Abstract
The reuse of greywater has significant potential to reduce the demand on potable water. The greywater produced from laundry is free from oil and grease and hence makes it attractive to reuse for irrigation. This study investigates the adsorption of surfactant-rich laundry greywater into the soil surfaces during irrigation. A series of miscible displacement column experiments was conducted under water-saturated condition using non-reactive (NaCl solution of concentration 0.650 g/L) and reactive tracers (greywater solution of concentration 0.26–0.442 g/L with same background electrolyte). Plasterer’s sand was used as the porous medium. Samples collected at the column outlet every two minutes were measured for pH, electrical conductivity and greywater concentrations. Hydraulic conductivity for each experiment was also determined using constant head method. Separate experiments were conducted to determine the surface tension of greywater solution (with same background electrolyte) and modelled using Gibbs adsorption isotherm. Surface tension of greywater reduces with increasing greywater concentration and becomes constant at greywater concentration of 0.440 g/L. The results revealed that pH is improved and electrical conductivity decreased indicating it may increase the soil salinity. The comparison of breakthrough curves of reactive and non-reactive tracers showed that the greywater adsorptions occur into the soil surfaces and it increased with greywater concentrations, which may make the soil water-repellent. This may be a concern if soil becomes water-repellent, increases hydraulic conductivity and enhances the risk of groundwater pollution. The concentration of greywater needs to be checked before irrigation and if needed, it should be diluted to avoid any risk of soil water-repellence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the climate variability and ever-increasing population, there is a projected shortage of potable water in the coming decades, especially in the arid and semi-arid regions of the world. In many countries, campaigns for smart water use have been introduced (Beal and Flynn 2015; Stewart et al. 2010). In many places, people are becoming more aware to use the potable water in a conservative manner. In Western Australia, people are experiencing water use restriction due to current drought condition and critical low levels of surface water storage in dams. Loh and Coghlan (2003) reported that gardening consumes 54% of the total water usage in Western Australia. The average household (based on 3.3 persons per house) water usage is 459 m3 per year or approximately 1.259 m3 each day. This equates to approximately 117 L of greywater per person per day, of which 24 L is used in the kitchen, 42 L in laundry and 51 L in the bathroom. Approximately 707 L each day is used outdoors for garden watering, pools, car washing, etc. (DHWA 2005). This data clearly shows that alternative water resources are to be sourced to reduce the demand on potable water. Alternative water resources may include stormwater (e.g., rainwater harvesting), greywater and treated wastewater (Alam and Anwar 2020; Harmayani and Anwar 2016). Among these alternatives, greywater is mostly produced in residential households on an everyday basis. Greywater is the wastewater resulted from domestic activities such as laundry, dishwashing, kitchen sink and bathing. This greywater could be reused for irrigation and thus it could reduce the demand on garden watering. But greywater may contain oil and grease, dissolved salts and other particles such as soil, sand, bone, soap, and minerals of original water. These components of greywater may have adverse effects on soil, plants and groundwater if it is discharged to ground surfaces. That is why, the reuse of greywater without treatment may cause health hazard or environmental pollution (Gross et al. 2005, 2007).
It is now becoming more common to reuse greywater in many arid and semi-arid regions including Australia. As it was stated earlier, in a typical residential household in Western Australia, 89% of total greywater is generated from laundry and bathroom (total generation 117 L/d per person) (DHWA 2005). Among the greywater produced in a household, laundry greywater may be considered suitable for using it in irrigation because other greywaters (especially kitchen water) may contain oil and grease (Travis et al. 2008; Friedler 2004). Laundry greywater contributes around 34% of the total greywater volume of a household in Western Australia (DHWA 2005). Composition of laundry detergent generally comprises of six different kinds of substances such as surfactants, builders, enzymes, bleaching agents, fillers, and other minor additives such as dispersing agents, fabric softening clay, dye-transfer inhibiting ingredient, and optical brighteners. The laundry detergent or the personal care products contain almost half of the surfactant ingredients. Thus, the surfactants are the major constituents found in domestic greywater. The surfactant molecules (known as monomers) have hydrophilic head and hydrophobic tail (Shafran et al. 2005, 2006). The hydrophobic means “water-hating” and the hydrophobic group comprises of a long alkyl chain of C10–C20. The hydrophilic group may be polarized with an electrical charge and it can form hydrogen bonds (Shafran et al. 2005). For example, Alkylbenzene sulfonates are the type of anionic surfactants which consist of a hydrophilic sulfonate head-group and a hydrophobic alkylbenzene tail-group. These are widely used in formulating laundry detergents and dishwashing liquids. The dual nature of hydrophilicity and hydrophobicity of surfactant monomers can lead to accumulation in different interfaces such as liquid/liquid, liquid/air or solid/liquid in an oriented fashion and thus reducing the surface tension of aqueous solutions (Anwar et al. 2000). The reduced surface tension may have adverse impacts on the soil quality if irrigated with surfactant-rich greywater due to the reduction in capillary pressure. This thus further reduces the availability of moisture in the soil for plant growth (Anwar 2011, 2012; Mohamed et al., 2018).
When a surfactant-rich aqueous solution is present in an unsaturated soil medium, the surfactant monomers accumulate first at the soil/water and air/water interfaces. When these interfaces are filled up with monomers, the excess monomers remain in the bulk aqueous phase. But for the saturated soil, there is no air/water interface and the surfactant monomers accumulate only at the soil/water interface and the rest remains in bulk aqueous phase. This concept is elaborated in Anwar et al. (2000) and shown in Fig. 1. Anwar et al. (2000) calculated the air/water interfacial area in unsaturated glass bead medium using surfactant adsorption method and the results show increasing air/water interfacial area with decreasing soil-moisture content. In addition, Anwar (2012) investigated the effect of greywater irrigation on air/water interfacial area using soil characteristics curve developed from irrigating in a sand column composed of several circular rings. However, Anwar (2012) found that the greywater has no effect on the air/water interfacial area in unsaturated porous medium. But the accumulation of surfactants from greywater irrigation into the solid-water interfaces may leave the soil water repellent, which could have significant impact on agricultural productivity and environmental sustainability (Shafran et al. 2005). The surfactant adsorption into the solid-water interfaces is mainly responsible for causing the soil to be water-repellent (See Fig. 1). Currently, there is very limited knowledge on the causes and characteristics of water-repellent soil from greywater irrigation. There is no study which investigated the adsorption of greywater into the soil surfaces during irrigation and therefore, limited information is available about the soil hydrophobicity. Thus, the objective of this study is to investigate the adsorption characteristics of greywater into the soil surfaces during the irrigation of surfactant-rich laundry greywater in a saturated soil.
Schematic representation of surfactant distribution in a unsaturated and b saturated soil mediums (Redrawn Anwar et al. 2000)
Theoretical background
It is assumed that the surfactant-rich laundry greywater will follow the same distribution shown in Fig. 1 for saturated and unsaturated porous mediums. For unsaturated medium, the greywater adsorption into the air/water interface may be modelled by the Gibbs adsorption isotherm with common background electrolyte concentration as (Adamson and Gast 1997; Rosen 1989):
where σ is the surface tension of greywater solution (dyn/cm), C is the greywater concentration (mol/cm3), R is the ideal gas constant and T is the temperature in Kelvin and Γ is the surface (excess) concentration or the number of molecules adsorbed at the air/water interface per unit area (mol/cm2). Surface tension of greywater solution with different concentration under constant temperature and constant background electrolyte concentration can be measured to develop this equation.
For the case of a saturated soil, the surfactant-rich greywater will be adsorbed only into the solid/water interfaces because of no air/water interface. The one-dimensional advective–dispersive solute transport equation for saturated mass transport is given by (Bear and Verruijt 1987):
where, Rt is the retardation factor, D is the hydrodynamic dispersion coefficient (cm2/min) and v is the pore-water velocity (cm/s). For a saturated porous medium, retardation factor (Rt) is represented by:
where \({\rho }_{b}\) is the bulk density of the porous medium (g/cm3), \({k}_{d}\) is the linear distribution coefficient (also known as soil distribution coefficient) for the solid/liquid interface (g/cm3) and \(n\) is the porosity. The retardation factor \({R}_{t}\) can be determined by comparing the breakthrough curves (BTCs) of reactive and non-reactive solutes.
Materials and methods
Materials
To determine the greywater adsorption into the solid surfaces, a non-reactive tracer and a reactive tracer were irrigated into a soil medium. A pre-washed plasterer’s sand was chosen for this study because of its lower content of clay and foreign particles than the typical cement sand. The soil was washed further with tap water and oven dried at 105 °C for 24 h before sieve analysis. The grain size distribution of this sand is shown in Fig. 2. Sieve analysis shows it as a poorly graded soil and the physical properties of the soil were found as the mean grain diameter (\({d}_{50})=0.44 mm\), coefficient of uniformity (\({C}_{u}\)) = 1.72, coefficient of curvature (\({C}_{c}\)) = 0.89 and the bulk density (\({\rho }_{b}\)) = 1.42 g/cm3. The Sodium Chloride (NaCl) solution of 0.650 g/L was chosen as the non-reactive tracer, which was also used as background electrolyte of greywater solution. The reason for maintaining the fixed NaCl concentration in greywater is that the NaCl varies with respect to space and time producing a band of electrolyte concentration gradients across the solute front in miscible displacement (Kim et al. 1997). Again, the substances in greywater (mainly surfactant) and the NaCl are not transported in the same velocities and their dispersion coefficients may also vary. To avoid this, a uniform NaCl concentration (0.650 g/L) is used as background electrolyte to prepare greywater as in Kim et al. (1997) and Anwar et al. (2000).
The DUO 2X Ultra Concentrated Top Loader Aromatic Detergent Powder was selected as a laundry detergent for preparing synthetic greywater in this study. DUO was chosen because it is one of the top and common detergent brands in Australia and also the availability of required information in the literature (Lanfax Lab 2018). While calculating the greywater concentration, the washing instructions given in the DUO washing powder box was used considering a full washing capacity of a washing machine. The normal load wash and three cycles of wash and rinse were assumed to calculate the greywater concentration. The concentrations tested were 0.260 g/L, 0.316 g/L. 0.368 g/L and 0.442 g/L and all of these greywaters also contained the same background electrolyte concentration (0.650 mg/L NaCl).
Miscible displacement experiments
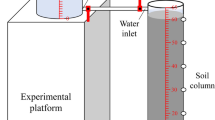
A series of miscible displacement experiments was conducted in a soil column with non-reactive (NaCl) and reactive tracers (greywater with NaCl). The laboratory soil column was constructed using several acrylic rings of 9 cm inner diameter and 3 cm length as shown in Fig. 3. The acrylic rings were prepared from an acrylic pipe of same diameter. A total of 19 rings was used to construct the column. Each ring joint was wrapped with multiple layers of sealing duct tape to ensure there are no leaks. The column is then tested for leaks by filling it with tap water and left aside for 10 min. Upon confirmation of no leakage, the column was packed successively with the soil under water-saturated condition and tapped with a small stick. This procedure ensures no entrapment of air in the medium and makes the medium homogeneous along the column length. Whilst adding the soil into the column, the water used to test for leaks will overflow as the volume of sand increases. This was prevented by incrementally lowering the outlet container as the volume of soil increases inside the column. After filling the column, it was then irrigated with tap water through a showerhead overnight to flush out any foreign substances in the solid medium under saturated condition and to equilibrate the system with constant flow rate (0.7 mL/s). This allows the soil column to reach a fully saturated condition which enables the testing of the interaction of greywater in solid/water interface. The tap water was then displaced with NaCl solution of 0.650 g/L and then NaCl solution was displaced with greywater solution containing same NaCl background concentration. A variable speed peristaltic dosing pump (VSPDP) was used to irrigate the soil column. The column effluent sample was collected in every two minutes and measured for pH, EC and greywater concentrations. The pH and EC were measured using WP-81 Waterproof Conductivity / TDS / pH / Temperature meter (TPS Pty Ltd.). The greywater concentration was measured using UV spectrophotometer (Thermo Fisher Scientific) at 235 nm wavelength. Separate experiments were also conducted with the same greywater concentration and background NaCl concentration to determine the soil hydraulic conductivity using constant head method. Each time, duplicate samples were taken, and the mean value of readings was considered for data analysis. All experiments were conducted under a room temperature of 22 ± 1 °C.
Surface tension measurements
The experiments were performed under water-saturated condition to determine the greywater adsorption into the soil surfaces. As there is no air/water interfaces, surface tension is not required for determining the greywater adsorption into the soil surfaces. But to check the greywater behaviour with increasing concentration, surface tension of different greywater (plus NaCl) solution was also measured using a surface tensiometer (Sigma 700). The surfactant is the main component of laundry detergent. The surfactant solution decreases the surface tension with increasing surfactant concentration and the surface tension becomes constant at a particular concentration which refers to the Critical Micelle Concentration (CMC) of the surfactant. This measurement technique here follows the Wilhelmy plate method (Adamson and Gast 1997). The measurements were taken at the same room temperature of 22 ± 1 °C for greywater concentrations range of 0–0.5 g/L.
Results and discussion
Surface tension of greywater
As the major constituent in laundry greywater is surfactant, the surface tension of greywater solution decreased with increasing greywater concentration. The surface tension of various greywater concentrations was measured and presented with the logarithmic value of greywater concentration in Fig. 4. The Gibbs adsorption isotherm (Eq. 1) was fitted with the data and the constant surface tension (33.75 dyn/cm) was found at the greywater concentration of 440 mg/L. This value may be referred to the CMC of greywater as CMC of a surfactant solution is a point when all the interfaces are saturated and no more surface tension reduction occurs. The composition of the laundry detergent only considers two main important types of surfactant such as, the anionic and non-ionic surfactants. The anionic surfactant used in Duo 2 × ultra concentration detergent is Linear Alkyl benzene Sulfonic acid (LAS). This type of surfactant is sequestered and precipitated from wash solution by divalent cations under high water hardness conditions which reduces the cleaning power of the detergent. However, the LAS is used globally in many detergents and personal care products. Adsorption of LAS into the soil was investigated by Ou et al. (1996) and they also found the CMC of LAS 433.5 mg/L corresponding to the surface tension of 33.5 dyn/cm, which are very similar to this study. In addition to surfactants, laundry detergent also comprises of other substances such as builders, enzymes, bleaching agents, fillers, and other minor additives-dispersing agents, fabric softening clay, dye-transfer inhibiting ingredient, and optical brighteners. But, Shafran et al. (2005) reported that the surfactant in laundry detergent is the only one substance that has the influence on the reduction of surface tension in aqueous solution. There is no other ingredients in laundry detergents that have such influence. According to the capillary theory, surface tension reduction enhances the reduction of capillary rise in soil when it is irrigated with surfactant-rich greywater (Anwar 2012). The decrease in capillary pressure in soil is also embedded with the mechanism of surfactant adsorption into the soil (Shafran et al. 2005).
Effect of greywater on soil pH
The pH tests on the soil are an effective way to determine what degree of surfactants are being adsorbed into the solid medium. The pH value at the column outlet was measured in every two minutes and presented in Fig. 5. The results revealed that the pH values initially started relatively high but reduced gradually as the soil neutralizes pH and adsorbs greywater, then increasing up to when the soil can no longer adsorb surfactant. As expected, the increasing concentrations of greywater solution resulted in high alkaline solution (pH = 8.6–10). This is due to washing powder having alkaline substances to dissolve organic matter from the clothes. But when the miscible displacement experiment began, the greywater displaced the original water and the pH level of greywater leaving the soil column became lower than the initial concentration. Over time, the pH level began to decrease slowly which suggests alkalinity is being transferred into the solid medium. The time taken for the pH level to drop increased as the concentration of surfactant increased. This also suggests that the higher alkaline solutions are resulting in higher soil pH. As the surfactant concentrations increased, the time taken for rising pH also increased. At this stage, no more alkalinity was adsorbed into the solid medium. The final levels of pH leaving the column were greater for higher concentrations of surfactants but were significantly less than the pH of initial concentrations entering the column.
This increase in pH has the potential to impede the ability for plants to grow in the soil. Higher alkaline soil can cause essential plant nutrients such as phosphorus, copper, iron, manganese, molybdenum and zinc to become unavailable. This may result in a significant decrease in plant growth or health. The optimum pH level for plants to grow is between 6 and 7.5 (Pinto et al. 2010). The results shown in Fig. 5 suggest that pH levels in the soil for higher concentrations of surfactant (0.442 g/L) were approaching to 7.2 and above. This has the potential to be detrimental to plant growth in some cases if it increases more. To continue the biological activity in soil, the normal pH range should be 5– 9. If it exceeds the value of 9, biological activity will no longer take place in a normal way and the dissolved organic materials will be leached out of the soil (Shafran et al. 2005). The dissolved organic materials could further degrade with time and become plant nutrients, while loss of organic materials might be detrimental to plant health (Shafran et al. 2006). However, Pinto et al. (2010) suggested that, high pH levels in soil irrigated with greywater can be avoided by the use of potable water and then with greywater. They also suggested to correct high pH level by adding gypsum to lower the pH.
Electrical conductivity at column outlet
The effluent water samples collected every two minutes were also analysed for their electrical conductivity (EC) and shown in Fig. 6. Tap water was tested first and produced a constant reading of EC (365 ± 4 µs/cm) in column experiment. The EC of initial NaCl solution (0.650 g/L) was measured as 1966 µs/cm, which was much faster to achieve higher EC at column outlet when irrigated. Next, NaCl solution (0.650 g/L) was mixed with the greywater solution as background electrolyte and the EC of initial greywater solution was in the range of 1856–2568 µs/cm for the greywater concentration range of 0.26–0.442 mg/L. Higher concentrations of greywater provided a higher value of EC. As the NaCl is a non-reactive solute, there was no adsorption and thus the effluent EC also reached the initial EC faster. The column experiments with greywater irrigation were performed until the effluent EC and greywater concentration became constant. The constant EC of effluent greywater was obtained after 180 min with 0.26 g/L greywater and after 230 min with 0.442 g/L greywater, respectively. The higher concentrations of greywater took longer time but reached higher values of electrical conductivity. Measuring the salinity levels of the greywater is essential to determine what levels of sodium are being adsorbed into the solid surface. Shafran et al. (2006) investigated the potential changes of soil properties following irrigation with surfactant-rich greywater and reported that high level of sodium may be detrimental to plant growth. Overall EC represents the presence of salt and the salinity of soil–water environment. Greywater increases the value of EC by increasing the salt presence in soil when used for irrigation. Salt usually dissociates in water easily to form anions and cations. The salt present in laundry detergent is made up of cations such as, calcium, magnesium, and potassium and anions of sulphate, phosphate, nitrate, chloride and carbonate (Shafran et al. 2005). Increasing salt presence in soil will affect the soil structure and hence the general plant health. Thus, it is recommended to use the greywater after some initial treatment to lower the EC value.
Greywater adsorption into the soil surfaces
The greywater adsorption into the solid medium is modelled assuming the linear equilibrium sorption isotherm in saturated medium (Eqs. 2 and 3). This method compares the BTCs for reactive (greywater) and non-reactive (NaCl) tracers to determine the adsorption into the solid medium. The BTCs are plotted for NaCl solution and greywater solution (with same background electrolyte) and are shown in Fig. 7. The retardation factor was then calculated as a ratio of the average travel times (\(\overline{t}\)) based on the first temporal moments for the reactive and non-reactive tracer BTCs (Kim et al. 1997):
where, Rt is the retardation factor in Eq. 3, C is the flux averaged tracer concentration (mg/L), C0 is the influent concentration (mg/L) and t is the average travel time (min). Subscripts NaCl and GW are the sodium chloride and greywater, respectively. The degree of surfactant adsorption can then be determined by calculating the linear distribution coefficient for solid–liquid interaction (\({k}_{d}\)) using Eq. 3. The soil distribution coefficient and retardation factor were found to be increasing with greywater concentration indicating more adsorption into the soil surfaces (Fig. 8). But it did not have significant effect on the soil bulk density or porosity.
The amount of increasing surfactant adsorption (LAS) into the soil surfaces with increasing surfactant concentration indicates a cooperative adsorption mechanism (House and Farr 1989). With increasing surfactant solution, active site of adsorption soil surface becomes less available. On the other hand, it causes more hemimicelles (monolayer of surfactant monomers attached to a surface) to form, which in turn changes the soil surface to become hydrophobic (Ou et al. 1996; Gangula et al. 2010). The increase surfactant concentration increases both the monomer concentration and the hydrophobicity of soil surfaces which makes the system unstable. To make the system stable, hydrophobic tails of surfactant monomers form a bilayer (known as admicells; see Fig. 1 for solid/liquid interface) through van der Walls force, hydrophobic binding or thermodynamics mechanism (Ou et al. 1996). Shafran et al. (2005) also conducted experiments with the pure surfactants (LAS) which are normally present in the laundry detergent. They reported that the electrostatic bonds of negatively charged sulfonate groups interact with the positively charged sand surfaces and it causes the adsorption of hydrophobic tails of LAS monomers into the soil surfaces. This phenomenon of surfactant behaviour makes the soil to be water-repellent. A water-repellent soil does not wet up spontaneously when water comes in contact with soil and this becomes a problem of future irrigation by water leaching. It reduces the soil moisture content and thus the field capacity for plant root to uptake water and nutrients but it may increase the soil hydraulic conductivity. The hydraulic conductivity for each experiment was determined in this study and found to be increasing with greywater concentration. Increasing hydraulic conductivity means the overall water transmitting capacity increases and the water retention capacity of soil decreases. Hydraulic conductivity is a very important soil property but if it increases, it elevates the risk of potential groundwater contamination.
Conclusions
To investigate the greywater (surfactant) adsorption into the soil surfaces, a series of soil column experiments was conducted with non-reactive (NaCl solution) and reactive (surfactant-rich laundry greywater with same background NaCl concentration) solution under water-saturated conditions. Most greywater concentrations used in the experiments were less than the concentration where surface tension was found constant. This concentration may be referred to CMC of greywater as surfactant is the main component of greywater and no interface adsorption occurs beyond the value of CMC of a surfactant. The pH results revealed that greywater irrigation neutralised the pH due to the acidic nature of the soil surface. The final EC value decreased in the soil outlet indicating the increase in soil salinity. The soil distribution coefficients (\({k}_{d})\) and retardation factor (\({R}_{t}\)) results revealed that the greywater (surfactant) adsorption into the soil surfaces increased with greywater concentration. The adsorption process slowed down when the concentration reached towards the CMC value. This behaviour of surfactant adsorptions enhances soil to be hydrophobic. Hydrophobic soils are water-repellent, increases the soil hydraulic conductivity and enhances the leaching of soil contaminants. However, still the information on soil-repellence is inadequate and further research is suggested to understand the soil hydrophobicity. To avoid any potential environmental risk; it is also suggested to treat the greywater or dilute it before using for irrigation.
References
Adamson AW, Gast AP (1997) Physical chemistry of surfaces. Wiley, New York
Alam M, Anwar AHMF (2020) Nutrients adsorption onto biochar and alum sludge for treating stormwater. J Water Environ Technol 18(2):132–146. https://doi.org/10.2965/jwet.19-077
Anwar AHMF (2011) Effect of laundry greywater irrigation on soil properties. J Environ Res Develop 5(4):863–870
Anwar AHMF (2012) Effect of greywater irrigation on air-water interfacial area in porous medium. Int J Civil Environ Eng 6(1):53–57
Anwar AHMF, Bettahar M, Matsubayashi U (2000) A method for determining air-water interfacial area in variably saturated porous media. J Contam Hydrol 43(2):129–146
Beal C, Flynn J (2015) Toward the digital water age: Survey and case studies of Australian water utility smart-metering programs. Utilities Policy 32:29–37
Bear J, Verruijt A (1987) Modeling groundwater flow and pollution. Reidel, Tokyo
DHWA (Department of Health, Western Australia) (2005) Code of practice for the reuse of greywater in Western Australia. Produced by Environmental Health Service with assistance from Marketing and Campaign Support, Population Health Division, HP 8122, p 54
Friedler E (2004) Quality of individual domestic greywater streams and its implication for on-site treatment and reuse possibilities. Environ Technol 25(9):997–1008
Gangula S, Suen A-Y, Conte ED (2010) Analytical applications of admicelle and hemimicelle solid phase extraction of organic analytes. Microchem J 95:2–4
Gross A, Azulai N, Oron G, Ronen Z, Arnold M, Nejidat A (2005) Environmental impact and health risks associated with greywater irrigation: a case study. Water Sci Technol 52(8):161–169
Gross A, Shmueli O, Ronen Z, Raveh E (2007) Recycled vertical flow constructed wetland (RVFCW)—a novel method of recycling greywater for irrigation in small communities and households. Chemosphere 66(5):916–923
Harmayani KD, Anwar AHMF (2016) Adsorption kinetics and equilibrium study of nitrogen species onto radiata pine (Pinus radiata) sawdust. Water Sci Technol 74(2):402–415. https://doi.org/10.2166/wst.2016.217
House WA, Farr IS (1989) Adsorption of sulphonates from detergent mixtures on potassium kaolinite. Colloids Surface 40:167–180
Kim H, Rao PSC, Annable MD (1997) Determination of effective air–water interfacial area in partially saturated porous media using surfactant adsorption. Water Resour Res 33(12):2705–2711
Lanfax Lab (2018) http://www.lanfaxlabs.com.au/. Accessed 26 Apr 2018
Loh M, Coghlan P (2003) Domestic water use study In Perth, Western Australia 1998–2001. Water Corporation, ISBN 1-74043-1235, p 33
Mohamed RM, Al-Gheethi AA, Noramira J, Chan CM, Amir Hashim MK, Sabariah M (2018) Effect of detergents from laundry greywater on soil properties: a preliminary study. Appl Water Sci 8:16. https://doi.org/10.1007/s13201-018-0664-3
Ou Z, Yediler A, He Y, Jia L, Kettrup A, Sun T (1996) Adsorption of linear alkylbenzene sulfonate (LAS) on soils. Chemosphere 32(5):827–839
Pinto U, Maheshwari BL, Grewal HS (2010) Effects of greywater irrigation on plant growth, water use and soil properties. Resour Conserv Recycl 54(7):429–435
Rosen MJ (1989) Surfactant and interfacial phenomena. Wiley, New York
Shafran AW, Gross A, Ronen Z, Weisbrod N, Adar E (2005) Effects of surfactants originating from reuse of greywater on capillary rise in the soil. Water Sci Technol 52(10–11):157–166
Shafran AW, Ronen Z, Weisbrod N, Adar E, Gross A (2006) Potential changes in soil properties following irrigation with surfactant-rich greywater. Ecol Eng 26(4):348–354
Stewart R, Willis R, Giurco D, Panuwatwanich K, Capati G (2010) Web-based knowledge management system: linking smart metering to the future of urban water planning. Australian Planner 47(2):66–74
Travis MJ, Weisbrod N, Gross A (2008) Accumulation of oil and grease in soils irrigated with greywater and their potential role in soil water repellency. Sci Total Environ 394(1):68–74
Acknowledgements
The authors would like to thank the anonymous reviewers for their constructive and professional comments on the first draft of the paper.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
AHMFA is the first author who wrote the first draft of this paper. He did most of the data analysis of this paper. He also made subsequent changes discussing with the co-authors, UR is the 2nd author who reviewed the paper several times, provided feedback and made significant contribution to this paper. WB is the 3rd author who did the actual experiments as a part of his final year engineering project at Curtin University. He also did the data analysis and reviewed the paper.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anwar, A.H.M.F., Rathnayake, U. & Bowyer, W. Greywater adsorption into soil during irrigation. Appl Water Sci 12, 95 (2022). https://doi.org/10.1007/s13201-022-01605-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01605-7