Abstract
The quality of groundwater samples around Igando area, Lagos State, Nigeria was investigated with the aim of detecting possible influence of dumpsites on nearby aquifers. Thirty-seven (37) groundwater samples were evaluated for their suitability in drinking and agricultural purposes. Physical parameters were determined in situ using portable multi-meter. Flame absorption was used to determine the concentrations of major cations while ion chromatography and titrimetry was used for anions’ concentration. Bacteriological test was conducted using multiple tube technique. Results of in-situ physical parameters revealed pH range of 7.01–7.79, EC range of 20–880 μS/cm while TDS range from 13–590 mg/L. Laboratory analyses showed Ca2+ concentration ranging 1.60–72.80 mg/L and HCO3− ranging 19.52–204.96 mg/L as the dominant ions. None of the ions’ concentrations exceed the WHO drinking limit. Water Quality Index (WQI) classification showed that all samples fall within good to excellent water categories. The majority of the samples are suitable for irrigation using indices such as Sodium Adsorption Ratio (SAR), Kelly’s ratio (KR), and Magnesium Hazard (MH). Bacteriological analyses revealed low total bacterial count and E-coli absence making the samples safe for drinking. Piper diagram indicated two main water-facies (Ca–HCO3 and the Mixed Ca–Mg–Cl). Gibbs’s plots showed that the samples are primarily influenced by rock weathering, while precipitation also contributed. Chloro-Alkaline Index confirmed direct ion-exchange process. Cluster analyses revealed four groups: the first revealed influence of groundwater by anthropogenic activities in the study area, the second and third revealed lithological origin of ions in the water while group 4 indicated pH low influence on the dissolved ions in the waters. The groundwater quality can be said to be fair-good, thus suggestive of minimal interactions between the dumpsites and the groundwater in the area at the time of testing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Life expectancy is largely dependent on the potability of water available for drinking and agriculture (Oloruntola et al., 2018; Ige et al., 2020, 2021). However, increasing population, urbanization and industrialization are major threats to harnessing good quality water. This is because waste generated from these symbols of societal deveploment consistute environmental nuisance, especially to water, soil and the air (Fig. 1). It is very common to find open dumpistes within and around major/capital cities in Nigeria with heterogeneous type of wastes. The waste will be chemically broken down in the presence of water to form leachate. Leachates are organic in nature but might carry some chemical constituents whose activities have negative effects on the engineering properties of underlying soil. The groundwater system suffers the major negative impact of leachate through the introduction of heavy metals (Pb, Cd, Fe, Zn etc.), dissolved minerals (Na, Mg, K, salts), suspended particles (clay, silt, sand) and development of leachate plumes (Mepaiyeda et al., 2019). Although unengineered landfills are synonymous to groundwater pollution, the potential and degree of pollution most times rest largely on the properties of saturated zone, atteneuation capacity and amount of precipitation (seasonal effect). In Lagos Nigeria, Igando (“Solous”) I–III (Fig. 1), Abule Egba and Oloshosun are the major dumpsites that are approved by the government for operation. However, the operations are not without environmental impacts. Several environmental studies (Ayolabi et al, 2013; Odukoya et al., 2013) have been carried out around “Solous” dumpsite in Igando to monitor its impacts on soil, water and air. Impacts of solid waste dumpsite were evaluated at “Solous” dumpsite III, Lagos state by Olorode and Alao (2013b, a) using VES and VLF methods. Results revealed the presence of porous sandy unit extending beyond 20 m below the base of landfill and that leachate has laterally moved about 300 m away from the dumpsite. Coker et al (2019) assessed the impact of solid waste on groundwater quality in “Olusosun” dumpsite, Lagos, Nigeria. A combined geophysical and geochemical methods was carried out at the dumpsite. Results of ten Vertical Electrical Sounding (VES) and five 2D electrical traverses revealed vertical percolation of leachate beyond 40 m in the southeastern direction. The flow direction was a serious concern because of the high population density of humans impacted negatively by the leachate migration. Elevated concentration of some heavy metals above World Health Organisation (WHO) maximum permissible limits were recorded. Salami and Susu (2019) conducted a comprehensive study of leachate characteristics from three “Solous” Dumpsites in Igando Area of Lagos State, Nigeria. This work was carried out with a view of establishing correlation matrix for the leachate parameters in each of the dumpsites. Aside from values of biological oxygen demand (BOD) and chemical oxygen demand (COD) which are higher than the recommended standards, the correlation matrices showed a strong relationship among the parameters and it was therefore recommended that the practice of waste dumping in unengineered open spaces should be discouraged. Aderemi et al. (2011) evaluated degree of groundwater contamination by leachate near open dumpsite in Abule Egba, Lagos. Concentrations of physical and chemical parameters of samples of water within and around the site were measured. Results from the analysis showed minimal impact of leachate from the landfill on groundwater body due to natural attenuation property of the clayey unit in the stratigraphic setting.
The focus of this paper is to assess physicochemical parameters of groundwater in the vicinity of waste dumpsites in Igando area to monitor negative impact on the quality and make appropriate recommendation for usability. This shall be achieved by examining effects of distance from dumpsite on the properties of groundwater, determining and isolating regions of pollution, determing spatial variation in the quality of groundwater and compare obtained groundwater results with approved stardands. This study is restricted to the southeastern arm of the “Igando” dumpsite III, which includes Alimosho local Governemt Secretariat (Fig. 1). This study will assist in the understanding of the groundwater hydrochemical properties, unravel sources of water solutes and reveal the discernible patterns, dissimilarities of water composition and determine interference of the activities, if any, from the dumpsite.
The study area
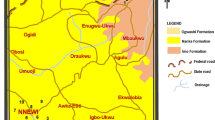
The area of study is situated within longitudes 3° 15’ E and 3° 18.5’ E and latitudes 6° 35.9’ N and 6° 38.8’ N Lagos State (Fig. 1) in southwestern Nigeria. The area is drained by River Abesan (flowing from the North of the area toward the west). It has a relatively and generally low relief with topography ranging between 50 and 150 m above sea-level.
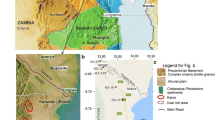
Geologically, the study area falls within the Mesozoic-Cenozoic Dahomey basin. It consists of Abeokuta, Oshosun, Ilaro, and Benin as major stratigraphic Formations. The formation unconformably lying over the crystalline basement is the Abeokuta Formation (Fig. 2). It is essentially made up of conglomerates, sandstones, and grits. Although its groundwater potential is satisfactory, there is a compromise of its quality due to a mixture of sand with some bituminous components (Jones and Hockey 1964). Overlying the Abeokuta Formation is the Oshosun Formation, which is made up of sandstone, shale, limestone, and clays. The groundwater potential is categorized as poor because of the fine-grained nature of the lithos (Longe et al. 1987). Above the Oshoshun Formation are the clays, shales, and fine to coarse-grained sandstone of the Ilaro Formation. The youngest formations are the recent alluvium and the coastal plain sand. The later is essentially made up of clays, shales, and sands. The Coastal plain sand is the most paramount aquiferous unit which supplies water to most parts of Lagos State. The Abeokuta Formation, however, supplies water to a few number of boreholes (Jones and Hockey, 1964).
Materials and method
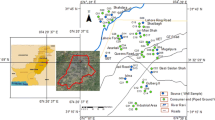
Thirty-Seven (37) water samples were randomly collected from groundwater sources within residential area (Fig. 1). Sampling process began right at the boundary of the dumpsite and extended over 1 km North-southwesterly away from the centre of the dumpsite (Fig. 1) following the procedure of APHA (1995). Samples from wells and boreholes were collected about 500 m distance away from the dumpsite as control following the sampling protocol for groundwater set out by APHA (1995). Boreholes were sampled after being pumped for 5–10 min. Samples were collected in New 60 cl and 100 ml bottles. Rinsing of the containers was done about 3–4 times with the same water to be sampled before filling and corking them. Corking was done immediately after all bubbles have escaped to prevent air-trapping. To prevent absorption and precipitation of mobile metals (ions) before their hydrochemical analyses, trioxonitrate five acid (HNO3) was added to samples to be tested for cations. The sample bottles were labeled immediately after collection on the field using permanent marker and paper-tape. The pH, temperature, Electrical Conductivity (EC), Total Dissolved Solids (TDS) and salinity were measured in-situ using a portable multi-meter. A portable GPS was used to accurately note sampled points’ locations. All the samples were kept in a cool environment to maintain a temperature of about 20 °C before their movement to the laboratory for analyses. The collected samples were tested for the following anions: NO3−, SO42−, HCO3−, Cl−; cations: Ca2+, K+, Na+, Mg2+ and heavy metals Pb, Zn, Cd, Cu and Fe.
Suitability of water for drinking purposes was evaluated using Water Quality Index (WQI). The WQI is a method that helps to understand the contribution of each Physico-chemical parameter on the holistic quality of water. It is thus a tool for rating and classifying water into excellent, good, poor, very poor, and unsuitable for drinking.
For this study, all Physico-chemical parameters were given weighting value between 1 and 5 depending on its significance in drinking water analysis. Hence, NO3, TDS, Cl, SO4, Ni, Pb, Cu, and Co were allotted 5. On the other hand, hardness and phosphate were given 1 while Ca, Mg, Na, and K were, respectively, allotted 3, 3, 4, and 2. The relative weight (Wi) is computed from the following equation:
where qi is the quality rating, Ci is the concentration of each chemical parameter in each water sample in mg/L and Si is the WHO drinking water standard for each chemical parameter in mg/L according to the guidelines of the WHO (2011).
For computing the WQI, the SIi is first determined for each chemical parameter, which is then used to determine the WQI according to the following equation (Kumar et al., 2014):
where SIi is the sub-index of ith parameter, qi is the rating based on the concentration of ith parameter, and is the number of parameters.
Results and discussion
Data reliability test
Data obtained from the laboratory were subjected to reliability evaluations to ensure accuracy of data interpretation process. Charge Balance Error (CBE) was performed so as to check the proportion of the cations to ions in a given sample of water; Charge Balance Error > ± 5% is indicative of imbalance chemical constituents or significant presence of some ionic species that were not identified (Fetter 2001). Another reliability test conducted was to estimate the difference between the measured (in-situ) TDS and estimated TDS (0.66*EC). The results of both tests showed that data from the laboratory are reliable.
Hydrogeochemistry
Statistical analyses were conducted on the physicochemical parameters of the water samples. The statistical analyses as shown in Table 1 reveals that pH ranged from 7.01 to 7.79 and varied slightly between the samples Coefficient of Variation (Cv) < 15% but with an average of 7.43. This implies that the water samples are generally slightly alkaline (Kumar et al., 2014). The EC (μS/cm) values ranged from 20 to 880 (average value of 198.92) and thus point toward low to moderate mineralization of water. TDS (13–5900 mg/L) varied considerably between the samples (Cv > 100%) with an average of 132.05 mg/L (Table 1). This is indicative of potable water. The values of total hardness ranged from 12 to 260 mg/L with an average of 80.4 mg/L indicating soft—moderately hard groundwater. The samples whose physical parameters appeared as outliers were those strongly influenced by the dumpsite in the study area. Expectedly, water chemistry varied outwardly with distance from the source of point pollution.
Major cations and anions
The order of mean concentration of cations’ was Ca2+ > Mg2+ Na+ > K+; with Ca constituting 55% in most cases. Ca concentrations ranged between 1.60 and 72.80 mg/L and a mean value of 16.04 mg/L (Table 2). Mg values ranged between 1.14 and 22.88 mg/L and a mean value of 7.67 mg/L. Na concentrations ranged between 0.05 and 22.10 mg/L and a mean of 3.64 mg/L. K ranged between 0.05 and 23.60 mg/L (mean of 3.68 mg/L). The alkaline earth metals had greater concentrations compared to alkali metals. Carbonate dissolution is suspected to be the major contributor to the high Ca concentration in these places.
The anions had an abundant distribution of HCO3− > Cl− > SO42− > NO3 > CO3− > OH− with the range (mean values) of 19.52–204.96 mg/L (77.55 mg/L), 1.00–67.74 (13.51), 1.00–20.00 (4.38), 0.16–7.65 (1.80), respectively. The anions CO3− and OH− concentrations were below detectable limits (Table 2). The presence of HCO3− in drinking water is not known for any adverse health challenge. The elevated concentration of NO3 is an indicaton of anthropogenic pollution (sewage and agricultural practices), however, its low concentration (< 50 mg/L) implies minimal impact of the point source pollution probably due to the geology of the area.
Principal component analysis (PCA)
Statistical data reduction were applied on chemical parameters using Varimax rotation with Kaiser Normalization that uses Eigenvalues (EV) > 1; only results with EV > 1 were retained. Two (2) principal components (PC1 and PC2) were extracted for the Eigenvalues > 1, with both components constituting 79.93% of the total variance (Table 3).
Principal Component 1 revealed parameters EC, TH, TDS, Cl and Ca, NO3 and Mg had very high positive PC loadings of 0.977, 0.960, 0.964, 0.917, 0.927, 0.820 and 0.887, respectively. While the ions Na, K, and SO4 had a medium positive PC loading of 0.644, 0.629, and 0.710, respectively. These high PC loadings reflect the influence of hydro-geochemical processes (weathering, rock-water interaction, leaching) on the chemistry of the water. Moreover, the high correlation between Cl, Ca, Mg, NO3, TDS, and EC (Table 4) corroborated by very high PC loadings (Table 3) reflect their principal contribution to the mineralization of groundwater in the study area (Sahu et al., 2021). Electrical Conductivity tends to aid the mineralization of the groundwater through ionic strength activities which are then masked by anthropogenic inputs from the urbanization activities in the study area. The high loadings of these dissolved ions (Na, K, Mg, and Ca) could be traced to the intense weathering/dissolution of the country rocks. Furthermore, the high positive loading of TDS, Cl, and NO3 can be a pointer to anthropogenic influence such as run-off from the nearby dumpsite, sewage on groundwater (Wang et al. 2020). Therefore PC 1 could be interpreted as a combination of geogenic and anthropogenic influences on Groundwater geochemistry.
Principal Component 2 has almost all the chemical variables characterized by very low positive PC loadings except for Na and K with medium positive PC loadings of 0.682 and 0.687, respectively (Table 4). This could be an indicator to the weathering of the feldspathic contents in the parent rocks.
Statistical correlation
The relationship between physico-chemical parameters was studied using the Pearson Correlation. Generally, correlation values lesser than 0.5 were considered weak and vice versa. The correlation in Table 4 shows a strong relationship between SAR and KR (0.98), KR and SSP (0.99), and TDS with EC (0.97); these indicate that the concentration of Na influences groundwater chemistry in the study area. Furthermore, an increase in the concentration of Na in the groundwater of the study area may portend a negative impact on plant life and productivity in the future. High TDS concentration represents high concentration of dissolved ions in the groundwater samples. Table 5 shows that TDS strongly correlates with EC (0.97), Ca2+ (0.91), Mg2+ (0.85) NO32−(0.79), SO42−(0.71) and Cl− (0.87). It can be inferred that these ions were derived from the same source. The weak correlation between HCO3− and Cl− (0.23) reflects Cl source from atmospheric inputs such as rainfall, marine aerosols, sea-salt spray, etc. (Manjusree et al., 2017).
The effect of anthropogenic activities on groundwater quality can be evaluated using the behavior of nutrients (mainly NO3− and PO43−) with other ionic species. In this study, NO3− moderately correlates with K+ (0.57) and SO42− (0.50) implying a little impact of agricultural activities on groundwater chemistry in the area. This is confirmed by a mean value of 1.80 mg/L for NO3− indicating minimal influence by pollution from agricultural sources. A high correlation (0.88) between NO3− and Cl− indicates a strong impact of domestic sewage on groundwater quality (Helena et al., 2000; Silva-Filho et al., 2009).
ION exchange process
Groundwater chemical composition experiences a variety of changes as it travels in the subsurface through varying lithology (Srinivasamoorthy et al., 2014). The ion exchange between groundwater and its host environment can be studied using the chloro-alkaline indices (CAI-1 and CAI-2) whose values can be positive or negative (Fig. 3). The results from the Chloro-Alkaline Index (1) values ranged from − 1.15 to 0.98 with an average value of 0.50. Six (6) samples had negative ratios while the remaining thirty-one (31) samples were of positive ratios. The water samples with negative ratios signify cation–anion exchange while the dominant positive ratios of the Chloro-Alkaline Index confirm the direct ion-exchange process acting in the water.
The ionic plot of (Ca + Mg)–(HCO3 + SO4) vs (Na + K)–(Cl + NO3) gives a straight line, a slope of -1.14, 0.14 as the intercept and a weak correlation of 0.23 (Fig. 4). This implies that the ion exchange reaction process is taking place between the present Ca, Mg and Na. This confirms that groundwater is influenced by ion exchange is in the study area.
Suitability for drinking
The groundwater samples’ potability was determined by comparing (Table 6) the ionic concentrations to relevant standards such as WHO (2011) and Standard Organization of Nigeria (SON 2007). Generally, the comparison revealed that the groundwater samples are within acceptable limits. Therefore, they pose no potential health concern. A low concentration of Na+ and K+ is attributed to neutral to slightly basic pH condition of the water samples. The pH, EC, and TDS of all water samples are within desirable to permissible limits for drinking. Furthermore, the evaluation using the WQI classification also reveals that all the samples categorize as good to excellent making them suitable for drinking (Table 7).
Suitability for irrigation
A water body considered suitable for drinking may not necessarily be fit for irrigation (Haritash et al. 2008). Some of the useful parameters that can be used to evaluate water for this purpose are Sodium Adsorption Ratio, TDS, EC, and Magnesium Ratio.
The Electrical Conductivity (in µs/cm) measures salinity hazard to crops. The values ranged between 20 and 880 and a mean of 198.82 (Table 6). These values fall within excellent—doubtful for irrigation use (Manjusree et al., 2009). 75% of the samples are characterized as C1 (Excellent), 22% as C2 (good) while 3% as C3 (doubtful) (Table 8). The TDS values ranged from 13 to 590 with average values of 132.05 (Table 6). Using Davis and De Wiest (1966) classification, 100% of water samples are evaluated as fit for irrigation (Table 8).
The hazardous effect of Carbonate and Bi-carbonate on irrigation water was evaluated using Residual Sodium Carbonate (RSC). RSC values ranged from − 2.44 to 1.08 meq/L with an average of − 0.40 meq/L. All the samples had RSC values < 1.25 characterizing them as suitable for irrigation (Eaton 1950; Wilcox 1955; Ayers and Westcot 1985).
Magnesium Adsorption Ratio (MAR) measures the effect of Magnesium in irrigation water. A high concentration of Mg adversely affects crop yield (Nagaraju et. al., 2006). Its concentration ranged from 12.511 to 70.37 meq/L with an average of 38.858 meq/L. Eighty-four (84) % of the samples are suitable for irrigation purposes while the remaining sixteen (16)% are unsuitable for irrigation purposes since they exceed the MAR limit of 50% (Ayers and Westcot, 1985).
Assessment of contamination
Groundwater contamination of the study area was assessed using Revelle Index. It is calculated using the ionic ratio Cl/(CO3 + HCO3) in meq/L (Revelle, 1941). A Revelle index value of < 1 is indicative of good water quality while > 1 is indicative of contamination. 95% of the samples collected had Revelle Index < 1, indicating good quality water; these samples were taken some distance from the dumpsite with no visible agricultural activities close to them. However, the remaining 5% indicating contamination or poor water quality were taken close to the dumpsite.
SAR and Kelly’s Ratio (KR) values revealed 100% of water samples to be fit for irrigation as all KR values were lower than 1 while all SAR values were less than 10. Sodium Solubility Percentage values ranged 0.22–36 with an average of 9.17; 92% of the samples are classified as safe for irrigation.
Percentage Sodium (Na%) usually expresses Sodium presence in irrigation water (Tank and Chandel, 2010). High Na concentration results in a reduction in permeability of soils and a negative effect on internal draining (Tiri and Boudoukha, 2010). The computed Na% for the study area ranged from 0.00 to 0.48% with an average of 0.13%. 73% of the samples are classified as excellent, 19% fall in the good category while 8% are permissible. Therefore, these samples can be used for irrigation.
Hydrogeochemical classification
The Piper (1944) diagram is very useful in the classification of water based on its chemical character (dominant cations and anions) and determining relationships between different dissolved constituents. The piper diagram involves plotting the relative abundance of major ions (in meq/L) on cationic and anionic triangles which are then projected into a quadrilateral. The groundwater samples from the study area are categorized as two major water facies within the Piper diagram. They include the dominant Ca-HCO3 facie (which is a temporary hard water with Alkalinity values greater than their Total Hardness) and the Mixed Ca-Mg-Cl facie which is represented by few samples. The Ca–HCO3 type is generally indicative of freshwaters derived from rainfall recharge associated with low EC. The abundance of this water facie could be indicative that the dissolved load of groundwater in the study area is mainly contributed by geogenic sources. Furthermore, the triangular cationic field showed that 95% of the samples fall into (Ca) dominant type while only 5% of the samples fall into the no dominant cation domain. However, in the anionic triangle, 95% of the samples fall in the HCO3- type (Fig. 5); 4% of the samples fall into the no-dominant type while 1% of the samples fell into Cl type.
Diverse processes such as rock weathering, precipitation, and ion exchange can affect the chemistry of the groundwater system. These processes can be identified using the Gibbs plots. According to Gibbs (1970), a plot of TDS vs Na+/(Na+ + Ca2+) and Cl−/Cl− + HCO3− can be used to highlight the effect of these processes on groundwater. The plots revealed that 54% of the samples were influenced by rock weathering, 40% were influenced by precipitation while 5% were influenced by a combination of rock weathering and precipitation. (Fig. 6). However, the contribution of precipitation toward the recharging of the unconfined shallow groundwater system of the study area cannot be ignored due to the large numbers of samples falling in the atmospheric dominance.
Chadha’s plot (Fig. 7) was used to further interpret hydro-chemical processes occurring in the study area. It usually distributes samples into four-quadrant graphs which are summarized as follow:
The plot (Fig. 7) and Table 9 reveals that 89% of the samples fall into Field 1 (Ca-HCO3 field) indicative of freshwater that percolates the subsurface while carrying dissolved carbonate in the form of HCO3 and the geochemically mobile Ca. 11% of the samples fall into field 4 (NaHCO3 water type). This is indicative of groundwater mainly derived from base-ion exchange processes.
The Schoeller diagram is a semi-logarithmic plot showing the ionic concentrations of groundwater samples from the study area (Schoeller 1967). Each ion concentration is represented using a point which is then connected by a line. The Schoeller diagram showed that Ca2+ and HCO3− are the dominant ions in the study area (Fig. 8a, b).
Microbiological analysis
Bacteriological test in water involves testing for the presence of bacteria and coliform count in water. They, unlike certain physical parameters like turbidity, odor, and salinity cannot be detected by sight, smell, or taste but only through a laboratory test. Failure to detect bacteria in water does not mean they do not exist or that the water is safe (WHO, 2011). It is important to note that the most sensitive method of assessing water’s hygienic condition is a frequent measure of its fecal content (WHO, 2003).
The microbiological assessment of the samples mirrors the physical on-site water source inspection. WHO standard for potable water indicates there should be < 1 Most Probable Number (MPN)/100 ml of total coliforms and no E.coli in 1 ml of water. Laboratory results showed that no sample was found to contain E. coli. Five samples (13, 26, 33, 36, and 37) indicated the presence of bacteria (0.1 × 101, 0.2 × 101, 0.1 × 101, 0.1 × 101 and 0.2 × 101 respctively) although all within WHO recommendation for drinking water. The presence of bacteria could result from poorly constructed wells, wells close to septic tanks, wells with poor covering, wells close to abandoned dumpsites, and wells where the use of different containers to draw water from the well is allowed. The absence of E-coli in the samples is indicative of no fecal contamination as there was no open defecation practice. Low total bacterial count and E-coli absence make these samples suitable for drinking.
Conclusion
From this study, the laboratory results for groundwater samples collected from the area indicated the order of the abundance of the cations as: Ca2+ > Mg2+ Na+ > K+; with Ca constituting 55% in most cases. This imply that the alkaline earth metals have greater concentrations compared to alkali metals. The order of anions’ abundance revealed: HCO3− > Cl− > SO42− > NO3. Results from the Chloro–Alkaline Index revealed dominant positive ratio confirming the direct ion-exchange process acting in the water and this corroborated the fact that the host rocks in the study area were the major origin of the dissolved ions in the groundwater. Effects of weathering, rock-water interaction, leaching procssess on the chemistry of the water were confirmed by high PC I loading of the parameters. The Piper diagram indicated the occurrence of two main water-facies namely: the dominant Ca–HCO3 and the Mixed Ca–Mg–Cl. This was corroborated by the results of Schoeller’s diagram where Ca2+ and HCO3− were identified as the dominant ions in the study area. Gibbs’ plot revealed that 54% of the samples were influenced by rock weathering, 40% were influenced by precipitation while 5% were influenced by a combination of rock weathering and precipitation. Groundwater contamination in the study area was assessed using Revelle Index (RI). The results revealed that 95% of the samples collected had RI < 1. This indicated good quality. Samples collected close to the dumpsite had RI > 1. This showed a direct influence of this anthropogenic activity on groundwater quality. Furthermore, the groundwater samples' potability and suitability for irrigation were evaluated. Results revealed that the ionic concentrations of all the samples were within WHO and SON permissible limits for drinking water. This was corroborated by results from the WQI classification. All samples were classified as excellent to good water. The groundwater samples' usefulness for irrigation was evaluated using different irrigation parameters (SAR, KR, MH, etc.). The results from these irrigation indices revealed that most of the samples were suitable for irrigation. In addition, the total bacterial count and absence of E. coli presence obtained from the bacteriological analyses reinforced the potability of the groundwater from the study area.
References
Aderemi AO, Oriaku AV, Adewumi GA, Otitoloju AA (2011) Assessment of groundwater contamination by leachate near a municipal solid waste landfill. Afr J Environ Sci Technol 5(11):933–940
APHA (1995) Standard methods for the examination of water and wastewater, 21st edn. In: American Public Health Association, American Water Works Association, Water Environment Federation, Washington
Ayers RS, Westcot DW (1985) Water quality for agriculture FAO. Irrigation Drain 29(1):1–109
Ayolabi EA, Folorunso AF, Kayode OT (2013) Integrated methods for environmental Assessment of municipal dumpsite system. Int J Geosci 4(5):850–862
Coker JO, Alausa SK, Odunaike RK, Alabi AA, Ogunpola AE (2019) The impact assessment of solid waste on groundwater quality in olusosun dumpsite, Lagos Nigeria. J Nig Geophys Soc 2(1):1–12
Davis SN, DeWiest RJM (1966) Hydrogeology. Wiley, New York
Eaton FM (1950) Significance of carbonate in irrigation waters. Soil Sci 67(3):128–133
Fetter CW (2001) Applied hydrogeology, 4th edn. Prentice-Hall Inc, p 615
Gibbs RJ (1970) Mechanism controlling world water chemistry. Sciences 170:795–840
Haritash AK, Kaushik CP, Kaushik A, Kansal A, Yadav AK (2008) Suitability assessment of groundwater in some villages of Rewari district in Haryana. Environ Monit Assess 145(1–3):397–406
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Wat Res 34:807–816
Ige OO, Owolabi TA., Fatoyinbo IO, Ayodele OE, Obasaju DO (2020) Characterization of factors influencing water quality in Federal University of Agriculture Abeokuta and its Environ, Southwestern Nigeria. Int J Energy Water Res. https://doi.org/10.1007/s42108-020-00093-2
Ige OO, Adewoye FO, Obasaju DO (2021) Hydrochemical evaluation of groundwater quality - a case study from parts of North-Central. Nigeria. Sustain Water Res J 7:101. https://doi.org/10.1007/s40899-021-00577-x
Jones HA, Hockey RD (1964) The geology of part of southern-western Nigeria. Nigeria Geol Survey Bull 31:9–55
Kumar SK, Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2014) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci. https://doi.org/10.1007/s13201-014-0196-4
Longe EO, Malomo S, Olorunniwo MA (1987) Hydrogeology of lagos metropolis. J Afr Earth Sci 6(2):163–174
Manjusree T, Sabu J, Jobin T (2009) Hydro-geochemistry and groundwater quality in the coastal sandy clay aquifers of Alappuzha District, Kerala. J Geol Soc India 74:459–468
Manjusree T, Sabu J, Marco P, Jobin T (2017) Integrated approach for identifying the factors controlling groundwater quality of a tropical coastal zone in Kerala, India. J Environ Earth Sci 76(486):1–18
Mepaiyeda SI, OO, Madi K, Gwavava O (2019) Integrated geophysical and geochemical methods for environmental impact assessment of a municipal dumpsite in king Williams town, Eastern Cape, South Africa. Water Resou 29: 37–51
Nagaraju A, Suresh S, Killham K, Hudson-Edwards K (2006) Hydrogeochemistry of waters of Mangampeta a baryte mining area, Cuddapah basin, Andhra Pradesh India. Turk J Eng Environ Sci 30:203
Odukoya AM, Folorunso AF, Ayolabi EA, Adeniran AE (2013) Groundwater quality and identification of hydrogeochemical processes within University of Lagos, Nigeria. J Water Resour Prot 5(10):930–940
Olorode DO, Alao AO (2013a) Geo-electrical investigation of underground water contamination by solid waste: Case study of Solous III Dumpsite, Igando, Lagos, Nigeria. Adv Appl Sci Res 4(4):420–431
Olorode DO, Alao AO (2013b) Geo-electrical investigation of underground water contamination by solid waste: case study of Solous III Dumpsite, Igando, Lagos Nigeria. Adv Appl Sci Res 4(4):420–431
Oloruntola MO, Obasaju DO, Yusuf MA (2018) Hydrochemical, microbiological and biochemical assessments of groundwater quality in some parts of lagos metropolis, Southwestern Nigeria. Ethiop J Environ Stud Manag 11(1):58–72
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928
Revelle R (1941) Criteria for recognition of sea water in ground water. Trans Am Geophys Union 22:593–597
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. In: Agric. Handbook 60, USDA and IBH Publishing Company Limited, New Delhi, pp 98–99
Sahu S, Gogoi U, Nayak NC (2021) Groundwater solute chemistry, hydrogeochemical processes and fluoride 700 contamination in phreatic aquifer of Odisha. India. Geosci Front 12(3):10109. https://doi.org/10.1016/j.gsf.2020.10.001
Salami L, Susu AA (2019) A comprehensive study of leachate characteristics from three soluos dumpsites in igando area of Lagos State, Nigeria. Greener J Environ Manag Public Saf 8(1):01–14
Silva-Filho EV, Barcellos RGS, Emblanch C, Blavoux B, Sella SM, Daniel M, Simler R, Wasserman JC (2009) Groundwater chemical characterization of a Rio de Janeiro coastal aquifer, SE–Brazil. J S Am Earth Sci 27(1):100–108
SON (2007) Nigerian standard for drinking water quality. Nigerian Industrial Standard (NIS 554–2007). Standards Organisation of Nigeria, Abuja
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu, India. J King Saud Univ Sci 26(1):37–52
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th international congress of ISSS, Trans II, pp 803–812
Tank DK, Chandel CPS (2010) A hydrochemical elucidation of the groundwater composition under domestic and irrigated land in Jaipur City. Environ Monit Assess 166:69–77
Tiri A, Boudoukha A (2010) Hydrochemical analysis and assessment of surface water quality in Koudiat Medouar reservoir, Algeria. Euro J Sci Res 2:273–285
Wang Y, Peng J, Cao X, Xu Y, Yu H, Duan G, Qu J (2020) Isotopic and chemical evidence for nitrate sources 731 and transformation processes in a plateau lake basin in Southwest China. Sci Total Environ 711(732):134856. https://doi.org/10.1016/j.scitotenv.2019.134856
Wilcox LV (1955) Classification and use of irrigation water, USDA, Washington DC (Circular 969)
World Health Organization (2003) Guidelines for drinking-water quality, vol (1). Recommendations. 2nd edition. World Health Organization, Geneva.
World Health Organization (2011) Guidelines for drinking water quality, 4th. Ed. World Health Organization, Geneva, 1:117–153
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no funding was received for this research and that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ige, O.O., Owolabi, A.T., Olabode, O.F. et al. Groundwater quality evaluation: a case study of Igando waste dumpsite, southwestern Nigeria. Appl Water Sci 12, 79 (2022). https://doi.org/10.1007/s13201-022-01601-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01601-x