Abstract
This study seeks to improve the QMRA of drinking water sources in the context of developing countries. Existing QMRA dose–response models were modified to use data generated from the developing country environment and scenarios. The modified model assessed drinking water borehole supplies in Afikpo North Local Government Area, Nigeria. Water samples were taken every three days from July 2019 to December 2019. They were assessed for concentrations of E. coli, Salmonella spp, Shigella spp, Campylobacter, Giardia lamblia and Cryptosporidium parvum. Other input parameters to the modified model were obtained in the study environment through survey instruments; they include per capita water consumption per day, % exposed population, % vulnerable population and pathogen strike rate. The daily mean risk of infection was determined to be 0.236, standard deviation, 0.056, while the daily mean risk of diarrhea was 0.039, standard deviation, 0.016. The predicted mean diarrhea risk values showed a positive correlations (C = 0.74) with the observed diarrhea disease prevalence rate among the study communities. Mean values of diarrhea risk obtained using the modified model were compared with those obtained using formulations adopted by some recent studies that used existing QMRA models in the developing countries. The mean risk values were further compared with values obtained by using other existing QMRA dose–response models/parameters. The study found no statistical significant difference in the predicted risk of diarrhea of the two types of models. The modification is intended to facilitate a better interest on and the acceptability of QMRA in the developing countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is an inevitable necessity of life. Access to sufficient quantity and quality of water is required for the maintenance of health and other human activities. For water used for drinking, including domestic uses such as for cooking and personal hygiene, microbial quality of water is of paramount importance. Since 1855, when John Snow scientifically established the link between cholera epidemic and microorganism (Vibrio cholera) in a drinking water source in England, many other microorganisms continue to be associated to other waterborne disease outbreaks across the world. Consequently, a number of water treatment and extraction technologies have developed to improve raw water quality, to make it safe for drinking and other domestic uses.
Nevertheless, drinking water treatment failures continue to occur, even in the most developed countries (e.g., Schuster et al. 2007) and many people, especially in poor countries still obtain drinking water from contaminated sources. A major consequence of using or consuming water contaminated by microorganisms is high prevalence of gastro-intestinal diseases, leading to high morbidity and mortality globally. The prevalence of gastro-intestinal diseases claims more than 2.2 million people annually, mostly in developing counties (WHO 2020a). According to the World Health Organization [WHO] (2020b), Global Health Observatory (GHO) data for 2016, diarrhea disease, commonly caused by gastro-intestinal infections, ranks 9th among the 10 leading causes of death globally. Diarrhea can be caused by inadequate sanitation, poor hygiene, contaminated food or unsafe drinking water. It is estimated that contaminated drinking water alone causes 485,000 diarrheal deaths annually (WHO 2019). The United Nation Children’s Fund [UNICEF] estimates that 57 million Nigerians lack access to portable drinking water (Adebowale 2017). According to a Nigerian public health expert, Uche Ewelike, about 60 million Nigerians lack access to safe drinking and are exposed to diseases such as “diarrhea, cholera, dysentery and hepatitis A and hepatitis B” (Akor 2017, p. 1).
Water treatment and extraction technologies are necessary systems for improving quality of drinking water supplies, but they have not proven to be sufficient for eliminating microbial water contamination and the associated health risks to water consumers. Raw water extraction and treatment decisions are usually guided by factors such as the water source, physical/human environment, and the raw water quality. However, these factors together with the available technologies operate in human environment and are therefore subject to a great deal of variability and uncertainty (Li 2007). Thus, a drinking water supply system output does not consistently deliver water with no risk of microbial contamination. The situation becomes worse when the water supply system such as unprotected spring or a water borehole is assumed to supply safe drinking water continually, and so left with no periodic assessment. Therefore, water supply systems at both the raw water and treatment levels must be consistently monitored to detect and control imbedded microbial hazards.
The WHO (2007) water quality guidelines emphasize a preventive risk-based management approach of microbial hazards in the water supply process. Hence quantitative microbial risk assessment of drinking water supply system is seen as a useful tool for identifying microbial hazards, analyzing vulnerabilities and estimating associated health risks and selecting preventive and mitigation measures for maintaining pathogen concentrations at safe levels throughout the drinking water supply cycle.
Quantitative microbial risk assessment (QMRA) has gained wide acceptance as a scientific means of understanding human health risk that are associated with microbial hazards in water supply sources. QMRA can apply to the past, the present or the future waterborne disease prevalence or outbreaks. According to the WHO (2016), “QMRA has developed as a scientific discipline over the last two decades and has been embedded in the WHO water-related guidelines.”
However, QMRA is yet to gain acceptance in developing countries (Dong et al. 2015). Major concerns include the availability of data that are relevant to the socio-cultural environment of the developing countries. In addition, the expertise and resources required to generate site-specific data (such as outbreak data, human trial data, clinical data) are lacking in most developing countries. According to Howard et al. (2006, p. 50) “if QMRA is to gain acceptance and be used in developing countries, it must be offered in a workable and simple form.” Therefore, QMRA models require modification for appropriate application in the developing country environment.
Apart from cost, expertise and dearth of data, there are other factors that may make the approaches undertaken for QMRA of drinking water in the developed and developing countries to be different. The first has to do with the control of exposure to gastrointestinal pathogens. Exposure to gastrointestinal pathogens typically comes from contaminated food and unsafe drinking water. There exist high sanitary and food handling policies in the developed countries, and therefore, food and drinking water sources in these countries usually contain very low doses of pathogens compared to the developing countries. On the other hand, sanitary and drinking water quality policies are often ineffective or even nonexistent in the developing countries. Thus, many people in the developing countries are more frequently exposed to higher doses of gastro-intestinal pathogens than those in the developed countries. Many drinking water sources in the developing countries contain pathogen concentrations far above the low concentration known to have caused outbreaks in the developed countries. Nevertheless, people in the developing countries seem to be more adapted to resist progression of exposure to infection/illness.
The second has to do with vulnerability/susceptibility to infections. Whereas the citizens of the developed countries have low susceptibility to known infection and better health due to access to proper health care and good nutrition, majority of people in poor income countries are encumbered with low immune system and high susceptibility to diseases due to poor nutrition and lack of access to adequate health care. Howard et al. (2006) assert that improving access to water supply, sanitation and hygiene, improving nutrition and boosting immune system can reduce waterborne disease burden and chances of severe waterborne disease cases in the developing countries. Havelaar and Melse (2003) consider the immune status to be very significant when undertaking waterborne disease burden assessments for developing countries, especially in Africa. According to Hunter et al. (2003), severe morbidity or mortality caused by waterborne disease exposures is common in many developing countries, but such is relatively rare in industrialized countries.
However, travelers from industrialized countries staying in developing countries, where there are relatively very poor sanitary and hygienic conditions, are at a high risk of contracting waterborne diseases, especially diarrhea (so-called traveler’s diarrhea) (Connor 2020; Nwabor et al. 2016; Wolfe 1992). This is because many waterborne pathogens are self-limiting (e.g., Campylobacter, Giardia, Cryptosporidium, Hepatitis A virus, etc.). After infection and recovery, patients (mainly healthy local people) develop resistance or immunity to such pathogens over time. Therefore, QMRA in the developing countries must address the risk for infections and disease of the people in most vulnerable conditions (infants, pregnant women, the aged, immune-suppressed people, the malnourished, etc.), including visitors from less endemic countries.
The third factor is the objective of QMRA. The QMRA in the developed countries tend to focus on understanding specific outbreaks and preventing future occurrence, understanding the health effects of new pathogens, or developing or assessing water treatment targets for specific pathogens in a very low or sporadic risk events. In the context of the Nigerian situation, a QMRA model that can gain acceptance most likely will be capable of providing verifiable quantitative risk information: to convince drinking water supply stakeholders and the public health agencies on the need to regulate and monitor drinking water sources. On part of the citizenry, it should create the awareness for understanding the health risk inherent in their drinking water sources and treating raw water before drinking or direct consumption. Overall QMRA should be a community-specific tool for decision-making, at both the individual and governmental levels, for reducing waterborne disease prevalence in the developing countries.
A few QMRA studies have been conducted in Nigeria and other developing countries (e.g., Chukwuemeka et al. 2021, 2013b; Machdar et al. 2013a). These studies used a combination of the exponential and beta-Poisson models to predict the risk of infection of gastrointestinal infection from consumption of contaminated drinking water. The input parameters used in these models are the volume of water consumed per person per day, concentration of pathogens, infectivity/shape parameters. In these studies, only one input variable, namely the concentration of pathogens, is obtained from the study environment, and the rest are obtained from literature based on studies carried out in the developed countries. Thus, the QMRA studies done in the developing countries so far seem to assume that objective of the assessment together with the resistance to gastro-intestinal infection by human host or progression of diseases in the host (dose–response) is precisely the same with dose in the developed countries. However, these parameters are functions of the characteristics of the population Therefore, modifications in the existing QMRA model are required to cater for the peculiar needs of the developing countries.
This paper improves on the existing dose–response QMRA models by introducing an environmentally based susceptibility parameter and selecting the infectivity parameter as 0.5, which is a middle course probability between no risk (0) scenario and worst risk scenario. Susceptibility is a function of exposure, sensitivity and adaptability—factors that are peculiar to specific environments. A novel environmentally based approach to QMRA addresses the needs of developing countries by factoring in their peculiar environment and water supply scenarios. It provides simple verifiable risk assessment site-specific information and enables a comprehensive understanding of the issues surrounding drinking water supply and water users, which contribute to high risk of gastro-intestinal diseases in the developing countries. It is hoped that an environmentally based QMRA can motivate decision makers and other stakeholders in the developing countries to develop interest in drinking water risk assessment, and most importantly to take timely actions in addressing issues pertaining to access to safe drinking water and reduction of waterborne diseases in local communities.
The study used the improved model to assess the risk from gastro-intestinal pathogen contamination of the domestic drinking water supply from water boreholes in a growing urban area of Afikpo North Local Government Area of Ebonyi State, Nigeria.
The aim of this study is to improve the existing QMRA of drinking water sources for appropriate application and wide acceptability in the developing countries. The specific objectives include the following:
-
1.
Modify the existing QMRA dose–response models to accommodate the peculiar environment and needs of the developing countries in the estimation of the probability of infection and risk of diseases due to exposure to gastro-intestinal pathogens in water supply sources.
-
2.
Determine, in the study area, the major source of drinking water, the concentration of specific pathogens in the drinking water source, the volume of drinking consumed by oral ingestion per capita per day, the pathogen strike rate in water sources, the vulnerable fraction of the population and the prevalence of waterborne diseases.
-
3.
Use the modified model to assess the risks of some water borne pathogens whose presence in specific drinking water sources could play significant roles in the prevalence of gastro-intestinal diarrhea diseases in the study area.
-
4.
Compare the risks estimated for exposure to specific pathogens obtained using the modified model with those obtained for similar pathogens using existing QMRA dose–response model formulations, including those formulations that have been adopted by some recent studies carried out in developing countries.
Literature review
Quantitative microbial risk assessment of water supply systems evolved from chemical risk assessment in the 1990s, when some data became available on the identification and occurrence of infectious microorganisms, the potential for human exposure, dose–response and the associated health effects (USDA/FSIS and EPA 2012). Since then, QMRA has been applied in various water systems including an entire urban water systems (Labite et al. 2010), water distribution networks (Godfrey and Smith 2005; Howard et al. 2006; Hunter et al. 2009) isolated drinking supply sources (Kouamé et al. 2017) and source to tap system (Dunn et al. 2014). Most common QMRA models are currently based on the single-hit theory, which assumes that every ingested unit of pathogen acts independently with individual probability of causing infection (Haas 1983; Teunis and Havelaar 2000). In this theory, morbidity is commonly assumed conditional on infection but independent of dose. Usually, a fixed morbidity or mortality ratio to the risk of infection is used to obtain the risk of diseases or mortality probability (Haas et al. 1999). The fixed ratio is usually determined from infectivity and illness data, as an estimate of the fraction of the infected population that becomes ill after infection.
The one-hit models have been described as the most relevant for microbial dose–response assessment. However, there still exists a great deal uncertainty surrounding how well these models represent the reality of the environmental system. Most of the existing QMRA models for drinking water were developed based on environmental systems and scenarios in the developed countries.
A few recent studies of QMRA of drinking water system done in the developing countries are identifiable in the literature. Kouamé et al. (2017) assessed the health risks associated with consuming salad irrigated with wastewater in Côte d’Ivoire, using the QMRA procedure described by Haas et al. (1999). In the study, G. lamblia and E. coli O157:H7 were selected as diarrhea causing organisms, and the risk of diarrhea resulting from the two pathogens were estimated using the exponential and the beta-Poisson models, respectively. The study predicted that the risk of diarrhea resulting from ingesting of water during farming activities in Côte d’Ivoire ranged between 0.002% and 0.046% for E. coli O157:H7, and from 0.0064% to 0.027% for G. lamblia. Similarly, the risks of diarrhea arising from consumption green salad consumption, the annual risk linked to G. lamblia ranged from 0.0 to 1.0 per person per year; and the risk of diarrhea linked to E. coli O157:H7 varied from 0.0 to 1.00 per person per year. Of input parameter used in the assessment, only the concentrations of G. lamblia and E. coli were directly related to or obtained from the study environment. According to the authors, the volume of water ingested and the mass of salad consumed during each type of exposure were estimated from the literature. Also obtained from the literature are the shape parameters—α and β parameters (0.0571 and 2.2183, respectively, from Mok et al. 2014; the dose–response parameter—r (0.0198), from Haas and Eisenberg 2001; the risk of illness given infection (Pill = 0.25) for E. coli O157:H7 from Howard et al. (2006), and Pill = 0.67 for Giardia lamblia from Rose et al. 1991).
Most of the QMRA done in the developing countries adopt the same approach as Kouamé et al.’s, such that once the concentration of pathogens are known the existing dose–response models (developed using developed countries’ water supply scenarios) are applicable in the developing countries’ environment (e.g., Table 1). This approach seems to be an over simplification because the water supply scenarios and factors that affect exposure, infection and progression to diseases are different between the developed and developing countries. For instance, factors like frequency and concentration of water contaminants, frequency of exposure, health status (including nutrition), hygiene practices and adaptability to waterborne diseases are unfortunately different for the two types of countries. Whereas people in the developing countries manage to live with exposure to high levels of pathogens in drinking water, very low levels of pathogens are known to have caused outbreaks in the developed nations. Consequently, performing QMRA studies in the developing countries using the existing dose–response formulation most likely will end up with only an alarming and discouraging conclusion: the annual risk of infection per person is approximately 1.0 against the target of 10–4 per person per year in the developed countries. However, studies that provide verifiable quantitative details of all the peculiar environmental factors that facilitate high exposure of pathogens in drinking water system and the spread of waterborne diseases in human communities of developing countries can encourage government/local preventive and proactive actions on water safety and possibly improve the relevance of QMRA in developing countries.
Each water supply scenario tends to be unique, and there is yet no general guidance regarding when the available dose–response data are sufficiently representative for a particular scenario. The dose–response data obtained from human trials are a reflection of the characteristics of the unique population in the trials. Therefore, the data are more appropriate if the population defined in the scope of the assessment is comparable with the ones used to obtain the dose–response data (USDA/FSIS 2012). The progression of exposure to infection/illness depends primarily on the host defense against the pathogen. This defense is a function of the ingestion medium or food matrix (e.g., food structure and fat content) of the host. Ingestion medium that raises the pH of the stomach contents to raise the probability of survival of many organisms by some orders of magnitude relative to the extent of acid buffering (USDA/FSIS 2012). It can be noted that food matrix for human communities is largely a characteristics of a population. Current QMRA studies carried out in the developing countries have not been able to provide comprehensive site-specific information or verifiable data that stakeholders need, not only to understand the gravity of water borne diseases contracted from drinking water but to take proactive actions to address issues surrounding drinking water source contamination and vulnerability of individuals and communities to waterborne diseases. This is perhaps, the reason QMRA has not gained wide acceptance in the developing countries. This study therefore includes an attempt to modify the existing QMRA for use in the developing countries.
Materials and methods
Formulation of the Modified Dose–Response Model
This study assumes that for any ingested waterborne gastro-intestinal pathogens, the resistance of any human host to infections is mathematically uncertain and is a probability between 0 and 1. The study therefore presumes the infectivity parameter, to be 0.5, as a 50% chance that a pathogen of every type will survive to initiate an infection once ingested by a human host. The computed individual risk of waterborne pathogen infection or diseases is an average for the total population within an environment setting. For pathogens of known health effects, such as most gastro-intestinal pathogens, the risk of an adverse health effect is a function of susceptibility, infectivity and exposure dose. A “comprehensive susceptibility” parameter is a characteristic of the water supply environment, consisting of the fraction of the exposed population, percentage of the vulnerable population, and pathogen strike rate (fraction of positive presence of organisms in water samples taken over a reasonable period, at least three months).
The modified dose–response model for microbial risk assessment is then formulated as:
where PI is the daily risk of infection; \(\zeta\) is a comprehensive susceptibility parameter (which is characteristic of the exposed population and pathogen strike rate in the water source of the exposed population); \({\uplambda }\), is the concentration of specific pathogenic organism in the water samples; V is the volume of water ingested by an individual per day. The product λV is the exposure dose.
The comprehensive susceptibility parameter can then be stated as:
where pp is the fraction of population of the study area exposed to the contaminated drinking water under study; \(p_{v}\) is the percentage of the population vulnerable to pathogenic infection among the exposed population; and \(s_{d}\) is the pathogen strike rate in the contaminated water sources, which is the percentage of samples which test positive to a specific pathogen in total water sample.
The mean of the combination of the three parameters of \(\zeta\) is assumed to be a constant for a specific pathogen, for a given population and a given environmental setting. Each parameter value ranges between zero and one.
The input values for \(p_{p } , p_{v} \,{\text{and}}\,s_{d}\) were obtained with the aid of Water Supply and Waterborne Disease Survey of the study area.
Annual risk of infection can be obtained from the daily risk of infection as:
where Py is annual risk of infection and n is the number of exposures per year due to a single pathogen dose. For a point estimate of risk, a mean dose of a specific pathogen can be applied to Eqs. 1 and 3 to calculate the daily and annual risk of infection, respectively.
The risk of disease is determined as:
where k is the probability of illness-Pill (diarrhea) factor given an infection. The values k for this study were obtained from the literature (Table 2). The lowest values of k were applied to this study because it was assumed that people in Nigeria and other developing countries are better adapted to gastro-intestinal infections than their counterparts in developed countries as explained in the previous section.
The formulated model was applied in Afikpo North Local Government Area of Ebonyi State, Nigeria. This is also in pursuant of the urgent need to provide a simple objective basis for sensitizing water consumers and providing quantitative information that can enable stakeholders to take urgent steps toward improving access to safe drinking water to household communities.
Calibration and validation of the modified model
Diarrhea is one of the most common waterborne diseases caused by gastro-intestinal pathogen infections. Infection to many waterborne pathogens has been associated with diarrhea (Table 2). Therefore, the modified model was calibrated by comparing the model predictions of risk of diarrhea diseases for eleven communities in Afikpo North Local Government Area of Ebonyi State, Nigeria, with the observed diarrhea disease prevalence in those communities. Risk was calculated by point estimate. A positive correlation of about 74% was obtained and was considered satisfactory.
Application of the modified QMRA dose–response model in Afikpo north local government area of Ebonyi State, South Eastern Nigeria
The study area
Afikpo North Local Government Area [AFNLGA] is located in Ebonyi State, Southeastern Nigeria, on latitude six degrees, north and longitude eight degrees, east. The study area covers approximately 164 square kilometers, and its distance from Abakaliki, the Ebonyi State Capital, is about 67 km. The population of Afikpo North Local Government Area, based on the figures of last Nigerian census of 2006, is about 157,000 people (National Bureau of Statistics 2013). Afikpo North Local Government Area hosts two major peri-urban towns of Ebonyi State, which are Afikpo (the local government headquarters) and Unwana (the site of Akanu Ibiam Federal Polytechnic). The study area has no municipal or centralized drinking water supply. Drinking water supply in Afikpo North Local Government Area is largely a private affair, as many households still, inevitably, secure drinking water from natural water bodies such as ponds, streams, rivers and springs and rainwater. Over 50% of the households in the study area currently rely on water boreholes for supply of drinking water. There are no available formal epidemiological or waterborne diseases outbreak/prevalence data. However, the research observed a high waterborne gastro-intestinal diseases prevalence rate in the study area. Figure 1 shows the location and explosive map of the study area indicating the selected water borehole sampling sites.
Hazard identification
The prevalence of waterborne disease in Afikpo North Local Government Area of Ebonyi State Nigeria was obtained using a Water Supply and Waterborne Disease Survey Questionnaire. Eleven drinking water boreholes (labeled A–K) were purposively selected for determination of waterborne pathogen concentrations. The criteria for the selection included active daily production of water, service to a minimum of 15 households and accessibility for water sampling. The pathogen of interest was those that pose significant health risk and was considered most likely to contaminate water due to vulnerability of the borehole system environment.
Expert opinion aided selection of pathogens. A structured semi-quantitative instrument described the borehole environments and enabled experts to score the vulnerability of the boreholes’ water to various waterborne disease-causing microorganisms. Experts in the fields of water resources engineering, microbiology and public health assessed each borehole. Weighted expert opinions were combined using the linear opinion pool method (Robert and Robert 1999). In addition to expert opinions, the pathogens selected for the QMRA satisfied some criteria. These include occurrence and persistence of the specific pathogen-associated waterborne diseases in the study area environment (determined by waterborne disease prevalence survey and borehole vulnerability assessment survey), adequate literature on the pathogen, and low infectious doses, feasibility for detection and quantification of the pathogen, and use of the pathogen as an indicator organism for gastrointestinal diseases. The pathogens selected for analysis in the water samples were Escherichia coli [E. coli], Salmonella spp, Shigella spp and Campylobacter, which represented bacteria and Giardia lamblia and Cryptosporidium parvum which represented protozoa. Viruses were not selected due to some envisaged constraints in sampling and laboratory analysis.
Sample collection and analyses
Water samples were collected every three days from each water borehole site from July 15, 2019, to December 15, 2019, and so 52 samples were collected per site, making a total number of 572 samples. The water samples were collected in clean sterilized 1500-ml bottles (with caps) from the points of delivery to consumers. The water samples were analyzed using standard laboratory procedures for concentrations of E. coli, Salmonella spp, Shigella spp, Campylobacter, Giardia lamblia and Cryptosporidium parvum (Bichi and Amatobi 2013; Cheesebrough 2000).
Exposure assessment
It was assumed that the microbial health risks from exposure by dermal contact and inhalation were minor relative to that through oral ingestion. Hence, only exposure by oral ingestion was considered. Average water consumption per person per day was obtained using a daily drinking water survey questionnaire. Consumption of water was estimated for water used directly for drinking without treatment. The concentration of suspect pathogens in water was obtained directly from water sampling and laboratory analyses. Concentrations of E. coli were multiplied with a factor of 0.08 and used for E. coli O157:H7 (Haas et al. 1999). The exposure or dose, d, of ingested pathogens was obtained as the product of the volume of water ingested by the consumer per day, V, and the concentration, λ, of pathogens detected in the water samples. The values for V and λ were obtained by survey of water consumers and laboratory analysis of water samples, respectively.
Dose–response assessment
The daily risk of pathogen infection is as stated in Eq. (1). The annual risk of infection is as stated in Eq. (3). The data inputs for Pp and Pv were obtained through survey, while Sd was obtained through water sampling and analyses. Equation 1 was used to calculate the daily risk of infection.
The risk of disease, given an infection, was calculated based on diarrhea. This is because the most common symptom of gastro-intestinal infection is diarrhea. The sampling population for the prevalence of waterborne diseases among communities was drawn from individuals considered to have basic sanitation services and practice good hygiene. This was determined through a hygiene practice survey, in order to attribute observed diarrhea incidences to contaminated drinking water source only.
The risk of diarrhea disease given a waterborne gastro-intestinal infection was calculated using the Eq. (4)
The k values were obtained from current assumptions available in the literature (Table 2) as explained in the previous section.
Risk characterization
The product of mean concentration of each pathogen (units/liter) and mean daily water consumption in (liters per capita per day) was put into the dose–response relations (Eqs. 1 and 4) to obtain a point estimate of risk of pathogen infection and risk of diarrhea disease. However, the concentration of pathogen and volume of water ingested tended to exhibit wide variability within the study environment. The extent and mechanism of this variability were uncertain. Therefore, to address the variability and uncertainty in the exposure estimates, ten thousand Monte Carlo iterations were made for each identified pathogens using a program written in Microsoft Office Excel 2007 spreadsheet. The Monte Carlo simulation generated 10,000 variations (samples) of each of the model input variable and created 10,000 estimates of the risk of infection and risk of diarrhea disease given an infection which were summarized. The risk characterization expressed risks of infection and diarrhea diseases, in terms of mean, mode, median, 95th percentile, and 5th percentiles.
Comparison of the modified model with the existing models
First, the risk results for some pathogens obtained using the modified QMRA model were compared with the results that could have been obtained if the formulations of the existing QMRA models adopted by some recent studies carried out in the developing countries (Table 1) were followed. Hence, the results were compared with the works of Kouamé et al. (2017), Ahmed et al. (2020), Machdar et al. (2013a, b), and Chukwuemeka et al. (2021).
Second, for further comparisons, the mean risk of diarrhea for the six waterborne pathogens—E. coli O157:H7, Salmonella spp, Shigella Spp, Campylobacter, Giardia lamblia and Cryptosporidium parvum—was estimated separately using the existing model formulations selected randomly from the literature (Table 3). The student’s t-distribution was used to test the difference of two means of the diarrhea risk values of the existing model and the modified model, at 95% confidence interval. The predicted incidence of diarrhea was compared with the observed incidence for both existing and the modified model by using simple variance analysis.
Results and discussion
Drinking water sources for consumers in Afikpo north local government area of Ebonyi State Nigeria
More than 62% of the sampled population used water boreholes as a source of drinking water, but approximately 58% do not subject the water to any form of treatment before direct consumption (Table 4). This is a trend in Nigeria due to lack of treated water supply systems, and the erroneous assumption that clear/odorless water connotes safe drinking water. However, most of the boreholes are poorly constructed and operate in poor sanitary environment. The high incidence of waterborne diseases in Nigeria calls for risk assessment of water from these boreholes.
Table 5 presents the mean concentration of pathogens detected in samples taken from each of the studied boreholes over six months period. The presence of the enteric organisms (E. coli, Salmonella spp, Shigella spp, Campylobacter, Giardia lamblia and Cryptosporidium parvum) in the water samples portend public health risk since they are food and water-related pathogenic agents (Anyanwu, and Okoli 2012). The enteric organisms in water supply indicate fecal contamination. Their presence in water boreholes could be because of the poor construction and location of majority of drinking water borehole operating in the study area. Most of the boreholes sites in study area are in residential quarters and are located at less than the recommended distances of 15 m from septic tanks or less than 30 m from pit latrines. The implication is that consumption of untreated or unboiled water from the boreholes in the study area (Afikpo North Local Government Area of Ebonyi State, Nigeria) will most likely contribute to the high incidence of waterborne gastrointestinal diseases in the area.
Drinking water consumption per person/day in Afikpo north local government area
The mean water consumption is estimated as 1.54L/person per day (Table 6). The most likely water consumption per person per day, determined by this study, using 10,000 Monte Carlo simulations ranged between 1.06 and 2.47 l/person per day. The modal water consumption is estimated to be 1.77 l/person per day. These values fall within the range used for QMRA by some researchers (e.g., WHO 2017; Howard et al. 2006; Masago et al. 2004).
Pathogen strike rate
Pathogen strike rate for any pathogen represents the percentage of “positives” detected from water samples for the pathogen. The strike rates determined from 572 samples are presented in Table 7. This study believes that the population consuming drinking water from sources of high pathogen strike rates will be vulnerable to repetitive exposure. Therefore, strike rate should be factored into the calculation of risk of infection.
Percentage of vulnerable population among people who consume untreated borehole water
About 19% of the sample population are among the group of individuals likely to have low immune system (Table 8). These group are most susceptible to pathogen infection and therefore needs to be factored into QMRA estimations.
Prevalence of waterborne diseases among study population
The prevalence of waterborne disease in the study area was determined to be 12.3%, with diarrhea diseases being 9.3% (Table 9). This agrees with findings of Akinyemi (2019) and Akinrotoye and Uzal (2018). Individuals obtaining drinking water from untreated sources in high waterborne disease prevalent environment could be at a higher risk of infection. Gastro-intestinal pathogen infections spread through the fecal–oral route, and so the chance of contaminating water sources will be higher where the water sources are not adequately protected from fecal contamination as found in the developing countries.
Risk of pathogen infection and risk of diarrhea
Table 10 presents the risk of pathogen infection and risk of diarrhea determined using the modified QMRA model for consumers of untreated borehole water in Afikpo North Local Government Area of Ebonyi State in Nigeria. The estimated risk of infection due to presence of E. coli O157:H7 pathogen is 0.325; due to Salmonella, spp is 0.227; due to Shigella, spp is 0.240; due to Campylobacter is 0.255; due to Giardia lamblia is 0.218; and due to Cryptosporidium, parvum is 0.153. The mean risk of infection for a combination of all pathogens is 0.236 with a standard deviation of 0.056.
Risk of disease due to the presence of E. coli O157:H7 pathogen is 0.065; due to Salmonella spp is 0.045; due to Shigella spp is 0.031; due to Campylobacter is 0.026; due to Giardia Lamblia is 0.044; and due to Cryptosporidium parvum is 0.021. The mean risk of diarrhea disease based on all the mentioned pathogens is 0.039 with the standard deviation of 0.016.
The mean daily risk of infection of 2.36 × 10–1 translates to annual risk of infection of approximately 1.0, for an exposure episode as low as 30 days, i.e., one month during the year (using Eq. 3). This suggests a very high-risk scenario because as this study can show, exposure episodes are usually more than 30 days in the developing countries. Conventionally acceptable annual risk of infection for waterborne pathogen is 10–4 (WHO 2016). The risk of diseases is normally a factor of risk of infection. These results suggest that consumers of untreated water from boreholes in the study area are at a very high risk of being infected and contracting diarrhea and other gastro-intestinal diseases. Hunter et al. (2009) predicted that for relying on untreated drinking water for 10 days in a year, the annual risk of infection was 0.745 for E.coli and 0.999 for Cryptosporidium; the respective risk of infection for one-day exposure being 0.128 and 0.791. Due to unavailability of treated drinking water in the study area (and in many communities in Nigeria), many people rely on untreated drinking water from boreholes for hundreds of days in a year. Therefore, it is not surprising that the annual probability of infection of gastro-intestinal pathogens from exposure to drinking water from water boreholes could approach 1.0.
Data on quantitative microbial risk assessment of water boreholes in Nigeria are not readily available. However, other forms of risk assessments of boreholes carried out in Nigeria also infer a high human health risk for consumers of untreated borehole water (e.g., Okoye et al. 2016; Enoh et al. 2016).
Risk characterization
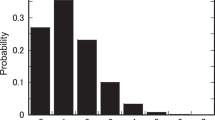
Risk characterization results based on 10,000 Monte Carlo simulations are presented in Tables 11 and 12. Graphs of 10,000 iterations for calculation of risk of diarrhea disease based on the concentration of specific pathogens determined from water samples are presented in Fig. 2.
All the pathogens assessed in the studied boreholes manifested a high-risk scenario in all the dimensions of risk characterization (adopted for the study), for both risk of infection and risk of diarrhea diseases. It is only in 5% of the population that exist little or no risk, and this may be due to chance or existence of some people with strong immunity. Nevertheless, this result is expected in a situation where people use raw water (untreated water) as a source of drinking water. The result agrees with Hunter et al. (2009), and Murphy et al. (2015). A major implication of these results is that consumers of untreated borehole water in Afikpo North Local Government Area of Ebonyi State, Nigeria, are at high risk of contracting gastro-intestinal infections and diarrhea diseases.
Figure 2 shows a Monte Carlo simulation of percentile risk of diarrhea diseases contribution for the six pathogens studied. The curves indicate that E. coli O157:H7, Giardia lamblia and Salmonella spp contribute the major diarrhea risk for consumers of borehole water in the study area. These pathogens are majorly of fecal origin, and their high concentrations in water samples reflect the poor construction and poor sanitary conditions of the drinking water boreholes in the study area. Most of the boreholes are sited in living residences at less than 12 m distance from septic tanks.
Comparison of the risk results obtained using the modified QMRA dose–response formulation with the results predicted using the existing dose–response formulations
Table 13 shows results of the application of QMRA by recent studies on some waterborne pathogens based on the concentrations determined in the drinking water boreholes sampled in Afikpo North local Government Area. These concentrations are extracted from Table 5. Table 13 compares the risk of diarrhea diseases predicted using the modified model for each specific organisms with the risk of diarrhea that would be predicted if considerations followed by other in developing countries were followed. From the results, it can be observed that the difference of the results obtained using the modified model and following the existing model are within an order of magnitude or less. The results are close due to high levels of pathogens that are typically found in many drinking water supply sources of developing countries. However, the authors adopting the existing dose–response model used different dose–response parameters for same pathogen, for instance, parameters for E. coli O157:H7 in Table 1. The criteria for selection of dose–response parameters from the literature were not explained and therefore seem to be based on personal judgment. This is a trend followed by many authors, but the procedures have no adjustment for different water supply scenarios that exist in the developing countries, and therefore cannot provide site-specific results. On the other hand, the modified model produces site-specific results.
The risk results obtained from the modified QMRA models based on the concentration of some specific pathogens were compared with the results obtained using the existing QMRA model formulation adopted by some recent studies in the developing countries. For E. coli, the risk of diarrhea obtained using the modified model was 0.058; using the formulation of Kouamé et al. (2017, in Côte d’Ivoire), it was 0.021, for Ahmed et al. (2020 in Pakistan), 0.012, Machdar et al. (2013a, b in Ghana), 0.017, and Chukwuemeka et al. (2021, in Nigeria), 0.005. For Giardia lamblia, the modified model returned the risk of diarrhea value of 0.054 while using Kouamé et al. (2017) gave a risk value 0.07. For Campylobacter the modified model obtained the risk of diarrhea of 0.029; following, Machdar et al. (2013a, b) gave 0.082 and Ahmed et al. (2020), 0.167. The difference between results obtained using the modified model and using the existing model for estimating the risk of diarrhea is within an order of magnitude or less.
Further comparison of the results obtained on the concentration of pathogens using the modified QMRA dose–response model with the results obtained using some of the common QMRA models/formulations
Linear comparison of risk estimates obtained using the existing and the modified QMRA models is presented in Tables 14 and 16. Result of t-test statistic (Table 15) at 95% confidence interval showed a significant difference between the mean risk of infection values obtained using the existing model and using the modified models (ttab = 2.28, tcal = − 5.117). The difference could be due to different assumptions employed for each model. There was, however, no significant difference between the mean of diarrhea diseases obtained by both the existing and the modified models (-ttab = − 2.228; tcal = − 0.2349, Table 17). This apparent agreement is possible because the modified model used the lowest available probabilities of sickness given an infection (Pill) in the computation of risk of diarrhea. Adaptability to waterborne infection/illness is mostly likely to be different between the developed and the developing countries, but previous QMRA studies seem to neglect this fact. Whereas very low doses of waterborne diseases could cause outbreaks in developed countries, people in the developing countries could cope with daily exposure of very high doses of pathogens in drinking water without outbreaks.
In real life, infections are not easily observable on individuals since there could be asymptomatic carriers, especially for gastro-intestinal infection (CDCP 2012). The incidence or prevalence of diseases is readily recognizable. In effect, the statistical agreement of the modified model with the existing model on predicted mean diarrhea is most remarkable. This is because disease incidences are physically observable, while infection incidences are usually difficult and sometimes impossible to observe without clinical/laboratory analyses. For the modified model, the result shows that local data input can quantitatively predict the risk of a known waterborne pathogenic microorganism in drinking water for developing countries without recourse to data or assumptions developed in the advanced countries.
Comparison of the predicted risk of diarrhea diseases using models with the observed prevalence values in the study communities by means of simple variance analysis
The results for variance analysis results obtained from the existing and the modified models are presented in Tables 18 and 19. They were obtained using point estimate for simplicity. The observed prevalence of diarrhea disease in the study communities ranged between 0.017 and 0.151, with a mean of 0.093. With existing models, the predicted risk of diarrhea in the study communities ranges between 0.012 and 0.066, with a mean risk of 0.037. When compared side by side with the observed diarrhea prevalence, this gives a mean variance of − 0.056. By using the modified model, predicted risk of diarrhea diseases in the study communities fall between 0.024 and 0.051, with a mean of 0.043; the variance from the observed diarrhea prevalence is 0.050. The prevalence ranges between 7.5% (Akinyemi 2019) and 18.8%. However, apart from contaminated drinking water, diarrhea can come to humans through other pathways such as improper sanitation and poor hygiene practices.
Conclusion
The application of modified model in the household communities of Afikpo North local Government Area of Ebonyi State Nigeria revealed the population-specific susceptibility and risk situations. In following the process of the modified QMRA, other population specific data were determined for the study population. These include the major source of drinking water, the concentration of specific pathogens in the drinking water sources, the volume of drinking water consumed by oral ingestion per capita per day, the pathogen strike rate in water sources, the vulnerable fraction of the population and the prevalence of waterborne diseases.
Due to the wide gaps in education, technology, resources, governance, and so on, most drinking water supply sources in the developing countries contain very high levels of pathogens relative to those of the developed countries. Susceptibility to waterborne infections and progression to waterborne diseases are also different for both countries because susceptibility is a function of exposure, infectivity and adaptability—characteristic of the population. Even though population characteristics are different, the existing QMRA models have been universally and uniformly applied to different types of populations. The exposure and infectivity parameters used to develop the existing dose–response models were produced in the developed counties’ environmental setting, and so such parameters will most likely not reflect the population characteristics of the developing country. This study has modified the exiting QMRA with the introduction of an environmentally (population characteristic) based susceptibility parameter into the dose–response function to make QMRA appropriate to the developing countries. Due consideration was given to different adaptability levels of progression to illness from exposure/infection of waterborne pathogen, which are likely to exist between the developed and the developing countries.
Apart from the concentration of pathogens, the population-characteristic susceptibility parameters (% exposed population, % vulnerable population and pathogen strike rate) introduced in the QMRA model offer water safety stakeholders, especially in the developing countries, verifiable data about issues which could be addressed to reduce waterborne diseases prevalence in the developing countries. Improving access to clean water sources, ensuring that pathogen concentration in drinking water sources is controlled within acceptable limits, and taking special interest on the vulnerable members of the society can reduce susceptibility and therefore risk of waterborne diseases in the developing countries.
In addition, making and enforcing improved sanitary and hygiene policies can enhance water quality, reduce contamination of food and water, and reduce the risk of waterborne diseases in the developing countries. For example, ban open defecation; provide adequate public toilets; regulate the siting of residential drinking water wells/boreholes and septic tanks/latrines; encourage business/public places to provide running water; enforce hand washing practices; engage sanitary inspectors, etc.
Thus, the modified QMRA dose–response model proposed by this study for the developing countries offers a credible alternative to the existing QMRA models, which will likely create a better interest on and the acceptability of QMRA in the developing countries.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article. Further details of the data are available on request from the corresponding author.
References
Abia ALK, Ubomba-Jaswa E, Genthe B, Momba MNB (2016) Quantitative microbial risk assessment (QMRA) shows increased public health risk associated with exposure to river water under conditions of riverbed sediment resuspension. Sci Total Environ 566:1143–1151
Adebowale N (2017) Over 57 million Nigerians lack access to potable water—UNICEF. Premium Times, Nigeria
Ahmed J, Wong PL, Chua PY, Channa N, Mahar RB, Yasmin A, VanDerslice JA, Gari JV (2020) Quantitative microbial risk assessment of drinking water quality to predict the risk of waterborne diseases in primary-school children. Int J Environ Res Public Health 17(2774):2–6
Akinrotoye KP, Uzal U (2018) Combating diarrhoea in Nigeria: the way forward. J Microbiol Exp 6(4):191–197. https://doi.org/10.15406/jmen.2018.06.00213/
Akinyem YC (2019) Spatial pattern and determinants of diarrhoea morbidity among under-five-aged children in Lagos State, Nigeria. Cities Health. https://doi.org/10.1080/23748834.2019.1615162
Akor O (2017) Nigeria: water borne diseases ravage communities. Daily Trust. https://allafrica.com/stories/201704030185.html
Anyanwu CU, Okoli EN (2012) Evaluation of the bacteriological and physicochemical quality of water supplies in Nsukka, Southeast, Nigerian African. J Biotechnol 11(48):10868–10873. https://doi.org/10.5897/AJB12.903
APHA (2004) Control of communicable diseases manual, 18th edn. APHA, American Public Health Association, Washington, DC
Bichi MH, Amatobi DA (2013) Assessment of the quality of water supplied by water vendors to households in Sabon-gari area of Kano, northern Nigeria. Int J Eng Sci 2(7):09–17
CDCP (2012) Giardiasis. Centre for Disease Control and Prevention: Iowa State University USA: College of Vetinary Medicine. http://www.cfsph.iastate.edu/Factsheets/pdfs/giardiasis.pdf/
Cheesebrough M (2000) District laboratory practice in tropical countries Part 2. Cambridge University Press, Cambridge
Chukwuemeka KJ, Jaan HP, Rodrigo M, Manish P (2021) Health-risk assessment for roof-harvested rainwater via QMRA in Ikorodu area, Lagos, Nigeria. J Water Clim Change (in press). https://doi.org/10.2166/wcc.2021.025/871790/jwc2021025.pdf
Connor BA (2020) Preparing international travelers: travelers' diarrhea. Centers for disease control and prevention (CDC). https://wwwnc.cdc.gov/travel/yellowbook/2020/preparing-international-travelers/travelers-diarrhea
Dong QI, Barker GC, Gorris GM, Tian MS, Song XY, Malakar PK (2015) Status and future of Quantitative Microbiological Risk Assessment in China. Elsevier Trends Food Sci Technol 42(1):70–80. https://doi.org/10.1016/j.tifs.2014.12.003
Dunn G, Harris L, Cook C, Prystajecky N (2014) A comparative analysis of current microbial water quality risk assessment and management practices in British Columbia and Ontario, Canada. Sci Total Environ 468–469:544–552. https://doi.org/10.1016/j.scitotenv.2013.08.004
DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W (1995) The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med 332(13):855–859
Enoh EA, Eneche PU, Uko NF (2016) Assessment of micro elements in borehole water sources in Oso Edda, Afikpo L.G.A Ebonyi State, Nigeria. Int J Multidisc Res Modern Educ 2(1):2454–6119. http://www.rdmodernresearch.com
Gale P (2005) Land application of treated sewage sludge: quantifying pathogen risks from consumption of crops. J Appl Microbiol 98(2):380–396
Godfrey S, Smith MD (2005) Improved microbial risk assessment of groundwater. Hydrgeol J 13(1):321–324. https://doi.org/10.1007/s10040-004-0412-7
Haas CN (1983) Estimation of the risk due to low doses of microorganisms: a comparison of alternative methodologies. Am J Epidemiol 118(4):573–582. https://doi.org/10.1093/oxfordjournals.aje.a113662
Haas C, Eisenberg JNS (2001) Risk assessment. In: Fewtrell L, Bartram J (eds) Water quality: guidelines, standards and health assessment of risk and risk management for water-related infectious disease. IWA Publishing, London, pp 161–183
Haas CN, Rose JB, Gerba CP (1999) Quantitative microbial health risk assessment. John Willy and Sons Inc, New York
Havelaar AH, Melse JM (2003) Quantifying public health risks: in the WHO guidelines for drinking-water quality: a burden of disease approach, Report 734301022/2003, RIVM, Bilthoven, Netherlands
Howard G, Pedley S (2004) Assessing the risk to public health fromwater supply using QMRA. In: Godfrey S, Howard G (eds) Health, institutional, social and mapping programmesto support WSPs. Leicestershire:WEDC, Loughborough University
Howard G, Pedley S, Tibatemwa S (2006) Quantitative microbial risk assessment to estimate health risks attributable to water supply: can the technique be applied in developing countries with limited data? J Water Health 4(1):49–65. https://doi.org/10.2166/wh.2005.058
Hunter PR, Payment P, Ashbolt N, Bartram J (2003) Assessment of risk. In: Dufour A (ed) Assessing microbial safety of drinking water—improving approaches and methods. IWA Publishing, London, UK, pp 79–109
Hunter PR, Zmirou-Navier D, Hartemann P (2009) Estimating the impact on health of poor reliability of drinking water interventions in developing countries. Sci Total Environ 407(8):2621–2624
Kouamé PKNguyen-Viet H, Dongo K, Zurbrügg C, Biémi J, Bonfoh B (2017) Microbiological risk infection assessment using QMRA in agriculture systems in Côte d’Ivoire, West Africa. Environ Monit Assess 189:587. https://doi.org/10.1007/s10661-017-6279-6
Labite H, Lunani I, van der Steen P, Vairavamoorthy K, Drechsel P, Lens P (2010) Quantitative microbial risk analysis to evaluate health effects of interventions in the urban water system of Accra, Ghana. J Water Health 8(3):417–430. https://doi.org/10.2166/wh.2010.021
Li H (2007) Hierarchical risk assessment of water supply systems (Doctoral dissertation), Loughborough University, Loughborough, Leicestershire, UK). https://dspace.lboro.ac.uk/2134/2735
Lim KY, Jiang SC (2013) Re-evaluation of health risk benchmark for sustainable water practice through risk analysis of rooftop-harvested rainwater. Water Res 47(20):7273–7286. https://doi.org/10.1016/j.watres.2013.09.059
Machdar E, van der Steen NP, Raschid-Sally L, Lens PNL (2013a) Application of quantitative microbial risk assessment to analyze the public health risk from poor drinking water quality in a low income area in Accra, Ghana. Sci Total Environ 449(2013):134–142. https://doi.org/10.1016/j.scitotenv.2013.01.048
Machdar E, van der Steen NP, Raschid-Sally L, Lens PNL (2013b) Application of quantitative microbial risk assessment to analyze the public health risk from poor drinking water quality in a low income area in Accra, Ghana. Sci Total Environ 449:134–142
Makri A, Mondarres R, Parkin R (2004) Cryptosporidiosis susceptibility and risk: a case study. Risk Anal 24:209–220
Masago Y, Oguma K, Katayama H, Hirata T, Ohgaki S (2004) Cryptosporidium monitoring system at a water treatment plant, based on waterborne risk assessment. Water Sci Technol 50(1):293–299
Medema GJ, Teunis PF, Havelaar AH, Haas CN (1996) Assessment of the dose-response 322 relationship of Campylobacter jejuni. Int J Food Microbiol 30:101–111
Messner MJ, Chappell,CL, Okhuysen PC (2001) Risk assessment for Cryptosporidium: a hierarchical Bayesian analysis of human dose-response data. Water Res 35(16):3934–3940
Mok HF, Barker SF, Hamilton AJ (2014) A probabilistic quantitative microbial risk assessment model of norovirus disease burden from wastewater irrigation of vegetables in Shepparton, Australia. Water Res 54:347–362
Murphy HM, Thomas MK, Schmidt PJ, Medeiros DT, McFadyen S, Pintar KDM (2015) Estimating the burden of acute gastrointestinal illness due to Giardia, Cryptosporidium, Campylobacter, E. coli O157 and Norovirus associated with private wells and small water systems in Canada. Epidemiol Infect 144(2016):1355–1370
National Bureau of Statistics (2013) Annual abstract of statistics, 2011. http://istmat.info/files/uploads/53129/annual_abstract_of_statistics_2011.pdf
Nwabor OF, Nnamonu EI, Martins PE, Ani OC (2016) Water and waterborne diseases: a review. Int J Trop Dis Health 12(4):1–14
Okhuysen PC, Chappell CL, Crabb JH, Sterling CR, DuPont HL (1999) Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis 180:1275–1281
Okoye JI, Ene GI, Ojobor CC (2016) Physico-chemical and microbiological evaluation of borehole water samples in Enugu, South-Eastern, Nigeria. IOSR J Environ Sci Toxicol Food 10(11):16–19
Robert TC, Robert LW (1999) Combining probability distributions from experts in risk analysis. Risk Anal 19(2):187–203
Rose JB, Gerba CP (1991) Use of risk assessment for development of microbial standards. Water Sci Technol 24:29–34
Rose JB, Haas CN, Regli S (1991) Risk assessment and the control of waterborne giardiasis. Am J Public Health 81:709–713
Schmidt PJ et al (2013) Harnessing the theoretical foundations of the exponential and beta-poisson doseresponse models to quantify parameter uncertainty using Markov Chain Monte Carlo. Risk Anal 33:1677–1693
Schuster CJ, Ellis AG, Robertson JR, Charron DF, Aramini JJ, Marshall BJ, Teunis PFM, Ogden ID, Strachan NJC (2007) Hierarchical dose response of E. coli O157:H7from human outbreaks incorporating heterogeneity in exposure. Epidemiol Infect 136(2008):761–770. https://doi.org/10.1017/S0950268807008771
Soller JA, Schoen ME, Bartrand T, Ravenscroft J, Ashbolt NJ (2010) Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res. https://doi.org/10.1016/j.watres.2010.1006.1049
Strachan NJC, Doyle MP, Kasuga F, Rotariu O, Ogden ID (2005) Dose response modelling of Escherichia coli O157 incorporating data from foodborne and environmental outbreaks. Int J Food Microbiol 103(1):35–47
Teunis PFM, Havelaar AH (2000) The Beta Poisson dose-response model is not a single-hit model. Risk Anal 20(4):513–520. https://doi.org/10.1111/0272-4332.204048
Teunis P, Takumi K, Shinagawa K (2004) Dose response for infection by Escherichia coli O157:H7from outbreak data. Risk Anal 24(2):401–407
Teunis P, van den Brandhof W, Nauta M, Wagenaar J, van den Kerkhof H, van Pelt W (2005) A reconsideration of Campylobacter dose–response relation. Epidemiol Infect 133:583–589
Teunis PFM, Ogden ID, Strachan NJC( 2008) Hierarchical dose response of E. coli O157:H7from human outbreaks incorporating heterogeneity in exposure. Epidemiol Infect 136(6):761–770. https://doi.org/10.1017/S0950268807008771
USDA/FSIS/ (2012) Microbial risk assessment guideline: Pathogenic organisms with focus on food and water. United States Department of Agriculture/Food Safety and Inspection Service & U.S. Environmental Protection Agency, FSIS Publication No. USDA/FSIS/2012-001; EPA Publication No. EPA/100/J12/001
U.S. EPA (2010) Quantitative microbial risk assessment to estimate illness in freshwater impacted by agricultural animal sources of fecal contamination. United States Environmental Protection Agency: Office of Water: EPA 822-R-10-005
Westrell T (2004) Microbial risk assessment and its implications for risk management in urban water systems [PhD dissertation]. University of Linköping, Faculty of Arts and Science, Linköping
WHO (2011). Guidelines for drinking-water quality (4th edn). World Health Organization. Geneva. http://whqlibdoc.who.int/9789241548151_eng/
Wolfe MS (1992) Giardiasis. American society for microbiology. Clin Microbiol Rev 5(1):93–100. https://cmr.asm.org/content/cmr/5/1/93.full.pdf
World Health Organization (2007) Combating waterborne disease at the household level. https://www.who.int/household_water/advocacy/combating_disease.pdf
World Health Organization (2016) Quantitative microbial risk assessment: Application for water safety management. WHO_Annex1/microbial-risk- assessment.pdf
World Health Organization (2017) Guidelines for drinking-water quality: fourth edition incorporating the first Addendum. Geneva, Switzerland. https://doi.org/10.1017/S0950268815002071 (Copyright 2015 by Cambridge University Press)
World Health Organization (2019) Drinking-water: key facts. https://www.who.int/news-room/fact
World Health Organization (2020a) Water-related diseases. https://www.who.int/water_sanitation_health/diseases-risks/diseases/diarrhoea/en/
World Health Organization (2020b) Global Health Observatory (GHO) data. https://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amatobi, D.A., Agunwamba, J.C. Improved quantitative microbial risk assessment (QMRA) for drinking water sources in developing countries. Appl Water Sci 12, 49 (2022). https://doi.org/10.1007/s13201-022-01569-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01569-8