Abstract
The geology of an area plays a significant role in the chemistry of groundwater. The quality of groundwater can also be adversely affected by anthropogenic activities if not properly monitored. The composition of groundwater in Akure was assessed and characterised in order to determine the chemical behavior of the groundwater system within the city. A total of thirty-six representative groundwater samples were randomly collected within Akure metropolis. The chemical composition of the water samples was analysed and determined using AAS, flame photometry and titration methods. The analysis of the results showed that the chemical concentrations of EC ranged from 35.5 to 1670.0µS/cm; TDS (in mg/l) ranged from 5.0 to 1700.0; Na+(1.0–122.0); K+(1.2–156.7); Ca2+(1.2–17.3); Mg2+(3.9–6.4); Fe3+(0.03–1.03); HCO3− (0.0–622.5); NO3−(0.01–0.25); Cl−(1.99–216.4); SO42−(22.6–`873.9); Mn2+(0.05–0.30); Zn2+(0.06–0.78); Cu2+(0.02–0.32); Pb2+(0.01–0.18); Ni2+(0.0–0.02); Cr3+(0.02–0.32); and Se2+(0.06–0.37). Statistical evaluation of the data showed that the water samples collected within the centre of the ancient city as well as the samples obtained from downstream of the major river draining the city have elevated EC and TDS higher than the WHO and NIS recommended limits. Further evaluation of the results by piper diagram, Gibb’s plot and Chadha classifications showed that the chemistry of the groundwater system in Akure metropolis is largely dominated by rock water interactions. The primary character of the water falls within the alkali metals exceeding alkaline earth metals and strong acidic anions exceeding weak anions. The indicates that the groundwater system in the study area has salinity challenges for both domestic uses and irrigation purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is very essential to the well-being of mankind, thereby making it an important necessity for everyday life (Majolagbe et al. 2014). It is also very essential to all forms of life, although it provides no form of calories or organic nutrients. The adequacy in quantity and quality determines its roles as a life-sustaining resource in human community. The quality of water influences the choice of water being used for household chores. Several authors have identified domestic and industrial effluents, agricultural chemicals and fertilisers, soil erosion, oil spills and leakages, mining activities, infiltration from dumpsites and salt water intrusions as possible sources of contaminants to groundwater (Majolagbe et al. 2014; Afolabi et al. 2018; Okoronkwo et al. 2006; Longe and Enekwech 2007). These studies have also shown that the quality of groundwater can be influenced by climate, geology of the watershed and aquifers and topography. Ayeni (2011) and Okoli et al. (2013) have indicated that about two million people mostly children die globally every year of water-related diseases due to poor sanitation and contaminated water.
Groundwater in Akure metropolis constitutes the major source of water for industrial production, domestic and agricultural uses (Ayodele 1998). The demand for water in Akure metropolis has increased significantly in recent years due to increase in population which has exacerbated the problem of contaminated water for man (Asiwaju-Bello and Akande 2004; Onipede and Bolaji 2004).
The lack of access to safe water is likely to impinge upon the economic growth by 2050, if not earlier (UNICEF/WHO 2014); access to safe water has a great impact on the health, economic productivity and quality of life. “The inadequacy of safe water to the entire population is manifested in the prevalence of water-related diseases such as guinea worm, cholera, diarrhoea, dysentery, etc.” More than half of the residents in Akure lack access to potable water. This populace depends largely on water from shallow wells or stream without any treatment, thereby making them prone to serious medical issues such as acute and chronic toxicity, liver, kidney and intestinal damage, anaemia and cancer, while the few ones that have access to safe water are either linked to a piped source from the local water board or from few well-treated boreholes (Nwakor, et al. 2013). Groundwater obtained through shallow wells is most susceptible to these contaminants compared to boreholes from deeper depths (Afolabi et al. 2019; Anudu et al. 2008).
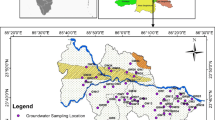
The study area falls within the tropical rain forest of Southwestern Nigeria, with wet and dry seasons. The wet season runs through the months of April to October, while the dry season is usually between November and March. The average annual rainfall is about 3000 mm (Iloeje 1980). Akure metropolis is underlain by Precambrian Basement Complex rocks of Southwestern Nigeria. The major rock types in Akure are mainly migmatite, granite and charnockite. These rocks are members of the migmatite–gneiss complex, and the Older Granite suits occupying about 45% and 55% of the total area of Akure metropolis, respectively (Afolabi 2010). The overall structural trend in Akure is largely dominated by NNW-SSE and NNE-SSW which is in conformity with the principal basement complex fracture direction (Oluyide 1988).
Materials and methods
A total of thirty-six representative water samples were collected from thirty-six boreholes within Akure metropolis for this study based on the land-use pattern and population density (Fig. 1). This was carried out during the dry season from December to February in order to avoid the dissolution effect on the groundwater. At each sampling point, the borehole water was allowed to pump for a few minutes before sampling, and there after two sets of water samples were collected into plastic containers for cations and anions analyses. Samples for cations were acidified with two drops of concentrated nitric (10% vol.) acid. Electrical conductivity (EC), total dissolved solid (TDS), pH and temperature were measured in situ using the HANA multi-meter (model 850). The analysed chemical parameters include major anions and cations. Chlorine (Cl−) was analysed for, using standard silver nitrate solution and potassium chromate indicator. Alkalinity (HCO3−) was analysed using standard sulphuric acid titration and phenolphthalein and methyl orange indicators. Total hardness of the samples was calculated using the Fournier’s equation (Fournier 1981).
The concentrations of both major and trace elements were measured using AAS with the exception of Na and K. The cations analysed are magnesium (Mg2+), manganese (Mn2+), iron (Fe3+), calcium (Ca2+), sodium (Na+) and potassium (K+), while the anions analysed are sulphate (SO42−), chloride (Cl−), bicarbonate (HCO3−) and nitrate (NO3−). Standard chemical analytical procedures suggested by the American Public Health Association (APHA, AWWA, WPCF 2005) were adopted for all the chemical analyses. The ionic balance error for both ions was within ± 7%, while analytical precision was less 2%. The base map used for this study was digitised from a topographical map of 1: 50,000 using Arc GIS. The data were analysed and evaluated using SPSS window 22.
Results and discussion
Physical and chemical parameters of groundwater samples
The result presented in Table 1 revealed that pH of the samples varied from slightly acidic to slightly alkaline with pH values ranging from 6.8 to 8.5 with an average value of 6.8 ± 1.1 suggesting slightly acidic water. The range of values and the average value recorded in all the water samples were within the limit of WHO and NIS standards for drinking water (Table 1). The electrical conductivity (EC) values of the water samples as measured in situ varied from 35.2 to 1670.2 μS/cm with an average value of 560.3 ± 52.1 μS/cm. About 30% of the samples collected around the ancient city centre have elevated EC values higher than the recommended limit of WHO and NIS standards for drinking water. The total dissolved solid (TDS) values varied from 5.0 to 1700.0 mg/l with a mean value of 492.6 ± 445.3 mg/l. All samples recorded TDS values that were within the WHO standard for drinking water, while a few of the samples fall out of the NIS standard. The total hardness of all the water samples varied from 8.2 to 382.4 mg/l with an average of 145.7 ± 16.2 mg/l. These values were within the WHO permissible limits for drinking water with the exception of some samples collected downstream of River Ala which has elevated values above the WHO recommended value. This can be attributed to infiltration and percolation of surface runoff into the boreholes. Water hardness occurs when there is reasonable amount of some cations such as calcium and magnesium in the presence of carbonate, bicarbonate, chloride and sulphate in water.
Major cations and anions concentrations of groundwater samples
Ca2+ ranged from 1.2 to 17.30 mg/l with an average of (9.6 ± 3.03) mg/l; Fe3+ ranged from 0.03 to 1.03 mg/l with an average of (0.14 ± 0.18) mg/l; Mg2+ ranged from 3.85 to 6.43 mg/l with an average of 4.75 ± 0.72) mg/l; Na+ ranged from 1.0 to 122.0 mg/l with an average of (34.6 ± 37.1) mg/l; K+ ranged from 1.2 to 156.7 mg/l with an average of (37.7 ± 38.5) mg/l; Cl− ranged from 1.99 to 216.41 mg/l with an average of (85.2 ± 65.1) mg/l; SO42− ranged from 22.56 to 873.85 mg/l with an average of (245.5 ± 215.1) mg/l; HCO3− ranged from 0.0 to 622.52 mg/l with an average of (143.3 ± 162.8) mg/l; and NO3− ranged from 0.01 to 0.25 mg/l with an average of (0.05 ± 0.07) mg/l in groundwater samples from the study area. The ranking order of the mass concentrations (meq/L) of the major ions analysed was in the following order of magnitude: K+ > Na+ > Ca2+ > Mg2+ > Fe3+ for the cations in groundwater, while the order of magnitude of anions in the same water samples is SO42− > HCO3− > Cl− > NO3− (Table 1). Comparing these ranges of values with the WHO and NIS recommended values for drinking water showed that all the major cations and anions analysed from most of the water samples from the study area were all within the limit of WHO and NIS standards with the exception of Mg2+ in three samples having elevated concentrations ranging from 32.2 to 42.4 mg/l. The SO42− and HCO3− were the dominant anions from all the water samples investigated suggesting that all the waters were of the bicarbonate type.
Heavy metal concentrations in groundwater samples
The chemical data of ten heavy metals consisting of Zn2+, Cu2+, Ni2+, Pb2+, Cr3+, Se2+, As2+, Mn2+ and Cd2+ derived from analysis of the groundwater show that Zn ranged from 0.06 to 0.78 mg/l with an average of (0.19 ± 0.12) mg/l; Cu2+ ranged from 0.02 to 0.32 mg/l with an average of (0.16 ± 0.07) mg/l; Ni2+ ranged from Nd to 0.02 mg/l with an average of (0.01 ± 0.01) mg/l; Pb2+ concentrations ranged from 0.01 to 0.18 mg/l with an average of (0.10 ± 0.04) mg/l; Cr3+ ranged from 0.02 to 0.32 mg/l with an average of (0.14 ± 0.07) mg/l; Se2+ ranged from 0.06 to 0.37 mg/l with an average of (0.18 ± 0.09) mg/l; As2+ ranged from 0.0 to 0.09 mg/l with an average of (0.02 ± 0.03) mg/l; Mn2+ ranged from 0.05 to 0.30 mg/l with an average of (0.12 ± 0.05) mg/l; and Cd2+ ranged from 0.0 to 0.007 mg/l with an average of (0.003 ± 0.001) mg/l (Table 1).
Comparing the spatial distributions of the heavy metals with WHO (2017) and NIS (2007) standards showed that Fe3+, Cu2+ and Cd2+ concentrations in most of the samples were within the limit of the WHO and NIS standards for safe water. Similarly, over 60% of the samples have metals such as Ni2+, Pb2+ and Mn2+ concentrations within the standards, but the remaining 40% were slightly above the standards. On the contrary, over 80% of the samples have Cr3+, As2+, Pb2+ and Zn2+ concentrations above the standards even the averages of these metals were above both the WHO and NIS standards for safe water. Based on the mean/average values ranking of the heavy metal concentrations, the following order of magnitude was observed; Se2+ > Zn2+ > Cu3+ > Cr2+ > Mn2+ > Pb2+ > As2+ > Ni2+ (Table 1).
The following parameters are generally used as indices of water pollution: NO3− and heavy metals such as Fe3+, Zn2+, Mn2+, Cr3+, Cu2+, Cd2+, As2+ and Pb2+. The presence of these parameters above the required or recommended values could portend health hazard to the general public. Spatial distributions of metals such as Cr2+, As2+, Zn2+ and Pb2+ revealed that out of the thirty-six water samples analysed, over 80% have concentrations greater than the WHO and NIS recommended standards, while all samples have NO3− concentrations within the standards.
Classification of the groundwater within the study area
Using conductivity to classify the groundwater from the study area according to Talling and Talling (1965), over 80% of the groundwater samples fell within freshwater category, while the remaining few samples are slightly saline in nature (Table 1). Similarly, all the water samples with the exception of four samples belong to the freshwater category using the range of TDS values (Table 1). This could be related to external input of materials derived from infiltration of surface runoff. About 50% of the water samples have TH < 75 mg/l; 23% falls within 75–150 mg/l and 150–300 mg/l, respectively, while the remaining 27% have values > 300 mg/l. These can be described as soft water, moderately hard water, hard water and very hard water, respectively.
Table 2 presents the classification of the groundwater samples based on the predominant chemical parameters (Petraccia et al. 2006). The result revealed that some of the water samples from the study area fell within the category of bicarbonate, sulphate and chlorinated water types in conformity with the Petraccia et al. Scheme. Based on the fact that sulphate and bicarbonate are the predominant anions from all the water sources, the water from the area can therefore be said to be of the sulphate–bicarbonate type (Table 2).
The hydrochemical data such as Cl−, HCO3−, Na+, Ca2+, Mg2+ and K+ derived from the water samples were plotted in Gibb’s diagram in order to determine the dominant chemical processes of the groundwater chemistry and also to assess the functional sources of the dissolved chemical constituents of the groundwater (Fig. 2). Over 70% of the data plotted clustered around the rock dominance suggesting that chemical weathering of rock-forming minerals within the study area has significant influence on the chemistry of the groundwater in the study area. The remaining 35 per cent spills into evaporation crystallisation domain.
The plots of Ca2+ + Mg2+ against HCO3− showed that Ca2+ + Mg2+ are in excess and the alkalinity of the water is balanced by these alkaline earth metals in the aquifers of Akure metropolis (Fig. 3a). Also, the plot of alkaline earth metals versus acidic ions (HCO3− + SO42−) revealed that over 90% of the data points plotted above the equiline which is still an indication that alkaline earth metals Ca2+ + Mg2+ are in excess of the acidic ions (Fig. 3b). The plot of alkaline earth metals against the total cation (Tz +) shows all data points plotted above the equiline, while the plot of alkaline metals against the total cations shows all data points plotted nearly on the equiline indicating slight contribution towards the charge balance (Fig. 3c and d). These plots revealed that the ions of the groundwater chemistry in Akure metropolis are largely controlled and governed by alkaline earth metals Ca2+ and Mg2+ rather than the alkaline metals, inconformity with the Gibb’s plot where the over 70% of the water samples fall within the rock dominance arising from weathering of rocks in the study area.
The data were used for the plotting of piper trilinear diagram, and this diagram consists of three distinct fields made up of two triangles and a diamond-shaped field. The cations are plotted on the left-hand triangle, while the anions are plotted on the right-hand triangle, the data point is then projected into the upper field, and their intercepts indicate the characters of the groundwater (Piper 1953). The piper diagram is dominated by mixed CaMgCl and CaMgSO4 water types or facies where alkaline earth metals Ca2+ + Mg2+ and strong acids Cl− + SO42− dominate over the alkali metals and weak acids (Mor et al. 2009) (Fig. 4).
The data were also subjected to Chadha plot in a bit to determine the characteristics of the chemistry of the groundwater samples. All data points fall within field −2, −4 and −7 where alkaline metals such as Ca2+ and Mg2+ exceed the alkaline earth metals and strong acids exceed weak acids (Fig. 5). Over 60% of the data points plotted within the negative axes indicate that alkali earth metals exceed alkali metals and strong acidic anions exceed weak acidic anions (Table 3).
Conclusions
The quality and characteristics of groundwater within Akure metropolis were evaluated. The result shows groundwater within the study area falls within the freshwater type with over 75% of the groundwater varying from soft through moderate to hard water, while the remaining 25% falls within the very hard category.
Comparing the result of the chemical parameters of the groundwater with the approved WHO and NIS standards for safe water shows that all the major cations and anions were largely within the limit of WHO and NIS standards with the exception of some samples having elevated concentrations of Na+, K+, Mg2+, Cl−, HCO3− and SO42−. These samples were mostly collected from the ancient city centre. Over 70% of the water samples fall within the rock dominance domain suggesting that the chemistry of the water is as a result of ion exchange reaction which occurs during rock weathering processes.
The Chadha plot analysis showed that the groundwater geochemistry is characteristics of alkali earth metals exceeding alkaline metals and strong acidic anions exceeding weak anions, while Gibb’s plot shows that the dominant geochemical process responsible for the groundwater chemistry from the study area is predominantly from rainfall. The piper diagram indicates that the evolution of the groundwater is largely dominated by mixed CaMgCl and CaMgSO4 water facies types.
Availability of data and materials
“Authors declare that all data supporting the findings of this study are available within the article”.
References
Afolabi OO (2010) Geochemical evaluation of some environmental media in Akure metropolis. Unplished M.Sc. Thesis in the department of geology, University of Ibadan. 168+xiv pp
Afolabi OO, Olutomiola OO, Ishiak JD (2018) Assessment of Groundwater system characteristics in Ilorin metropolis, southwestern Nigeria. Am J Water Sci Eng 4(1):1–8. https://doi.org/10.11648/j.ajwse.20180401.11
Afolabi, O.O., Ogundipe, I.E. and Kogam, N.M. 2019. Assessment of potentially harmful metals and hydrochemical characterisation of portable groundwater in iworoko and its environs, Southwestern Nigeria. J Min Geol, Vol. 55 (1).
Anudu GK, Obrike SE, Ofoegbu C (2008) Groundwater investigation across the crystalline bsement rocks in Rogo area, Kano State, northern Nigeria usng resistivity methods. J Min Geol 44(2):351–364
APHA/AWWA/WPCF (2005) Standard methods for the examination of Water and Waste water. 21st ed. Washington D.C. APHA/AWWA/WPCF
Asiwaju-Bello And Akande (2004) Urban groundwater pollution: case study of a disposal sites in lagos metropolis. J Water Resources 12:22–26
Ayeni AO, Balogun II, Soneye ASO (2011) Seasonal assessment of physic-chemical concentrations of polluted urban river: case of Ala river in southwestern Nigeria. Res J Environ Sci 5(1):22–33
Ayodele JO (1998) Tropical hydrology and water resources. Macmillan publishers Ltd, London
Chadha DK (1999) A proposed new diagram for geochemical classification of natiral waters and interpretation of chemical data. Hydrogeol J 7:431–439. https://doi.org/10.1007/s:100400050216
Fournier RO (1981) Application of water Geochemistry to geothermal exploration and reservoir engineering. In: Rybach L, Muffler LJP (eds) Geothermal systems, principles and case histories. Wiley, New York, pp 109–144
Iloeje PN (1980) New Geography of west Africa. https://www.biblio.com/book/new-geography-west-africa-n-p/d/583780923
Longe EO, Enekwechi LO (2007) Investigation of potential groundwater impacts and influence of local hydrogeology on natural attenuation of leachate at a municipal landfill. Int J Environ Sci Tech 4(1):133–140
Majolagbe AO, Adeyi A, Osibanjo O (2014) Hydrochemical characterisation and quality assessment of groundwater in the vicinity of a major active dumpsite in Lagos, Nigeria: the use of multivariate analytical technique and water quality indices. Eur Water 48:29–42
Mor S, Singh S, Yadav P, Rani V, Rani P, Sheoran M, Singh G, Ravindra K (2009) Appraisal of salinity and fluoride in a semi-arid region of India using statistical and multivariate techniques. Environ Geochem Health 31:643–655
Nigerian Industrial Standards (NIS) 2007. “Nigerian Standards for Drinking Water Quality”. (Price group D., Copy right SON2007 NIS 554: 20; ICS 13.060.20.07). 30
Nwakor E.K. et al 2013. Assessment of seasonal groundwater quality for domestic use in akure metropolis, Ondo state, Nigeria. Int J Agric Biosci. E-ISSN: 2306–3599
Okoli CC, Cyril JA, Itiola OJ (2013) Geophysical and hydrogeochemical investigation of Odolomi dumpsite in Supare-Akoko, southwestern Nigeria. Pac J Sci Technol 14(10):492–504
Okoronkwo NE, Odemelam SA, Ano AO (2006) Levels of toxic elements in soil of abandoned dumpsite. Afr J Biotech 5:1241–1244
Oluyide P.O. 1988. Structural trends in the Nigeria basement complex “In Precambrian Geology of Nigeria by P.O. Oluyide (ed.). Geological Survey of Nigeria Publication, Nigeria 93–98.
Onipede AIM, Bolaji BO (2004) Management and disposal of industrial waste in Nigeria. Niger J Mech Eng 2(1):49–58
Petraccia L, Liberati G, Masciullo SG, Grassi M, Fraioli A (2006) “Water, mineral waters and health. Clin Nutr 25:377–385
Piper AM (1953) A graphic procedure in the geochemical interpretation of water analysis. Washington D.C, United States Geological Survey
Talling JF, Talling IB (1965) The chemical composition of african lake waters. Internat Rev Ges Hydrobiol 50:421–463
World Health Organization (WHO). Guidelines for Drinking Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017. 5(2):537–544.
World Health Organization (WHO)/United Nations Children’s Fund (UNICEF) 2014. Joint Monitoring Programme for water supply and sanitation. Estimates on the use of water facilities. Updated April 2014, Nigeria.
Funding
“The authors declare that no fund was received for this research”.
Author information
Authors and Affiliations
Contributions
OOA; AKO and IEO contributed to the study conception and design. Materials preparations, data collection and analyses were performed by OOA. The draft was written by OOA. OOA; AKO and IEO comment on the previous versions of the manuscript. OOA; AKO and IEO read and approved the final manuscript.”
Corresponding author
Ethics declarations
Conflict of interest
A declaration related to conflict of interests should be present and be included by the authors: “The Authors declare that there is no conflict of interest”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afolabi, O.O., Ogundana, A.K. & Ogundipe, I.E. Assessment and characterisation of groundwater quality in Akure metropolis, southwestern Nigeria. Appl Water Sci 12, 84 (2022). https://doi.org/10.1007/s13201-021-01544-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01544-9