low concentrations of those leachate pollutants (Fig. 10). Nevertheless, quite close positive correlations between some heavy metals and alkaline calcium could be seen. Negative correlations occurred between magnesium and chromium as well as between nickel and manganese, cadmium, zinc and iron. This should be interpreted by absent, or occurring in trace concentrations, nickel. It is beyond any doubt that strong correlation between lead compounds and sulphate as well as chloride ions indicated a possibility of generation of sparingly soluble compounds such as: PbSO4 (II) or PbCl2 (II) and also CaSO4 as well as its hydrated form CaSO4 × 2H2O. It is quite possible that in alkaline leachate (pH > 8) copper compounds of Cu(OH)2 × 5H2O type could be generated.
This allowed for making of two graphs associated with the analysis of principal components, whereof one 2 W graph of factor loadings for objects (Fig. 9) and the second one 2 W graph of variable factors loadings (Fig. 10). Both graphs can be aggregated and then a single graph, called biplot (Fig. 11), is obtained. This graph allows for penetration of the experimental data set structure, which facilitates evaluation of mutual interactions occurring in tested leachate and forecast possible structures obtained through use of the leachate as a medium applied in the preparation of technological water. Evaluation of such phenomena requires research by application of solid materials analysis techniques, for example XRF X-ray fluorescence spectroscopy or XRD X-ray diffraction, DTA differential thermal analysis and TG thermogravimetric analysis (Lesko et al. 2001). The statistical analysis can be deepened due to outliers, applying the Bartlett’s test (Bartlett 1954). The level of confidence p associated with the analysis of principal components equal to 0.00248 allows for such type operation. A preliminary analysis of projections of original objects on the plane created by two first components (F1 and F2), factor 1 and factor 2, was then performed (Lebart et al. 1982). Already a superficial analysis indicates occurrence of outliers, for example, Ni, Cr as well as ammonium nitrogen (Figs. 10, 11). Such outliers may have a negative impact on the analysis result. Nickel occurred here in trace amounts whereas Cr, with relation to other metals, in significant concentrations. It can be noted that ammonium nitrogen NH4+ ions were not completely transformed into nitrate ions NO3− or NO2−, which indicated also oxygen deficit in analysed leachate. Definitely, the best way was to group the object factor loadings of the basic physicochemical analysis cases and selected groups of heavy and alkali metals with locations of leachate sampling (A1 and A4). The averaged concentration of pollutants in those locations had the highest value. At the same time, the highest amounts of accumulated sewage sludge were noted there, which originated from nature of the substratum, which played the role of a sedimentation facility.
Conclusions
Examination of leachate from existing leachate pond located nearby construction waste heap indicated that the said leachate must not be used as a component of technological water for production of technical concrete and concrete elements. This may lead to negative impact on strength parameters of products manufactured in the analysed company.
-
Raw post-production leachate must not be discharged into the sewerage system due to increased concentrations of some mineral components; it should be initially purified.
-
A model was developed taking into account selected significant parameters of pollutants contained in leachate from the industrial waste heap based on the example of electrolytic conductivity and sulphate ions, in which increased concentration of lead compounds was determined. Using this model, its verification was carried out.
-
The variables used in the model explain significant percentage of variability noted for the dependent variable.
-
Inclusion of additional dependent variables into the model did not improve significantly particular model parameters.
-
Model significance was additionally verified based on the analysis of correlations between Pb measured values and sulphates as well as electrolytic conductivity.
-
To describe the phenomena occurring in leachate from construction materials production, elements of mathematical statistic, in the form of multiple regression module and principal components analysis, can be used.
Availability of data and material
Data collected by the author and secondary data are cited.
References
Asadollahfardi G, Asadi M, Jafari H, Moradi A, Asadollahfardi R (2015) Experimental and statistical studies of using wash water from ready-mix concrete trucks and a batching plant in the production of fresh concrete. Constr Build Mater 98:305–314. https://doi.org/10.1016/j.conbuildmat.2015.08.053
Baetzner S (2002) Ways of anallyzing iron (II) sulphate hydrate in respect of it chromate reducing action in cement. Z K G 55(7):80–88
Bartlett MS (1954) A note on the multiplying factors for various chi square approximations. J R Stat Soc A Stat 16:296–298
Batchelor B (2006) Overview of waste stabilization with cement. Waste Manag 26(7):689–698. https://doi.org/10.1016/j.wasman.2006.01.020
Bonaccorsi E, Merlino S, Taylor HFW (2004) The crystal structure of jennite, Ca9Si6O18(OH)68H2O. Cem Concr Res 34:1481–1488. https://doi.org/10.1016/j.cemconres.2003.12.033
Brennan RB, Healy MG, Morrison L, Hynes S, Norton D, Clifford E (2016) Management of landfill leachate: the legacy of European union directives. Waste Manage 55:355–363. https://doi.org/10.1016/j.wasman.2015.10.010
Brennan RB, Clifford E, Devroedt C, Morrison L, Healy MG (2017) Treatment of landfill leachate in municipal wastewater treatment plants and impacts on effluent ammonium concentrations. J Environ Manage 188:64–72. https://doi.org/10.1016/j.jenvman.2016.11.055
Chen QY, Tyrer M, Hills CD, Yang XM, Carey P (2009) Immobilisation of heavy metal in cement-based solidification/stabilisation: a review. Waste Manag 29:390–395. https://doi.org/10.1016/j.wasman.2008.01.019
Czarnecki L, Deja J, Flaga K, Jasiczak J, Kurdowski W, Małolepszy J, Radomski W, Śliwiński J (2015) The frost resistance of bridge concretes. BTA 1(69):66–69
Czerniak A, Poszyler-Adamska A (2006) Assessment of heavy metals immobilisation in cement-ground composites used for construction of country roads. Acta Sci Pol Formatio Circumiectus 5(1):29–38
Dai Y, Qian G, Cao Y, Chi Y, Xu Y, Zhou J, Liu Q, Xu ZP, Qiao S (2009) Effective removal and fixation of Cr(VI) from aqueous solution with Friedel’s salt. J Hazard Mater 170:1086–1092. https://doi.org/10.1016/j.jhazmat.2009.05.070
Gineys T (2011) Influence de la teneur en elements métaliques du clinker sur les proprieties techniques et environnementales du ciment Portland–these. Dissertation, Université Lille Nord France
Ida S, Eva T (2021) Removal of heavy metals during primary treatment of municipal wastewater and possibilities of enhanced removal: a review. Water 13:1121. https://doi.org/10.3390/w13081121
Kinuthia GK, Ngure V, Beti D, Lugalia R, Wangila A, Kamau L (2020) Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: community health implication. Sci Rep 10:8434. https://doi.org/10.1038/s41598-020-65359-5
Kowal LA, Świderska - Bróż M, (1996) Water purification. PWN, Warsaw
Król A (2012) Release of heavy metals from mineral composites considering environmental impact. Politechnika Opolska, Opole
Kurdowski W (2010) Concrete chemistry. PWN, Warsaw
Lebart L, Morineau A, Fénelon JP (1982) Traitement des données statistiques, méthodes et programmes. Dunod, Paris
Lesko S, Leśniewska E, Nonat A, Mutin JC, Goudonnet JP (2001) Investigation by atomic force microscopy of forces at the origin of cement cohesion. Ultramicroscopy 86:11–21
Magistri M, D’Arcangelo P (2008) New reducing agent for cement. Z K G 3:61–65
Meena K, Luhar S (2019) Effect of wastewater on properties of concrete. J Build Eng 21:106–112. https://doi.org/10.1016/j.jobe.2018.10.003
Nalet C, Nonat A (2016) Retarding effectiveness of hexitols on the hydration of the silicate phases of cement: Interaction with the aluminate and sulfate phases. Cem Concr Res 90:137–143. https://doi.org/10.1016/j.cemconres.2016.09.018
Naveen BP, Mahapatra DM, Sitharam TG, Sivapullaiah PV, Ramachandra TV (2017) Physico-chemical and biological characterization of urban municipal landfill leachate. Environ Pollut 220:1–12. https://doi.org/10.1016/j.envpol.2016.09.002
Nocuń-Wczelik W, Łój G (2006) Effect of lead compounds on alite hydration Cement Lime. Concrete 11/73:343–350
Regulation of the Minister of construction sector of 2006 (Journal of Laws 2006 No 136, item 964)
Regulation of the Minister of Maritime economy and Inland navigation of 12 July 2019 (Journal of Laws 2019 item 1311).
Stanisz A (2001) An Affordable Statistic Course Based on the STATISTICA PL Program on Examples of Medicine. StatSoft Polska, Krakow
Szymański K, Siebielska I, Janowska B, Sidełko R (2018) Variations in physical and chemical parameters of landfill leachates over time. Desal Wat Treat 117:149–155. https://doi.org/10.5004/dwt.2018.22201
Taghizadeh M, Asadollahfardi G, Salehi AM, Akbardoost J (2021) Feasibility study of reusing wash water and steel fibre simultaneously on workability, mechanical properties and fracture toughness of concrete. Aust J Civ Eng. https://doi.org/10.1080/14488353.2021.1899599
Vaičiukynienė D, Kantautas A, Tučkutė S, Manhanga F, Janavičius E, Ivanauskas E, Rudžionis Z, Gaudutis A (2021) The using of concrete wash water from ready mixed concrete plants in cement systems. Materials 14:2483. https://doi.org/10.3390/ma14102483
Varshney H, Khan RA, Khan IK (2021) Sustainable use of different wastewater in concrete construction: a review. J Build Eng 41:102411. https://doi.org/10.1016/j.jobe.2021.102411
Wdowczyk A, Szymańska-Pulikowska A (2020) Differences in the composition of leachate from active and non-operational municipal waste landfills in Poland. Water 12:3129. https://doi.org/10.3390/w12113129
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
Authors firmly abide by all ethical norms and follow the guidelines set by COPE. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szymański, K., Walendzik, B., Janowska, B. et al. The use of a regression model in the variability analysis of the leachate quality from heaps of the production of the building materials. Appl Water Sci 12, 97 (2022). https://doi.org/10.1007/s13201-022-01639-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01639-x