Abstract
The 'Tamnean' Plutonic Suite aquifer is the main public water supply for the Garu-Tempane District. Thus, hydrogeochemical characterization is essential to provide valuable insights into pollution sources and the main controls on groundwater chemistry. In this regard, multivariate statistical methods, conventional hydrochemical graphical methods, and various ionic ratios complemented with PHREEQC geochemical modelling were carried out using 38 groundwater samples collected from the Tamnean Plutonic Suite aquifers, Ghana. The ionic ratio plots, the chloro-alkaline indices, and the graphical diagrams indicate that the major sources of groundwater chemistry are silicate mineral dissolution and cation exchange coupled with the leaching of domestic solid waste and nitrogen-based fertilizers. The Q-mode hierarchical cluster analysis reveals three spatial groundwater zones. Groundwater from recharge areas consists of Ca–Na–HCO3 water types in cluster 1. The intermediate zone is characterized by Ca–Mg–Na–HCO3 water types of moderate ionic compositions in cluster 2, and this evolves into a discharge zone in cluster 3 mainly of Ca–Mg–Na–HCO3–NO3 water types. The principal component analysis (PCA) reveals three factors, which account for 81% of the total variance, and this suggests most of the groundwater chemistry had longer interaction with the lithological materials. The PHREEQC geochemical modelling consisting of mineral saturation index indicates that groundwater is mostly supersaturated with respect to dolomite and undersaturated with respect to calcite, anhydrite, fluorite, gypsum, and halite. Based on the water quality index, the groundwater in the district is generally suitable for drinking water purposes. All the samples are within the World Health Organization acceptable limits for drinking water except for lower pH, elevated nitrate and bromide concentrations in some of the wells. About 10.5% of the groundwater samples are contaminated with nitrate, which may pose a health danger to the inhabitants in the communities. The finding of this study will not only contribute to solving the research paucity regarding the Tamnean Plutonic Suite aquifers in the Garu-Tempane District but will serve as a valuable document for water managers and decision-makers in Ghana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sustainable and quality water to meet global demand for agricultural food production and drinking water supplies are goals the world at large is striving towards (Dennehy et al. 2015; Dişli 2018). It is estimated that around 2.5 billion people depend on groundwater as a means of drinking water in the world (Dhar Chakrabarti 2017; Holting and Coldewey 2019). Despite this huge number, approximately 783 million people, who correspond to 11% of the global population, still cannot find access to safe drinking water since 2012 (WHO 2012). Furthermore, there is a projection that by the year 2025 and beyond, an increasing number of the world population will experience water scarcity (Lautze and Hanjra 2014; World Health Organization 2018). This development would significantly undermine the efforts towards achieving the United Nations Sustainable Development goals 6 (ensuring clean water and sanitation for humankind) (UN 2015). Challenges arising from the ever-growing population and the global climatic changes have severely affected precipitation patterns and groundwater recharge, consequently affecting the available water resources (Mook and Rozanski 2000). In addition, the steady rise in excessive agricultural irrigation practices coupled with the abstraction of groundwater for industrial purposes has exacerbated the negative impacts of groundwater quantity and quality (Ounvichit 2011). While the challenge of ensuring sustainable management of groundwater resources has become a universal goal for most countries, the idiosyncrasies of groundwater use warrant holistic diagnosis and approaches (Okofo et al. 2021).
Groundwater availability for irrigation and drinking water purposes is often affected by geogenic and anthropogenic pollution, and these are reported in several studies (Ashraf et al. 2017; Kirschke et al. 2019; Suciu et al. 2020; Zango et al. 2019). The anthropogenic source of pollution includes mining, untreated solid and liquid waste, and intensive agriculture (Hölting and Coldewey 2019). In the absence of functional effluent and waste treatment facilities, these pollutants are usually facilitated by rainfall events and seep into the groundwater. This eventually causes an impairment of the groundwater quality (Xanke et al. 2020).
In Ghana, the zeal of the farmers to increase their agricultural yields through the use of chemical and organic fertilizers has inadvertently caused elevated nitrate concentrations in groundwaters in some agricultural communities (Anornu et al. 2017; Egbi et al. 2020). On the other hand, geogenic pollution includes solutes emanating from the water-bearing rocks, which change the chemistry of the groundwater in space and time (Appelo and Postma, 2005). The chemistry of groundwater is greatly affected by geology, groundwater–rock interactions, precipitation of minerals, and their compositions (Güler et al. 2002; Appelo and Postma 2005; Dişli and Gülyüz 2020). To safeguard groundwater quality against non-point and point sources of pollution, there is the need to assess groundwater periodically from a hydrogeochemical perspective.
Several techniques, including conventional hydrogeochemical graphical and multivariate statistical methods, have been copiously used to understand the geochemical processes in aquifer systems (Saravanakumar and Ranjith Kumar 2011; Sunkari and Abu 2019; Zakaria et al. 2020). Multivariate statistical methods such as principal component analysis (PCA) and hierarchical clustering are the most applied geostatistics in hydrological studies (Loh et al. 2020). Hierarchical cluster analysis is essentially used to discuss the groupings of groundwater samples that reflect the multiple sources, such as natural or anthropogenic activities on the groundwater. It also helps to elucidate the main factors controlling the groundwater chemistry of an area (Güler et al. 2002).`
The area under investigation falls under the White Volta Basin of Ghana. The primary source of public water consumption for the inhabitants is from the 'Tamnean' Plutonic Suite aquifer. Several boreholes are situated in this unique aquifer, where the people tap groundwater exclusively for drinking and irrigation purposes. Furthermore, the district is predominantly a rural setting, which has no groundwater protection zone and program. This has been a major driver worsening groundwater quality in most rural and peri-urban areas in Sub-Saharan Africa (Nyenje et al. 2010). It is, therefore, necessary to understand the water chemistry and quality in the area for groundwater resources planning.
Different researchers have reported hydrogeochemical studies in the White Volta Basin of Ghana. For example, Chegbeleh et al. (2020) studied the hydrogeochemistry of groundwater using principal component analysis (PCA) and hierarchical cluster analysis (HCA) in the Talensi District of the White Volta basin Ghana. The PCA and HCA revealed silicate and carbonate weathering as the main conspicuous minerals controlling groundwater chemistry in the Birimian rock terrain of the district. Koffi et al. (2017) also characterized the hydrochemistry of the groundwater and surface in the Vea catchment and concluded that the groundwater is suitable for drinking and irrigation purposes. The results also indicated that silicate weathering and cation exchange are the major processes controlling groundwater chemistry in the Upper Birimian and Bongo granitic terrain of the area. Similar work conducted by Anim-Gyampo et al. (2018) also studied the hydrochemistry of groundwater in the Atankwidi basin and suggested silicate weathering as the significant factor influencing groundwater chemistry in the Bongo granite terrain. Despite the numerous hydrochemistry studies in the White Volta Basin of Ghana, none to date has been written in the study area, and for this reason, there is a paucity of research on hydrochemistry studies in the Garu-Tempane District.
Therefore, this paper presents an approach to (1) ascertain the natural baseline chemistry in the groundwater of the area and (2) elucidate the main factors controlling groundwater hydrochemistry and its evolution using principal component analysis (PCA) and hierarchical cluster analysis (HCA). Combining these methods will help identify the pollution sources in the area for improved groundwater management.
Study area and climate
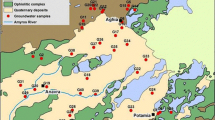
The hydrogeological catchment under investigation consists of two administrative areas: Garu and Tempane, located in the south-eastern part of the Upper East region of Ghana (Fig. 1). The catchment has three sub-basins: the Tamne River basin, the Morago River and Biankuri River basin, and lastly, the White Volta River and Nahau River basin (SNC-Lavalin/INRS 2011). The area shares an international boundary with the Republic of Togo to the east. The other boundaries are Bawku municipal to the north, Bunkpurugu- Yunyoo District to the south, and Bawku West District to the West. The study area is characterized by a low-lying agricultural area with a total land area of 1230 Km2 and a population density of 99 persons per Km2. The inhabitants are mainly farmers combining livestock rearing with the cultivation of onions, watermelons, and vegetables (Ghana statistical service 2010).
The rainfall season starts from May to September, accompanied by a long dry Harmattan period between October and April. The rainfall amount ranges between 669.8 and 1339.4 mm with an annual mean of 935 mm, whereas the temperature ranges between 26.9 and 29.9 °C with a yearly average of 28.7 °C (Asamoah and Ansah-Mensah 2020).
Geology and hydrogeology
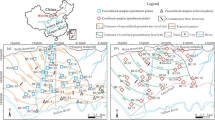
The area is underlain by two prominent geological formations: the Precambrian basement complex and the Voltaian supergroup (SNC-Lavalin/INRS 2011). The Precambrian basement complex is geologically subdivided into a Birimian supergroup consisting of volcanic and sedimentary rocks. These rocks have undergone systematic deformation and metamorphism and intruded by rocks of the Tamnean Plutonic Suite (mainly granitoids) formed during the Paleoproterozoic era ca. 2150–2070 ma (Feybesse et al. 2006). The Tamnean Plutoncic Suite covers about 80% of the district and comprises mainly of hornblende–biotite granitoids, alkali granite, syenite, minor granodiorite and minor quartz diorite and biotite tonalite (Fig. 2a). The rocks are generally exposed as tabular hills and are found in the northern and western parts of the area.
a The geological map of the area b the hydrogeology provinces of Ghana after Banoeng-Yakubu et al. (2011)
The Voltaian supergroup overlies the south-eastern part of the district and falls under the Morago River and Biankuri River sub-basin. The Voltaian supergroup lies unconformably on the Tamnean Plutonic Suite and consists exclusively of fine-grained and locally bedded sandstones from the Tossiego formations (SNC-Lavalin/INRS 2011). The study area forms an extension of the Gambaga scarp along the south-eastern portion, and the scarps are formed due to erosion and faulting of the sandstone rocks. There are small pockets of Mesozoic rocks that are rich in mafic dykes and dolerite in the area.
Hydrogeologically, the area under study is part of the Crystalline Basement Granitoid Complex Province and the Voltaian Province (Fig. 2b) (Banoeng-Yakubu et al. 2011). The Crystalline Basement Granitoid Complex Province consists of aquifers of the Tamnean Plutonic Suites, which are considered a powerful body of groundwater. These aquifers are intrinsically low in permeability; however, secondary porosity developed from chemical weathering and fracturing controls the groundwater occurrence in the area. According to Carrier et al. (2008), granitoids formations have borehole depths ranging from 35 m to 55 m with a mean depth of 50 m. Transmissivity has been reported to range from 0.3 m2/day to 114 m2/day with an average of 6.6 m2/day in granitoids terrains (Martin and van de Giesen 2005).
In the Voltaian Province, processes such as compaction and cementation have affected the primary porosity of the rocks. Thus, groundwater generally occurs in the fracture zones and along bedding planes. (Dapaah-Siakwan and Gyau-Boakye 2000; Carrier et al. 2008). Acheampong and Hess (1998) described the regolith thickness as relatively thin, circa 9 m, due to the mixtures of clay, quartz, and mudstones materials. This would imply that small amounts of water could be withdrawn locally in the Voltaian Province (Carrier et al. 2008). A study conducted by Acheampong (2017) in the Garu-Tempane District, using thirty-three drill logs from the World Vision GI-WASH program, indicates three main layers (Fig. 3). The first layer (residual soil) thickness is 6 m and consists of silty and sandy clay soil and lateritic sandy gravels mixed with clay. The second layer (saprolite) is slight- completely weathered granite that overlies a fresh, unweathered granite. The third layer comprises the saprock that overlie a fresh granitoid bedrock. The fracture zone is mainly found in the saprock under semi-confined and confined conditions. Groundwater generally occurs in the upper portion of the saprock and saprolite, and the groundwater table on average ranges between 12 m and 25 m (Acheampong 2017). There is no established groundwater recharge and groundwater flow regime in the district; however, groundwater recharge in the White Volta Basin ranged between 3.4 and 18.4% of the mean annual precipitation of 980 mm (Obuobie 2008). The groundwater flow regime in the Upper East region is from the north to the south. The aquifers in the district have been reported to have good potential to sustain the inhabitants with yields high as 5.5 m3/h (Gumma and Pavelic 2013).
Materials and methods
The Garmin ETrex was first used to record each sampling location's GPS coordinates (latitude, longitude, and altitude). Thirty-eight groundwater samples were collected from shallow wells, with depths ranging between 10 m and 60 m, during fieldwork carried out between October and November 2019 in line with the sampling protocols described by Barcelona et al. (1985). Sampling bottles were pre-treated in the laboratory and immersed in a 10% nitric acid solution for 48 h to prevent contamination. The sampling points were taken from the Tamnean Plutonic Suite aquifers.
Purging of the wells was initially done for 10–15 min to remove stagnant water and obtain uniform and well-mixed water. The in situ physical parameters such as pH, temperature, electrical conductivity (EC), and dissolved oxygen (DO) were regularly monitored and measured when a stabilized reading was achieved using a thorough calibrated Multi-Probe Meter (PCE - 1). The PCE -1 Multi-Probe Meter was rinsed with de-ionized water after each measurement at a sampling location to avoid cross-contamination. For alkalinity measurement, a bromocresol green indicator was added into a flask containing 1000 mL of water. Titration with sulphuric acid (1.60 Normality) was done until the colour changed to pink using the Hach digital field titration kit. The reading on the digital pipette was then expressed as equivalent concentrations of (CaCO3) in mg/L.
Groundwater was collected for both ions and trace metals in a clean 50-mL polyethylene bottle. The sampling bottles were rinsed with borehole water at least a minimum of two times before collection. The cations were acidified with a few drops of nitric acid to produce pH that stabilized trace metals and prevented bacteria growth. All the samples were filtered through a 0.45-µm syringe filter before bottling their chemical analysis.
The laboratory analysis for major anions and cations were analysed in January 2020 at the hydrogeochemistry laboratory-Institute of Applied Geoscience, Technical University of Darmstadt, Germany.
All the groundwater samples were analysed for major cations and anions using Metrohm 882 compact Ion Chromatography Plus. Trace metals such as iron, manganese, chromium, nickel, and copper were also analysed using Atomic Absorption Spectrometry ContrAA® 300. Distilled water and certified calibration standards were used to prepare the samples to achieve quality control. The results were reproducible within the ± 3% error limit.
Data analysis
SPSS software package version 26.0 was employed for the analytical methods to analyse the Pearson correlation, principal component analysis (PCA), and the hierarchical cluster analysis (HCA). Pearson's correlation coefficient (r) analysis was carried out to determine the correlation and similarity between the hydrochemical parameters. The correlation coefficient between pairs of the parameters generated and their significance was tested by the p-value. The correlation coefficient lies between -1 and 1, in which significantly correlated 30 parameter pairs have r > 0.5, and non-significantly correlated pairs have r values less than 0.5 (Liu et al. 2003).
The principal component analysis was performed to ascertain the key values in the dataset and to minimize the large datasets into a small dimensionality. This was used to explain the origin of the hydrochemical parameters and deduce geochemical meanings (Yidana et al. 2010). The dataset was first standardized, and a correlation matrix was produced to determine the interrelationships between variables (R-mode analysis). To ensure that the number of components is minimized to the model, the Kaiser criterion was adopted, which was constrained to only components greater than 1 (Kaiser 1960). Varimax rotation technique was chosen to increase the variance of the retained components greater than 1. In the current study, some of the datasets, such as PO43−, NO2−, NH4,+ and trace metals (Fe, Ni, Cr, and Mn), did have missing data, for which reasons they were excluded from the statistical analysis.
The HCA technique was further used to validate the results of the PCA by assembling the water samples into homogeneous clusters or groups on the assumptions of samples that are alike and at the same time unlike samples (Güler et al. 2002). To define these unique clusters, hydrochemical data were log-transformed and consequently standardized using Eq. 1. This was done to attain normal distribution, homogenize the variance and ensure that there is no bias in the sampling population. This is because, during the use of Euclidean distance in hierarchical cluster analysis, the chemical variables with the greatest variance usually influence the smallest variables (Güler et al. 2002)
where Z is the standardized measurement and X is an individual sample measurement.
The Euclidean distance and Ward's linkage method were applied to generate two hierarchical cluster analysis dendrograms: R-mode and Q-mode HCAs through assembling similarity and dissimilarity clusters using 38 groundwater samples and 14 variables. These methods were chosen because of their rigidity evidence, which is amply presented in numerous successful studies, where distinct homogenous clusters are joined such that an increase in the within-group variance is minimized (Cloutier et al. 2008).
Water quality index (WQI)
In this study, 15 chemical parameters were considered for the WQI. This computation is based on the premise of giving weights to each of the chemical parameters (wi) according to the relative importance of the WHO (2017) drinking water guidelines. For example, elevated fluoride and nitrate concentrations in drinking water are of health concern, so they were assigned the highest weight of 5. According to their importance, the other chemical parameters such as Ca2+, Mg2+, TDS, and Na+ were given different weight values between 1 and 5. The computed WQI is expressed in Eq. (2)
where Wi is the relative weight, wi is the individual parameter of a sample at a given location and n is the total number of parameters.
In the next step, the concentration of the individual parameter is divided by the WHO standard limit of that parameter and multiplied by 100 to obtain the quality rating (qi) in Eq. (3)
where qi is the quality rating, Ci is the concentration of the individual parameter and Si is the WHO standard limit value of the respective parameter.
The water quality index (WQI) of each location is finally determined from the subindex Si in Eq. (4) and (5)
where SIi is is the subindex
The spatial water quality distribution map was interpolated using the inverse distance weighted (IDW) in ArcGIS software 10.7.1. IDW interpolation was used because it is effective and robust, which can deal with densely even space points (Loh et al. 2020).
Chloro-alkaline indices
The chloro-alkaline indices (CAI) developed by Schöeller H (1965.) were used to determine the ion exchange process between the groundwater and the geological medium during its residence time. The chloro-alkaline indices (meq/L) were computed using the expression in Eq. (6) and (7).
Geochemical modelling
The mineral species in the groundwater were investigated by calculating the saturation index expressed in Eq. 8 (Parkhust and Appelo 2013).
where Ks (T) is the solubility constant for temperature-dependent of the mineral, IAP is the ion activity product.
Si = 0 mineral is in equilibrium with the solution.
Si < 0 mineral is undersaturated (may reflect mineral dissolution, if present in the water).
Si > 0 mineral is supersaturated (may reflect mineral precipitation and mineral formation, if present in the water).
The Wilcox diagram and Piper diagram were graphically interpreted using Aquachem software version 4.0.
Results and discussion
The descriptive statistics of the physicochemical parameters and the trace metals are presented in Table 1. Some of the on-site field parameters show a wide range of variance (Fig. 4). The groundwater temperature lies within 31.1–32.9 °C with a mean value of 31.9 °C. The groundwater temperature was higher than the recent average annual temperature (28.7 °C) of the area (Asamoah and Ansah-Mensah 2020). This suggests that the heating of the groundwater is influenced by the local geothermal gradient (Reiter 2001). Most of the areas have dissolved-oxygen concentrations less than 1 mg/L indicating the water is non-oxidized. The mean value of the dissolved oxygen is 0.57 mg/L and varies from 0.4 mg/L to 1 mg/L. Electrical conductivity measures all ionic solutes in water and is closely related to total dissolved solids (TDS). The conductivity of water can vary temporally and spatially because of processes such as temperature, ion exchange, dissolution, and adsorption. For instance, an increase in temperature generally shows an increase in electrical conductivity (Clark 2015). In the study area, specific conductance values range from 85.5 μS/cm to 593 μS/cm with a mean value of 249.86 μS/cm implying a general dilute to slightly mineralized water. The conductance displays high variance and standard deviation attributed to the mixing of diluted and mineralized water (Plummer et al. 2012). The total dissolved solids (TDS) values are less than 1000 mg/L indicating the water is very young and fresh (Freeze and Cherry 1979). According to Hounslow (1995), groundwater usually has a moderately acidic pH ranging between 4.0 and 6.5; neutral pH between 6.5 and 7.8; moderately alkaline pH between 7.8 and 9 and alkaline pH above 9. The groundwater in the study area is moderately acidic to moderately alkaline, with pH values ranging from 6.25 to 7.93, with a mean of 7.09. This indicates that about 10.5% of the samples have pH values below the WHO permissible limit of 6.5–8.5. The low pH value (acidic water) in the area is ascribed to carbon dioxide and man-made factors. Carbon dioxide derived from rain reacts with the water to form a weak carbonic acid (H2CO3) and hydrogen ion (H+). The hydrogen ion released into the water can decrease the pH of the water. Another source of acidity in groundwater is the reaction of oxygen with iron sulphide minerals (FeS2) (Candela and Morell 2009). The total alkalinity, expressed as the equivalent concentration of calcium carbonate (CaCO3) or bicarbonate (HCO3−), displays a high variance. A high value of 446 mg/L was recorded around the Yaratinga area in the northern part of the district. Low to medium bicarbonate values are dominant and scattered all over the area. The primary source of bicarbonate in groundwater is attributed to carbon dioxide in the atmosphere and in soil gases that dissolve in rain and surface water (Clark 2015).
Major ions' chemistry and correlations
Pearson's correlation coefficient (r) between the physicochemical parameters and other trace metals is presented in (Table 2). Pearson’s correlation coefficient reveals that the total dissolved solids (TDS) have a significant positive correlation with HCO3−(r = 0.89), K+(r = 0.74), Ca2+(r = 0.68), Na+(r = 0.70), Br−(r = 0.66), NO3−(r = 0.58), F−(r = 0.58), SO4−2 (r = 0.53), Mg2+ (0.52) and Sr2+ (0.51). This suggests that the major ions originate from the same source as the total dissolved solids, and they control the major groundwater chemistry in the area. A significant correlation between TDS and EC (r = 0.74) indicates that these parameters are closely related and that the water contains a relatively small amount of inorganic salts arising from domestic and agricultural waste (Rusydi 2018). The order of dominance of the cations is Ca2+ > Na+ > Mg2+ > K+ with their average concentrations of 20.32 mg/L, 17.7 mg/L, 9.8 mg/L and 1.7 mg/L, respectively.
Calcium appears to be the abundant cation in the area. Calcium has significant correlation with Mg2+ (r = 0.91), Na+(r = 0.85), F−(0.80), SO42 (0.76), NO3−, (0.76), K+ (0.69) and Cl− (0.55). The significant correlation between Ca 2+ and Mg2+ generally gives a clue about the hardness of the water. Water is classified as hard if the calcium carbonate concentration ranges between 120 mg/L and 180 mg/L (Ahn et al. 2018). Excessive water hardness can be mitigated by softening it with ion exchangers such as sodium zeolite, which displaces the Ca2+ and Mg2+. The calcium signatures into the groundwater generally originate from the dissolution of calcite, gypsum, and silicate mineral anorthite (Plummer et al. 2012). Intense agricultural activities may also introduce calcium into groundwater (Böhlke 2002). In this study, the groundwater samples were mainly taken from the host plutonic igneous rocks. Thus, the source of calcium in the groundwater could be attributed to silicate mineral dissolution.
Although sodium is not a conservative ion, it occurs abundantly in groundwater due to the weathering of feldspar and plagioclase minerals and, to some extent, cation exchange (Clark 2015). Other sources of Na+ in groundwaters could be attributed to the leaching of municipal solid waste and from the sea, where concentrations can be as high as 1000 mg/L (Hem 1985). The correlation between Na+ and Cl− exhibits a non-significant correlation (r = 0.46), which possibly indicates the dissolution of silicate mineral weathering other than halite as its origin. However, a significant correlation exists between Na+ and F−(r = 0.89), Na+ and Br− (r = 0.84), Na+ and K+ (r = 0.75) and Na+ and NO3− (r = 0.71). Na+ and S042− display a significant correlation (r = 0.68), which indicates a possible dissolution of sodium sulphate decahydrate Na2SO4.10H2O (Glauber's salt), that is of health significance to the body (Zhu et al. 2007). However, there are no known deposits of Glauber’s salts in the area.
Magnesium concentrations in groundwater could be attributed to the leaching of igneous and magmatic minerals such as olivine, pyroxene, and amphibole in groundwater (Clark 2015). Sedimentary origins such as dolomite and magnesite minerals may also contribute to magnesium in groundwater. There is a significant correlation between Mg2+ and Ca2+ (r = 0.91), indicating the weathering and dissolution of magnesium -bearing minerals such as biotite, chlorite, and hornblende, which may be found in the host plutonic rocks in the catchment (Zango et al. 2020). However, there is a non-significant correlation between Mg and EC (r2 = 0.36), suggesting minimum magnesium ions in solution as a single salt (Tutmez et al. 2006).
Potassium appears to be the lowest dominant cation in the district. Sources of potassium in groundwater are potassium feldspars, micas, and synthetic fertilizers from agricultural activities (Clark 2015). Generally, potassium concentrations in groundwater are very low due to the high resistance of potassium feldspar and biotite minerals to weathering (Appelo and Postma 2005). Potassium correlates significantly with Ca2+ (r = 0.69) Na+ (r = 0.75) and F− (r = 0.64), which indicates that these minerals may originate from common source in the host plutonic rock. The significant correlation between K+ and F− is consistent with the work of Anim-Gyampo et al. (2018), who found out that these minerals are from the potassium-rich Bongo granites in the Atankwidi basin—80 km from the study area.
The decreasing trends of the anions are HCO3− > NO3− > Cl− > S042− > F− with resulting mean values of 188.61 mg/L, 21.8 mg/L, 6.23 mg/L and 0.59 mg/L, respectively. Bicarbonate appears to be the dominant anion, and generally, the source may originate from silicate weathering that releases HCO3− in groundwater (Appelo and Postma 2005). Bicarbonate correlates significantly with TDS (r = 0.89). It is non-significant with Ca2+ (r = 0.30) and Na+ (r = 0.37), suggesting that the major source of bicarbonate is carbon dioxide in the atmosphere and soil gases that dissolve in rain and surface water. Nitrate correlates significantly with SO42− (r = 0.93) and Cl− (r = 0.82), indicating the possible leaching of domestic solid waste, fertilizer, and manure into the groundwater (Appelo and Postma 2005). Nitrate is present in groundwater in the form of organic nitrogen compounds synthesized in the presence of oxygen (Rees 1995). High nitrate concentrations are primarily from human and animal wastes, nitrogen-based fertilizers and precipitation-induced nitrogen (Anornu et al. 2017). Fluoride concentrations in groundwater may come from the dissolution of fluorite and apatite minerals or in igneous minerals such as amphibole and mica (Hounslow 1995). In Northern Ghana, elevated fluoride concentrations in groundwater have been of health concern to the people, and the source of fluoride has been discovered in the Bongo granites (Alfredo et al. 2014; Apambire et al. 1997). Fluoride has a significant correlation with the major cations, indicating that the weathering of apatite and fluorite minerals are common in the underlying igneous rocks, as has been espoused by (Alfredo et al., 2014). All the fluoride concentrations are within the WHO acceptable limit (0.5–1.5 mg/L) except that of the borehole situated at Holy English junior high school, which has fluoride a concentration of 1.61 mg/L.
Factor analysis
Principal component analysis (PCA) was carried out on the 38 groundwater samples, and the dimensionality of the 14 parameters was reduced to three principal components, which accounted for 81% of the total variance (Tables 3 and 4). PCA 1 explains 34% of the total variance and has high positive loading for, Ca2+, Mg2+, Na+, SO42−, F,− and Br−. The strong positive loadings, especially Ca2+ and Mg2+ in PCA1, suggest higher groundwater–rock interaction, which leaches appreciable quantities of silicate minerals such as anorthite, biotite, and hornblende in the groundwaters (Sunkari and Abu 2019). These rock-bearing minerals reflect the hydrochemical water composition of the plutonic rocks in the study area. The presence of Na+ can be explained as the weathering of silicate mineral albite (NaAlSi3O8) and also cation exchange process, in which Na+ tends to displace Ca2+ in water (Freeze and Cherry 1979). PCA2 also accounts for 24% of the total variance and is dominated by high positive loadings for NO3−, SO42−, Cl− and Sr2+ but low negative loading for pH. The result of the PCA2 corroborates with the findings of Cluster 1 in the R-mode HCA. The predominant chemical parameters (NO3−, SO42− and Cl−) in PCA2 suggest the application of inorganic and organic fertilizers such as animal waste, which may cause elevated amounts of nitrate in the groundwaters as is evident in places such as Baranatinga, Garu Zongo, and Duadinyediga in the study area. The low pH loading is attributed to the buffering activity of HCO3−. In a natural geochemical process, carbon dioxide derived from the atmosphere reacts with neutral water in the subsurface to form weak carbonic acid (H2CO3−). The carbonic acid dissociates into HCO3−, H+ and CO32−. The hydroxyl ion (H+) released in the water can decrease the pH, thus the low negative loading for pH. Besides, the movement of groundwater through the aquifer matrix can cause more reactions to decrease the carbonic acid and increase the HCO3− and CO32− concentrations. Thus, the high positive loadings of HCO3− for PCA3 (Clark 2015). The third component (PCA3) loads strongly for HCO3−, EC, K+ and TDS and accounts for 23% of the total variance. The presence of K+ in the groundwater may reflect the weathering of potassium feldspar associated with the igneous rocks of the Tamnean Plutonic Suites (SNC-Lavalin/INRS 2011).
Hierarchical cluster analysis (HCA)
The Hierarchical cluster analysis (HCA) was further used to validate the factor analysis results by assembling the distinct clusters into distinct groups. Thirty-eight groundwater samples and 14 variables were used for the hierarchical cluster analysis. The HCA produced Q-mode and R-mode dendrograms. The 14 parameters were used for the R-mode technique to define their relationships and generate homogenous clusters based on a subjective assessment. A phenon line was connected at a linkage distance of about 15 to avoid the generation of fewer or greater clusters. The visual observation of the R-mode HCA displays three main clusters (Fig. 5).
Cluster 1 is characterized by SO42−, NO3−, Cl−, and Sr2+, which indicates the groundwater appears to be influenced by anthropogenic activities at the near-surface groundwater, such as the use of domestic wastewaters and the application of chemical and organic fertilizers in the study area (Loh et al. 2020). These pollutants may reach a considerable amount, thereby increasing nitrate and sulphate concentrations in groundwater. The results of cluster 1 confirm the results of PCA2. The second cluster (Ca2+ Mg2+, Na+, K+, F−, and Br−) suggests groundwater–rock interactions largely influenced by silicate weathering. The weathering of rocks tends to increase these ions from upstream to downstream in the study area, which is characteristic of a semi-arid environment (Wu et al. 2017). Strontium and calcium are very close in terms of distance because strontium has identical physical and chemical properties as calcium and tends to replace Ca2+ in mineral structures. Strontium concentrations in groundwaters are mostly found in carbonate rocks when its bearing minerals such as celestite (SrSO4) and strontianite (SrCO3) precipitate out from calcite solution (Skougstad and Horr 1963). Granitic rocks showing high contents of rubidium are also characterized by strontium concentrations in groundwater (Kharaka and Hanor 2003). It can be added that anthropogenic activities such as burning coal and phosphate fertilizers can release strontium into the groundwater (Höllriegl and München 2011). The third cluster represents close associations between TDS, HCO3−, EC, K, and pH. This cluster, especially the bicarbonate, can indicate the dissolution of silicate minerals, which is accompanied by the release of bicarbonates in the groundwater, as espoused by previous researchers in Northern Ghana (Chegbeleh et al. 2020; Loh et al. 2020).
On the other hand, the Q-mode dendrogram represents the relationship between groundwater parameters in the different parts of the study area. Three spatial groundwater clusters were revealed from the Q-mode dendrogram (Fig. 6). Cluster 1 has the lowest mineralized water with mean TDS and EC values of 207 mg/L and 186 μS/cm, respectively. Cluster 1 samples are found in recharge areas because infiltration precipitation usually contains low ionic solutes and is in equilibrium with the groundwater in recharge areas (Salifu et al. 2012). Cluster 2 is a transition zone with an average TDS of 251 mg/L and EC of 271 μS/cm, respectively. Cluster 3 has relatively high concentrations of average TDS and EC values of 362 mg/L and 409 μS/cm, respectively, than the other clusters. The highly mineralised water of this cluster can be indicative of a discharge zone that possibly had longer residence time and greater rock interaction, thus leaching more ions in the groundwater (Appelo and Postma 2005). From Fig. 7, cluster 1 (recharge zone) occurs in the highest elevations, mainly around the northern and central parts of the study area. Cluster 2 (transition zone) is found in the northern and southern parts of the study area, whereas cluster 3 (discharge zone) is located around the central parts of the area.
Water types or hydrogeochemical facies
The Q-mode hierarchical cluster analysis (HCA) clusters were used to construct the Piper diagram (Fig. 8). The trilinear Piper diagram consists of the relative proportion of major cations and anions. The Piper plot reveals that all the three clusters are found in the no dominant cations water zone, except for two samples found in the Na+ + K+ zone. The anions are characterized mainly by HCO3− of all the three clusters. From the trilinear plot, it can be deduced that three water types exist in the area. The first type is Ca–Na–HCO3 water, indicating the freshwater type of the underlying plutonic rocks undergoing an initial stage of hydrogeochemical evolution (Tran et al. 2020). This water type is consistent with the findings of Anim-Gyampo et al. (2018), Sunkari and Abu (2019), Zakaria et al. (2020) done in the crystalline igneous terrains of Ghana, thus implying that these water types are predominately found in the igneous rocks terrains of Northern Ghana. The second type consists of Ca–Mg –HCO3 or Mg–Ca–Na –HCO3 mixed water types. This also highlights the greater role of the alkaline earth metals (Ca2+ + Mg2+) over the alkali metals (Na+ + K−). The third type consists of Ca–Mg–Na–HCO3–NO3 or Ca–Mg–HCO3–Cl–NO3 water types, suggesting higher rock interaction and the effect of anthropogenic activities on the groundwater. In the Garu-Tempane areas, the main occupation of the inhabitants is farming and cattle rearing. The use of different fertilizers such as phosphate and nitrogen on their farms may dissolve and enter the aquifers, causing anthropogenic pollution in the area.
Weathering process and the mechanism controlling the evolution of groundwater chemistry
To determine the influence of major geochemical processes such as water–rock interaction, precipitation, and evaporation on groundwater chemistry, the Gibbs (1970) plot was used. The Gibbs plot (Fig. 9) shows that all the groundwater samples are within the rock dominant boomerang-shaped area, consistent with most ionic compositions found in the world's major aquifer bodies (Marandi and Shand 2018). Groundwater quality evolution along its flow path typically contains freshwater enriched in Ca–HCO3− and evolves to Na–Cl type with increasing salinity during prolonged residence time. This evolution would move the freshwater to the evaporation domination zone due to the anthropogenic activities introducing Na–Cl− waters. Evaporation of minerals from groundwater usually occurs when the groundwater table is near the earth's surface, which is negligible in deep aquifers. The hydrochemistry data in the study area do not reflect this tendency, rather the ion compositions are characterized by water–rock interaction.
The genesis of groundwater chemistry requires the knowledge of aquifer forming minerals and weathering processes (Hwang et al. 2017). To understand the weathering process, the major ions are plotted against each other to deduce the important process controlling the evolution of groundwater chemistry in the area. The bivariate plot of Ca2+ + Mg2+ versus HCO3− + SO42− (Fig. 10a) should indicate ion exchange if the samples fall on the right section of the 1:1 line. On the other hand, if the samples fall on the left portion of the 1:1 line, the results should indicate a reverse ion exchange (Fisher and Mullican 1997). In addition, the samples above the 1:1 line suggest carbonate weathering, whereas the samples below the 1:1 line indicate silicate weathering. The samples (8%) along the 1:1 line depict carbonate and silicate weathering (Koffi et al. 2017). From Fig. 10a, most of the samples (72%) are below the 1:1 line, implying that silicate weathering and ion exchange are the controlling factors that affect the groundwater chemistry in the area. Here, HCO3− + SO42− is enriched relative to Ca2+ + Mg2+ in the groundwater (Rajmohan and Elango 2004). Few samples (20%) are above the 1:1 line, suggesting carbonate weathering from the Voltaian formations in the study area. The carbonate rocks in the study are hydraulically linked to the crystalline basement rocks and the associated granitoids (Dapaah-Siakwan and Gyau-Boakye 2000). The evolution of carbonate weathering begins when precipitation -induced CO2− gets in contact with soils containing carbonate minerals and releases HCO3− in the groundwater, as shown in the following reaction equations below (Zaidi et al. 2015).
Silicate minerals such as anorthite and albite are formed from magma cooling and crystallization at varying temperatures (Clark 2015). Silicate weathering is often deduced using the 1:1 molar ratios of Na/Cl to define the salinity levels in groundwater. It is generally accepted that if the data fall on the 1:1 plot, then it implies halite dissolution prevails in the groundwater (Meybeck 1987). The plot of Fig. 10b of Na (meq/L) against Cl (meq/L) is below the 1:1 line, and this suggests the dissolution of Na+ in groundwater, in which Na+ displaces Ca2+ during the cation ion exchange process. Therefore, groundwaters having high Na+ concentrations due to silicate weathering can be indicative of water enriched in HCO3− and this explains the predominance of HCO3− in the groundwater in the area, as shown in Eq. 12 (Yidana et al. 2018).
From Fig. 10b, three groundwater samples are above the 1:1 line, which indicates that reverse ion exchange involving sodium and chloride has taken place. These samples are enriched in Cl−, albeit the chloride concentrations are within the WHO acceptable limit (250 mg/L). The mechanism explaining reverse ion exchange is shown in Eq. 13.
To further substantiate the cation ion exchange reactions during silicate weathering, the plot of (Ca2+ + Mg2+) – (HCO3− + SO42−) against Na+ + K+–Cl− (Fig. 10c) was used. According to Zaidi et al. (2015), the relationship between these indices should give a slope of – 1 to confirm the active role of Ca2+, Mg,2+ and Na+ in the cation exchange process. Accordingly, the relationships between these indices in the study area give a slope of – 1.2, indicating that the majority of the water samples have undergone a cation exchange reaction (Anim-Gyampo et al. 2018). The mechanism explaining cation exchange is shown in Eq. 14.
Silicate and carbonate mineral weathering can be invoked using the bivariate plot of (Ca2+ + Mg2+) versus HCO3− (Fig. 10d). This is useful to determine the influence of different weathering processes in an aquifer. According to Sami (1992), the trend of groundwater samples falling along the y = 2 × of (Ca2 + Mg2+) versus HCO3− indicates that carbonate mineral dissolution is predominant in an aquifer. If the samples do not fall on that line, it could be the alteration of silicates. In this study, the bivariate plot of (Ca2+ + Mg2+) versus HCO3− in Fig. 10d shows only three samples falling on the y = 2 × line, indicating that carbonate weathering is not the dominant process occurring in the study area. The majority of the groundwater samples fall below the y = 2 × line, showing that silicate weathering is the predominant process controlling groundwater chemistry in the Garu-Tempane area.
Chloro-alkaline indices (CAI)
The chloro-alkaline indices CAI 1 and CAI 2 were computed for 38 water samples for this study to explain the type of ion exchange in the groundwater (Schöeller 1965). An assertion is made for positive CAI if Na+ and K+ ions of the water substitute the Ca2+ and Mg2+ of the aquifer material. Here the CAI will indicate reverse ion exchange. On the contrary, a negative CAI implies an ion exchange in which the Ca2+ and Mg2+ of the water substitute the Na+ and K+ of the aquifer material. According to Srinivasamoorthy et al. (2014), the negative CAI is also widely accepted as a cation–anion exchange reaction or chloro-alkaline disequilibrium due to low salt waters. If there is no exchange between the ions and the aquifer material, the relationship attains equilibrium, and CAI becomes zero. The computed CAI-1 has 93% negative values and 7% positive values ranging between − 43.26 and 0.597. The CAI-2 has 97% negative values and 3% positive values ranging between − 0.245 and 0.144. The results above suggest that the majority of the groundwater samples attain a negative chloro-alkaline index. Therefore, the cation exchange process is one of the factors influencing groundwater chemistry in the area. This is consistent with the plot of (Fig. 10b), in which three samples attain reverse ion exchange and the majority are cation exchange.
Water quality index (WQI)
Horton (1965) first used the water quality index in the USA to evaluate the suitability of drinking water. Since then, various researchers have used WQI to characterize groundwater for drinking water purposes (Tyagi et al. 2013; Kawo and Karuppannan 2018; Ponsadailakshmi et al. 2018). The water quality index agglomerates large and different chemical parameters into a single variable for geochemical inferences (Tyagi et al. 2013).
Thirty-eight groundwater samples and 15 chemical parameters were used for the water quality index (WQI) assessment and their relative weights, as shown in (Table 5). An adapted version of the WQI classification scheme postulated by Sahu and Sikdar (2008) was used for this study. The WQI classification for ‘excellent water’ ranges from (0–50); ‘good water’ from (51–100); ‘poor water’ from (101–200); ‘very poor water’ (201–300), and ‘unsuitable drinking water’ (> 300). The computed WQI ranges between 13.3 and 97.4. The WQI spatial distribution map (Fig. 11) shows two types of water quality in the area. Almost all groundwater samples (95%) can be described as ‘excellent water’, whereas 5% of the samples show ‘good water’ in the eastern part of the area. The influencing parameters of the ‘good water’ are associated with higher NO3−, F,− Fe, and TDS values. These values generally indicate that the groundwater in the 'Tamnean Plutonic Suite' aquifer is excellent for drinking.
Saturation index of mineral
The mineral saturation index of groundwater is very useful to determine the type of mineral present in groundwater and the precipitation and dissolution reactions (Appelo and Postma 2005). Three types of saturation indices/states can be distinguished. A saturation index of zero implies that the groundwater is in equilibrium with the precise mineral in the water. A saturation index less than zero means that the groundwater is undersaturated with respect to the mineral. Here, the mineral will continue to dissolve if that particular mineral is present in the water. A saturation index greater than zero implies the groundwater is deemed supersaturated, and the mineral may precipitate from the solution. In this particular case, the mineral should be forming, and the aquifer may contain a sufficient amount of minerals needed to attain equilibrium (Appelo and Postma 2005; Parkhust and Appelo 2013). The plausible minerals found in the 'Tamnean Plutonic Suite aquifer' are presented in Table 6.
From Fig. 12, groundwater in the 'Tamnean Plutonic Suite aquifer' clearly shows undersaturation with respect to gypsum, fluorite, halite, and anhydrite. These minerals indicate a shorter residence time in the groundwater, where these minerals will dissolve if present in the solution. Similarly, all the 38 groundwater samples are undersaturated with respect to calcite except for two samples, which show supersaturation. The calcite supersaturation indicates that the minerals are forming and may contain enough minerals to equilibrate with the groundwater. Again, nearly 66% of the groundwaters are supersaturated with respect to dolomite. This, together with the calcite undersaturation, reflects the conditions necessary for carbonate dissolution.
Nitrate levels in groundwater
Several studies have linked elevated nitrate concentrations in groundwaters to anthropogenic activities (Suthar et al. 2009; Wick et al. 2012; Sajedi-Hosseini et al. 2018; Suciu et al. 2020). In the study area, nitrate concentration ranges between 0.08 and 147.6 mg/L, with a mean value of 21.8 mg/L. The acceptable limit of nitrate in drinking water is 50 mg/L (WHO 2017). However, communities such as Garu Market (68.36 mg/L), Garu Zongo (77.8 mg/L), Baranatinga (85.7 mg/L), and Duadinyediga (147. 6 mg/L) have nitrate levels in groundwaters exceeding the WHO acceptable drinking water limit. The first two communities are located in the centre of the district, where there are no agricultural activities. Thus, the source of nitrate in the groundwater could be attributed to animal waste (cattle dung) and untreated household wastes (Anornu et al. 2017). The latter communities are agricultural areas, and the source of nitrate in groundwater is due to chemical and organic fertilizers. There is a significant correlation between NO3− and SO42− (r = 0.93) and NO3− and Cl− (r = 0.82), implying that these pollutants are commonly found in agricultural areas (Loh et al. 2020). In general, about 10.5% of the groundwater samples are contaminated with nitrate, and the spatial distribution is shown in (Fig. 13). The continuous use of water in these communities may be detrimental to people's health.
Conclusions
Groundwater characterization was carried out to investigate the natural baseline chemistry and the factors controlling groundwater chemistry in the Garu-Tempane hydrogeological catchment. The methods employed are the multivariate statistical method, hydrochemical graphical method, PHREEQC geochemical modelling approach, and the various ionic ratio plots. The graphical methods reveal an order of ion compositions Ca2+ > Na+ > Mg2+ > K+ and HCO3− > NO3− > Cl− > SO43− in the water. All the physicochemical parameters and trace metals are within the WHO acceptable limit for drinking water except for elevated bromide and nitrate concentrations in some of the wells. About 10.5% of the groundwater samples have pH levels below the permissible limit. In addition, one borehole has a fluoride concentration (I.61 mg/L) exceeding the WHO drinking water limit (1.5 mg/L). About 10.5% of the groundwaters are contaminated with nitrate due to anthropogenic activities in the area. The cation composition of the groundwater is predominantly of mixed types of calcium, magnesium, and sodium, where the first two arise from the weathering and dissolution of silicate minerals such as anorthite, hornblende, and biotite, and the latter (Na+) suggests the dissolution of silicate mineral albite that are all found in crystalline basement rocks of the area. The predominant anion (HCO3−) is characterized by the dissolution of silicate minerals by recharging waters through a carbonic acid reaction. Various ionic ratios suggest that the groundwater chemistry is mainly influenced by silicate mineral weathering and cation exchange process and, to a lesser extent, by the leaching of domestic solid waste and nitrogen-based fertilizers. The saturation index indicates that groundwater is undersaturated with respect to halite, calcite, anhydrite, fluorite, and gypsum, but supersaturated with respect to dolomite. The Q–mode HCA reveals three spatial groundwater clusters. Cluster 1 consists of Ca–Na–HCO3− water type from recharge areas. Groundwater recharge inflows occur from the northern to southern parts of the area. Cluster 2 is characterized by moderate ionic compositions of Ca–Mg–Na–HCO3 intermediate water types. The third cluster is mainly of Ca– Mg–HCO3 –Cl–NO3 water types and can be described as a discharge zone. Based on the water quality index (WQI), about 95% of the groundwater is excellent for drinking.
References
Acheampong A (2017) Borehole yield estimation from electrical resistivity measurement – A case study of Garu Tempane and Bawku West Districts, Upper East Region, Ghana By (BSc. Geological Engineering) A thesis submitted to the Department of Geological Engineering, Kwa. May, 71
Acheampong SY, Hess JW (1998) Hydrogeologic and hydrochemical framework of the shallow groundwater system in the southern Voltaian Sedimentary Basin, Ghana. Hydrogeology J. https://doi.org/10.1007/s100400050173
Ahn MK, Chilakala R, Han C, Thenepalli T (2018) Removal of hardness from water samples by a carbonation process with a closed pressure reactor. Water (switzerland). https://doi.org/10.3390/w10010054
Alfredo KA, Lawler DF, Katz LE (2014) Fluoride contamination in the Bongo District of Ghana, West Africa: Geogenic contamination and cultural complexities. Water Int. https://doi.org/10.1080/02508060.2014.926234
Anim-Gyampo M, Anornu GK, Appiah-Adjei EK, Agodzo SK (2018) Hydrogeochemical evolution and quality assessment of groundwater within the Atankwidi basin: the case of northeastern Ghana. Arab J Geosci. https://doi.org/10.1007/s12517-018-3753-6
Anornu G, Gibrilla A, Adomako D (2017) Tracking nitrate sources in groundwater and associated health risk for rural communities in the White Volta River basin of Ghana using isotopic approach (δ15N, δ18O[sbnd]NO3 and 3H). Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.01.219
Apambire WB, Boyle DR, Michel FA (1997) Geochemistry, genesis, and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environ Geol. https://doi.org/10.1007/s002540050221
Appelo CAJ, Postma D (2005) Groundwater, geochemistry and pollution, 2nd edn. AA Balkema Publishers, New York, USA
Asamoah Y, Ansah-Mensah K (2020) Temporal description of annual temperature and rainfall in the Bawku area of Ghana. Adv Meteorol. https://doi.org/10.1155/2020/3402178
Ashraf B, Aghakouchak A, Alizadeh A, Mousavi Baygi M, Moftakhari HR, Mirchi A, Anjileli H, Madani K (2017) Quantifying anthropogenic stress on groundwater resources. Sci Rep. https://doi.org/10.1038/s41598-017-12877-4
Banoeng-Yakubu B, Yidana SM, Ajayi JO, Loh Y, Aseidu D (2011) Hydrogeology and groundwater resources of Ghana: a review of the hydrogeology and hydrochemistry of Ghana. In: McMann JM (ed) Potable water and sanitation, vol 142. Nova Science New York, NY
Barcelona MJ, Gibb JP, Helfrich John A, Garske EE (1985) Practical guide for ground-water sampling (vol 600, No 2–104). Robert S. Kerr Environmental Research Laboratory, Office of Research and Development, US Environmental Protection Agency
Böhlke JK (2002) Groundwater recharge and agricultural contamination. Hydrogeol J. https://doi.org/10.1007/s10040-001-0183-3
Candela L, and Morell I (2009) Basic chemical principles of groundwater. Encyclop Life Support Syst 2:43–55
Carrier MA, Lefebvre R, Racicot J, Asare EB (2008) Northern Ghanahydrogeological assessment project. In: Access to Sanitation and Safe Water: GlobalPartnerships and Local Actions - Proceedings of the 33rd WEDC International Conference.
Chegbeleh LP, Akurugu BA, Yidana SM (2020) Assessment of groundwater quality in the Talensi District, Northern Ghana. Sci World J. https://doi.org/10.1155/2020/8450860
Clark I (2015) Groundwater geochemistry and isotopes. CRC press. https://doi.org/10.1201/b18347
Cloutier V, Lefebvre R, Therrien R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353(3–4):294–313. https://doi.org/10.1016/j.jhydrol.2008.02.015
Dapaah-Siakwan S, Gyau-Boakye P (2000) Hydrogeologic framework and borehole yields in Ghana. Hydrogeol J. https://doi.org/10.1007/PL00010976
Dennehy KF, Reilly TE, Cunningham WL (2015) Groundwater availability in the United States: the value of quantitative regional assessments. Hydrogeol J. https://doi.org/10.1007/s10040-015-1307-5
Dhar Chakrabarti PG (2017) People, planet and progress beyond (2015). The Energy and Resources Institute (TERI), New Delhi, p 462
Dişli E (2018) Evaluation of hydrogeochemical processes for waters’ chemical composition and stable isotope investigation of groundwater/surface water in Karst-Dominated Terrain, The Upper Tigris River Basin, Turkey. Aquat Geochem 24:363–396. https://doi.org/10.1007/s10498-019-09349-8
Dişli E, Gülyüz N (2020) Hydrogeochemical investigation of an epithermal mineralization bearing basin using multivariate statistical techniques and isotopic evidence of groundwater: Kestanelik Sub-Basin, Lapseki, Turkey. Geochemistry 80(4):125661. https://doi.org/10.1016/j.chemer.2020.125661
Egbi CD, Anornu GK, Ganyaglo SY, Appiah-Adjei EK, Li SL, Dampare SB (2020) Nitrate contamination of groundwater in the Lower Volta River Basin of Ghana: sources and related human health risks. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2020.110227
Feybesse JL, Billa M, Guerrot C, Duguey E, Lescuyer JL, Milesi JP, Bouchot V (2006) The paleoproterozoic Ghanaian province: geodynamic model and ore controls, including regional stress modelling. Precambrian Res. https://doi.org/10.1016/j.precamres.2006.06.003
Fisher RS, Mullican WF (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the Northern Chihuahuan Desert, Trans-Pecos, Texas, USA. Hydrogeol J. https://doi.org/10.1007/s100400050102
Freeze RA, Cherry J (1979) Groundwater. Prentive-hall, Englewood Cliffs, NJ, 604p.Groundwater.
Ghana statistical service (2010) Garu tempane district. Garu Tempane District.
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science. https://doi.org/10.1126/science.170.3962.1088
Güler C, Thyne GD, McCray JE, Turner AK (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J. https://doi.org/10.1007/s10040-002-0196-6
Gumma MK, Pavelic P (2013) Mapping of groundwater potential zones across Ghana using remote sensing, geographic information systems, and spatial modelling. Environ Monit Assess. https://doi.org/10.1007/s10661-012-2810-y
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. US Geological Survey Water-Supply Paper.
Höllriegl V, München HZ (2011) Strontium in the environment and possible human health effects. Encyclopedia of environmental health. Elsevier, Burlington, pp 268–275. https://doi.org/10.1016/B978-0-444-52272-6.00638-3
Hölting B, Coldewey WG (2019) Hydrogeology, Springer textbooks in Earth Sciences, Geography and Environment Berlin. https://doi.org/10.1007/978-3-662-56375-5
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–305
Hounslow AW (1995) Water quality data analysis and interpretation. Lewis Publishers, Boca Raton
Hwang JY, Park S, Kim HK, Kim MS, Jo HJ, Kim JI, Lee GM, Shin IK, Kim TS (2017) Hydrochemistry for the assessment of groundwater quality in Korea. J Agric Chem Environ. https://doi.org/10.4236/jacen.2017.61001
Kaiser HF (1960) The application of electronic computers to factor analysis. Educ Psychol Measur 20:141–151. https://doi.org/10.1177/001316446002000116
Kawo NS, Karuppannan S (2018) Groundwater quality assessment using water quality index and GIS technique in Modjo River Basin, central Ethiopia. J Afr Earth Sci. https://doi.org/10.1016/j.jafrearsci.2018.06.034
Kharaka YK, Hanor JS (2003) Deep Fluids in the Continents: I. Sedimentary basins. In: Treatise on geochemistry, Elsevier, pp 1–48. https://doi.org/10.1016/B0-08-043751-6/05085-4
Kirschke S, Häger A, Kirschke D, Völke J (2019) Agricultural nitrogen pollution of freshwater in Germany. Gov Sustain Complex Probl Water (switzerland). https://doi.org/10.3390/w11122450
Koffi KV, Obuobie E, Banning A, Wohnlich S (2017) Hydrochemical characteristics of groundwater and surface water for domestic and irrigation purposes in Vea catchment, Northern Ghana. Environ Earth Sci. https://doi.org/10.1007/s12665-017-6490-3
Lautze J, Hanjra MA (2014) Water scarcity. In: Key concepts in water resource management: a review and critical evaluation. Routledge, pp 22–44. https://doi.org/10.4324/9781315884394-12
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a Blackfoot disease area in Taiwan. Sci Total Environ 313:77–89
Loh YSA, Akurugu BA, Manu E, Aliou AS (2020) Assessment of groundwater quality and the main controls on its hydrochemistry in some Voltaian and basement aquifers, northern Ghana. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2019.100296
Marandi A, Shand P (2018) Groundwater chemistry and the Gibbs Diagram. In: Applied Geochemistry. https://doi.org/10.1016/j.apgeochem.2018.07.009
Martin N, van de Giesen N (2005) Spatial distribution of groundwater production and development potential in the Volta river basin of Ghana and Burkina Faso. Water Int. https://doi.org/10.1080/02508060508691852
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci. https://doi.org/10.2475/ajs.287.5.401
Mook WG, Rozanski K (2000) Environmental isotopes in the hydrological cycle. IAEA Publish, 39
Nyenje PM, Foppen JW, Uhlenbrook S, Kulabako R, Kulabako R, Muwanga A (2010) Eutrophication and nutrient release in urban areas of sub-Saharan Africa—A review. Sci Total Environ 408(3):447–455. https://doi.org/10.1016/j.scitotenv.2009.10.020
Obuobie E (2008) Estimation of groundwater recharge in the context of future climatechange in the White Volta River Basin, West Africa. Ecology and Development Series. No.62.
Okofo LB, Anderson NA, Bedu-Addo K, Armoo EA (2021) Hydrochemical peculiarities and groundwater quality assessment of the Birimian and Tarkwaian aquifer systems in Bosome Freho District and Bekwai Municipality of the Ashanti Region Ghana. Environ Earth Sci. https://doi.org/10.1007/s12665-021-10081-2
Ounvichit T (2011) Equal water sharing in scarcity conditions: The case of the Chaisombat Muang Fai Irrigation System in Thailand. Paddy Water Environ. https://doi.org/10.1007/s10333-010-0245-z
Parkhust DL, Appelo CA (2013) Description of input and examples for PHREEQC version 3: a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations (No.6-A43). U.S Geological Survey
Plummer LN, Bexfield Laura M, Anderholm Scott K, Sanford Ward E, Busenberg E (2012) Geochemical characterization of ground-water flow in the Santa Fe group aquifer the system, Middle Rio Grande Basin New Mexico. USGS 1(2):1–414
Ponsadailakshmi S, Sankari SG, Prasanna SM, Madhurambal G (2018) Evaluation of water quality suitability for drinking using drinking water quality index in Nagapattinam district, Tamil Nadu in Southern India. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2017.10.005
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol. https://doi.org/10.1007/s00254-004-1012-5
Rees DD (1995) Role of nitric oxide in the vascular dysfunction of septic shock. Biochem Soc Trans. https://doi.org/10.1042/bst0231025
Reiter M (2001) Using precision temperature logs to estimate horizontal and vertical groundwater flow components. Water Resour Res. https://doi.org/10.1029/2000WR900302
Rusydi AF (2018) Correlation between conductivity and total dissolved solids in various types of water: a review. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/118/1/012019
Sahu P, Sikdar PK (2008) Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal, India. Environ Geol. https://doi.org/10.1007/s00254-007-1034-x
Sajedi-Hosseini F, Malekian A, Choubin B, Rahmati O, Cipull S, Coulon F, Pradhan B (2018) A novel machine learning-based approach for the risk assessment of nitrate groundwater contamination. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2018.07.054
Salifu A, Petrusevski B, Ghebremichael K, Buamah R, Amy G (2012) Multivariate statistical analysis for fluoride occurrence in groundwater in the Northern region of Ghana. J Contam Hydrol. https://doi.org/10.1016/j.jconhyd.2012.08.002
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J Hydrol. https://doi.org/10.1016/0022-1694(92)90193-Y
Saravanakumar K, Ranjith Kumar R (2011) Analysis of water quality parameters of groundwater near Ambattur industrial area, Tamil Nadu, India. Indian J Sci Technol. https://doi.org/10.17485/ijst/2011/v4i5/30071
Schöeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and development. UNESCO, Paris, pp 54–83
Skougstad MW, Horr CA (1963) Chemistry of strontium in natural water: Occurrence and distribution of strontium in natural water. Geological Survey Water-Supply Paper, 1496-D, 55–97. https://pubs.er.usgs.gov/publication/wsp1496D
SNC-Lavalin/INRS (2011) Hydrogeological Assessment Project of the Northern Regions of Ghana (HAP). Final Technical Report, I(December), 383–403. https://doi.org/10.1016/S1570-8705(03)00040-4
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M, Sarma VS (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub-basin, Tamilnadu, India. J King Saud Univ Sci. https://doi.org/10.1016/j.jksus.2013.08.001
Suciu N, Farolfi C, Zambito Marsala R, Russo E, De Crema M, Peroncini E, Tomei F, Antolini G, Marcaccio M, Marletto V, Colla R, Gallo A, Capri E (2020) Evaluation of groundwater contamination sources by plant protection products in hilly vineyards of Northern Italy. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.141495
Sunkari ED, Abu M (2019) Hydrochemistry with special reference to fluoride contamination in groundwater of the Bongo District, Upper East Region, Ghana. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-019-00335-0
Suthar S, Bishnoi P, Singh S, Mutiyar PK, Nema AK, Patil NS (2009) Nitrate contamination in groundwater of some rural areas of Rajasthan, India. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2009.05.111
Tran TQ, Banning A, Wisotzky F, Wohnlich S (2020) Mine water hydrogeochemistry of abandoned coal mines in the outcropped Carboniferous formations, Ruhr Area, Germany. Environ Earth Sci. https://doi.org/10.1007/s12665-020-8821-z
Tutmez B, Hatipoglu Z, Kaymak U (2006) Modelling electrical conductivity of groundwater using an adaptive neuro-fuzzy inference system. Comput Geosci 32(4):421–433
Tyagi S, Sharma B, Singh P, Dobhal R (2013) Water quality assessment in terms of water quality index. Am J Water Resour 1(3):34–38
UN (2015) The human right to water and sanitation media brief. UN-water decade programme on advocacy and communication and water supply and sanitation collaborative council.
Wick K, Heumesser C, Schmid E (2012) Groundwater nitrate contamination: factors and indicators. J Environ Manag. https://doi.org/10.1016/j.jenvman.2012.06.030
WHO (World Health Organization) (2017) Guidelines for drinking-water quality: Fourth edition incorporating the first addendum 978-92-4-154995-0. https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf?sequence=1
World Health Organization (2018) Who water, sanitation and hygiene strategy 2018–2025. Who.
WHO (World Health Organization) UNICEF (2012) Millennium development goal drinking water target met p 58. Retrieved from the internet http://www.who.int/mediacentre/news/releases/2012/drinking_water_20120306/en
Wu X, Zheng Y, Zhang J, Wu B, Wang S, Tian Y, Li J, Meng X (2017) Investigating hydrochemical groundwater processes in an inland agricultural area with limited data: a clustering approach. Water. https://doi.org/10.3390/w9090723
Xanke J, Ender A, Grimmeisen F, Goeppert N, Goldscheider N (2020) Hydrochemical evaluation of water resources and human impacts on an urban karst system, Jordan. Hydrogeol J Lerner. https://doi.org/10.1007/s10040-020-02174-2
Yidana SM, Banoeng-Yakubo B, Akabzaa TM (2010) Analysis of groundwater quality using multivariate and spatial analyses in the Keta basin, Ghana. J Afr Earth Sci. https://doi.org/10.1016/j.jafrearsci.2010.03.003
Yidana SM, Bawoyobie P, Sakyi P, Fynn OF (2018) Evolutionary analysis of groundwater flow: application of multivariate statistical analysis to hydrochemical data in the Densu Basin, Ghana. J Afr Earth Sci. https://doi.org/10.1016/j.jafrearsci.2017.10.026
Zaidi FK, Nazzal Y, Jafri MK, Naeem M, Ahmed I (2015) Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: a case study from northwestern Saudi Arabia. Environ Monit Assess. https://doi.org/10.1007/s10661-015-4828-4
Zakaria N, Anornu G, Adomako D, Owusu-Nimo F, Gibrilla A (2020) Evolution of groundwater hydrogeochemistry and assessment of groundwater quality in the Anayari catchment. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2020.100489
Zango MS, Sunkari ED, Abu M, Lermi A (2019) Hydrogeochemical controls and human health risk assessment of groundwater fluoride and boron in the semi-arid North East region of Ghana. J Geochem Explor. https://doi.org/10.1016/j.gexplo.2019.106363
Zango MS, Pelig-Ba KB, Anim-Gyampo M, Gibrilla A, Sunkar ED (2020) Hydrogeochemical and isotopic controls on the source of fluoride in groundwater within the Vea catchment, northeastern Ghana. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2020.100526
Zhu GF, Li ZZ, Su YH, Ma JZ, Zhang YY (2007) Hydrogeochemical and isotope evidence of groundwater evolution and recharge in Minqin Basin, Northwest China. J Hydrol 333(2–4):239–251
Acknowledgements
The authors wish to thank Mrs. Zahra Neumann and Mrs. Stefanie Schmidt for their technical assistance and advice during the sample preparation and laboratory analysis at the Institute of Geoscience, Technical University of Darmstadt. The first author would like to thank the German Academic Exchange Service (DAAD) for the scholarship. Special thanks go to Nana Anderson of Bauer Engineering Ghana and Afrakoma Armoo of CSIR-Accra, Ghana for their invaluable contributions.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors did not receive support from any organizations for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okofo, L.B., Bedu-Addo, K. & Martienssen, M. Characterization of groundwater in the 'Tamnean' Plutonic Suite aquifers using hydrogeochemical and multivariate statistical evidence: a study in the Garu-Tempane District, Upper East Region of Ghana. Appl Water Sci 12, 22 (2022). https://doi.org/10.1007/s13201-021-01559-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01559-2