Abstract
The present study focused on the seasonal investigation of hydro-geochemical characteristics of groundwater samples collected from the vicinity of three tributaries of the Beas River, Punjab, India. Total 45 samples were analyzed during the pre- and post-monsoon season for physico-chemical parameters and heavy metals along with health risk assessment. Results revealed that the majority of samples were below the permissible limits set by the BIS and WHO. The relative abundance of major cations was Ca2+ > Mg2+ > Na+ > K+ and Ca2+ > Na+ > Mg2+ > K+, while that of the major anions was HCO3− > SO42− > Cl− > CO32− in the pre- and post-monsoon season, respectively. Groundwater was alkaline and hard in nature at most of the sites. Bicarbonate content exceeded the desirable limit having an average concentration of 337.26 mg/L and 391.48 mg/L, respectively, during the pre- and post-monsoon season. Tukey’s multiple comparison test was applied for finding significant differences among samples at p < 0.05. The dominant hydrochemical face of water was Ca–Mg–HCO3 type. US salinity (USSL) diagram indicated that during the pre-monsoon, 48.9% samples were C2S1 type and remaining 51.1% were C3S1 type while during the post-monsoon all samples were C3S1 type. It indicates that groundwater of the study area is at risk of salinity hazards in future and is not to be ignored. Such monitoring studies are recommended to design future safety plans to combat soil and human health risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater is the most important source for drinking and irrigation purpose but due to intensive farming, industrial discharge, pollution of rivers, its quality is deteriorating in North-West regions of Punjab, India for survival (Talabi and Kayode 2019; Adimalla et al. 2020). The Beas River, falling in the Northwest of Punjab, India and its tributaries adjoining agricultural fields loaded with contaminants like heavy metals, bicarbonates, chlorides, sulfates etc. due to inefficient agricultural practices (Kumar et al. 2017; Srivastava et al. 2019; Setia et al. 2020). Use of groundwater is very high in India, particularly for irrigation purposes (Singh et al. 2020). Indiscreet consumption and speedy rise of this contamination in groundwater is threat to supply of good quality water (Kumar and Shah 2006; Gupta et al. 2018; Jain and Mujawar 2019). Groundwater contamination is difficult to assess than that of surface water. It needs regular monitoring as contamination will remain for decades in the subsurface environment (Jha et al. 2020). India is the biggest consumer of groundwater in the world, with an estimated groundwater use of around 230 km3/year (CGWB 2017). The higher use has resulted in depletion of groundwater more prominently in the Punjab state (Krishan et al. 2014). Groundwater is intimately linked to surface water present in the rivers and water received as rainfall also contributes to groundwater reserves. So, pollution of surface water also affects the quality of groundwater (Srivastava et al. 2019).
About 80% of diseases in human beings are directly linked to consumption of polluted water (Ganiyu et al. 2018; Ali and Ahmad 2019). The heavy metals are also one of the major contaminants of water. Exposure to contaminants in groundwater via drinking water and cooked food had led to direct or indirect accumulation in human body resulting in health hazards like respiratory diseases (Jaishankar et al. 2014), osteoporosis (Rodriguez and Mandalunis 2018), hypertension (Scheelbeek et al. 2017), dermal problems (Sharma and Bhattacharya 2017), neurological (Bondy and Campbell 2017) and reproductive disorders (Agrawal et al. 2010), etc. According to the data compiled by the Central Groundwater Board (CGWB 2017), the total 2420 number of villages had been found to be affected by heavy metal contamination in India, out of which 2139 had fallen in Punjab (Sharma et al. 2019).The present study was therefore undertaken to investigate the quality of groundwater by determining the various hydro-geochemical characteristics of groundwater in the vicinity of the three tributaries of Beas River falling in Bist-Doab region, namely Pong left main canal, Odhra rivulet and Kali bein and determining the suitability of groundwater for drinking and irrigation purpose.
Materials and methods
Sampling and sample pretreatment
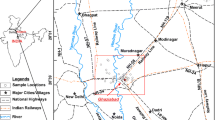
An array of 15 villages (5 villages/tributary) distributed across the study area was selected which are close to confluence of the selected tributaries of the Beas river, Punjab, India (Fig. 1). Total 90 groundwater samples (45 samples through each season) were collected from tubewells located in these villages (3 sampling sites i.e., 45 × 3 = 90) in June 2019 (pre-monsoon) and October 2019 (post-monsoon) from same locations to evaluate the seasonal variations. Samples were collected in clean pre-washed polyethylene bottles around 3–4 times with same groundwater to be collected. Samples were collected after pumping the tubewells for about 10 min so that water previously stored in pipes can be removed (Singh et al. 2013). For estimation of heavy metals such as manganese (Mn), lead (Pb), molybdenum (Mo), nickel (Ni), selenium (Se), aluminum (Al), arsenic (As), copper (Cu), cadmium (Cd), iron (Fe) , cobalt (Co) and zinc (Zn), a portion of water sample was acidified with conc. HNO3 (pH<2) and rest of the portion were used to analyze different physico-chemical parameters like pH, electrical conductivity (EC), total dissolved solids (TDS) and contents of anions and cations. The groundwater samples were transported in cooled ice box and stored in refrigerator at 5 °C until analysis.
Physico-chemical analysis of groundwater samples
Physico-chemical parameters of collected groundwater samples were analyzed by following the standard methods (Trivedy et al. 1987; APHA (2017); The portable kit was used for measuring pH and TDS at sampling sites. Carbonate (CO32−), bicarbonate (HCO3−) and chloride (Cl−) ions were determined by the titrimetric method while calcium (Ca2+) and magnesium (Mg2+) were quantified using versenate method.
First letter in name of each site represents the name of tributary and number represents the code of village as P1: Sadarpur, P2: Lamipur, P3: Jamalpur, P4: Bhagran, P5: Sunderpur, O1: Bhushan, O2: Pandori Aryian, O3: Odhra, O4: Takipur, O5: Sajjan, B1: Sarupwal, B2: Alluwal, B3: Bussowal, B4: Miani Bahadur, B5: Tarf Hazi
Sulfate (SO42−) was determined by turbidimetric method (Sheen et al. 1935) using colorimeter (make: Systronics; model: 115) at a wavelength of 420 mμ with a blue filter. Na+ and K+ contents in samples were analyzed using flame photometer (make: Systronics; model: 130).
Heavy metal analysis of groundwater samples
Heavy metals were determined in water samples by following standard method used by Oyekanmi et al. 2018 using microwave plasma-atomic emission spectrometer (make: Agilent; model- 4200). Before determination of concentration of heavy metals, water samples were filtered using Whatman filter paper no. 42 (Sharma et al. 2019).
Reagents and quality control
The chemicals and reagents used for analyzing physico-chemical parameters and heavy metal contents in present study were of analytical grade. Stock standard solutions (Agilent technologies, USA) were used to draw calibration curves for determination of heavy metal content in samples. Double distilled water was used at regular intervals to run instrument for washing to avoid the deposition of any analyst inside it.
Water quality assessment
Groundwater samples were categorized into various classes for irrigation and drinking purposes on the basis of physico-chemical parameters such as total dissolved solids (TDS) and total hardness (TH) etc. The hardness of water is due to dissolution of salts of Ca2+ and Mg2+. So, to determine TH, we have estimated Ca2+ and Mg2+ contents in water samples.
Hem (1989) had given following formula for calculating TH of water:
Groundwater samples can also be classified on the basis of indices such as electrical conductivity (EC), sodium adsorption ratio (SAR), residual sodium carbonate (RSC), Kelly’s ratio (KR), magnesium ratio (MR), permeability index (PI), corrosivity ratio (CR) and sodium percentage (Na%). Suitability of groundwater samples for drinking and irrigation purposes was determined by keeping in consideration the standard limits given by WHO (2011) and BIS (2012).
Sodium adsorption ratio (SAR) is used for estimating sodium hazard to crops and to classify groundwater to check its suitability for irrigating crop fields (Gholami and Srikantaswamy 2009). SAR values of all groundwater samples were estimated by using the equation given by Richards 1954 as:
Todd (1995) had given following equation to calculate sodium percentage (Na%) in water:
Magnesium ratio (MR) was evaluated using following formula (Palliwal 1972):
Kelly’s ratio (KR) was calculated by using equation given by Kelley (1946) which is expressed as follows:
Residual sodium carbonate (RSC) is the measure of excessive amount of sodium bicarbonate and carbonate over Ca2+ and Mg2+ which has affect on physical properties like pH, EC and SAR of the soil by dissolution of organic matter (Naseem et al. 2010). It has been evaluated using the following equation (Ragunath 1987):
Corrosivity ratio (CR) is used to check the corrosiveness of water. It was evaluated using following equation (Raman 1985):
Permeability index (PI) is a qualitative measure of rate of vertical movement of water from the ground surface through unsaturated zone to the subsurface layers of the soil (Kumar 2016; Rawat et al. 2018). It is given as:
Health risk assessment
Health risk assessment is essential to check the suitability of groundwater for drinking purposes. The US Environmental Protection Agency (USEPA 2004) had given following two equations to measure the exposure dose (E) through ingestion and potential non-carcinogenic risk of Hazard Quotient (HQ):
where E: chronic daily intake (mg/kg/day), CPW: concentration of contaminant (mg/L), IR: human ingestion rate (L/day: 0.78 L/day for children and 2.5 L/day for adults), ED: exposure duration (years: 12 and 64 for children and adults, respectively), EF: exposure frequency (days/years: 365 days for children and adults), ABW: average body weight (Kg: 18.7 and 57.5 for children and adults, respectively), AET: average time (days: 4380 and 23360 for children and adults, respectively), HQ: Hazard quotient, RfD: reference dose of pollutant (Adimalla and Qian 2019).
Statistical analysis
Analysis of variance (ANOVA) was applied on physico-chemical parameters and heavy metal content to assess variation among location and season of sampling site at p < 0.05 using SPSS 16.0 software. Further, Tukey’s multiple comparison test was used to confirm significant differences among samples at p < 0.05.
Results and discussion
Physico-chemical analysis
All the tributaries were in the Bist-Doab region where groundwater level falls systematically in various regions over the last two decades. The pH of the studied samples was within the maximum permissible limits given by WHO and BIS during both the pre- and post-monsoon season (Kamble and Saxena 2016). All the groundwater samples of both seasons were alkaline (average of 7.80 and 7.96) in nature. Dissolution of primary CO3− and silicate minerals after monsoon had led to increase in concentration of Ca2+, Na+, Mg2+ and HCO3− which had raised the value of pH (Rouabhia et al. 2011). During each season, values of EC remained higher than the permissible value of 250 μS/cm (WHO 2011). All samples have shown higher EC (μS/cm) values than permissible limits, i.e., 750.80 (pre-monsoon) and 891 (post-monsoon). TDS in mg/L of all the samples was observed to be less than the permissible limits set by WHO (2011) and BIS (2012) with average value of 316.09 (pre-monsoon) and 406.18 (post-monsoon). It was noticed a small increase in EC and TDS in the post-monsoon season than in pre-monsoon season.
It has been reported that leaching and dissolution or mixing of salts due to geochemical or anthropogenic activities was responsible for this variation in the study area (Sharma et al. 2017; Subba Rao et al. 2017ao et al. 2017). During summer, the water present in the interspaces of soil get evaporated and salts were accumulated in upper layer of the soil which were leached back again during monsoon period had resulted high EC and TDS values (Singh et al. 2013).
Total hardness (TH) of all the samples in the pre-monsoon was below the permissible limit but in the post-monsoon season, 31 samples of groundwater have high TH values than desirable value of 200 mg/L as per BIS (2012). It was probably due to increase in Ca2+ and Mg2+ ions in the studied samples. The pre-monsoon value (mg/L) of Ca2+ were ranged from 24.67 to 31.33 having average value 27.56 while in post-monsoon season it was from 31.33 to 41.33 having average value of 36.58. The Mg2+ concentration (mg/L) in the post-monsoon ranged from 19.60 to 28.40 having average value of 24.00 while in the post-monsoon it was from 22.80 to 29.20 with mean concentration of 26.27. The CO32− content was negligible in both the seasons of the study area while HCO3− was found beyond desirable limit in all the 45 samples having average concentration (mg/L) of 337.26 and 391.48 in the pre- and post-monsoon season, respectively. The carbonic acid helps in initiating acid–base reactions and fastens the weathering of rocks like limestone (CaCO3) and dolomite [CaMg(CO3)2] present in the study areas and similar results had been reported by Singh et al. 2013; Humbarde et al. 2014; Dev and Bali 2018. Due to this weathering process, the concentration of Ca2+ and Mg2+ were also increased after the monsoon (Sharma and Chhipa 2016; Sharma et al. 2019). The abundance of major cations was in the order of Ca2+>Mg2+>Na+>K+ and Ca2+> Na+>Mg2+>K+, respectively, in the pre- and post-monsoon season. On the other hand, abundance of major anions was in the order of HCO3−> SO42−> Cl−> CO32− in both the pre- and post-monsoon seasons as similarly reported by Humbarde et al. 2014; Thakur et al. 2016. The mean concentration of chloride (Cl−) in the study area was found to be 42.08 and 54.21(mg/L) in the pre- and post-monsoon season, respectively. The high concentration of Cl− could be due to the addition of sewage effluents directly into the tributary water and utilizing that water for irrigation (Saraswat et al. 2019). Leaching of salts along with rainwater was also reported as one of the main reasons behind high chloride concentration (Purushothaman et al. 2012; Pathak et al. 2014). Both the K+ and Na+ ions were found in concentrations (mg/L) lower than permissible limits having mean value of 11.80 and 12.20 for K+ and 24.22 and 26.88 for Na+ in the pre- and post-monsoon season, respectively. High concentration of K+ was noticed in the post-monsoon season may be due to weathering of saltpetre during the rainy season (Kumar et al. 2013). The average value (mg/L) of SO42− was 68.90 in the pre- and 78.85 during the post-monsoon, was lower than the permissible limit. The presence of high SO42− ion during the post-monsoon may be due to leaching, breakdown of organic matter in soil, excess use of fertilizers and anthropogenic activities. Similar findings were documented by Ganiyu et al. 2018 and Hejaz et al. 2020 in their studies.
Heavy metal analysis
The heavy metal content in samples of study areas during both the pre- and post-monsoon season is represented in Table 1. The average concentration of Fe, Al, Mn, Cu, As and Se in samples to be 0.30 mg/L, 0.02 mg/L, 0.02 mg/L, 0.03 mg/L, 0.19 μg/L and 0.78 μg/L, respectively during pre-monsoon and 0.33 mg/L, 0.03 mg/L, 0.03 mg/L, 0.03 mg/L, 0.25 μg/L and 0.84 μg/L, respectively, during the post-monsoon. Main sources of heavy metals in the present study area were due to indiscriminate use of agrochemicals and leaching of heavy metals along with the rainwater as reported by Sharma et al. 2019 and Virk 2019. Selenium was found in more concentration mainly at sites near Kali Bein, for the unknown reason but had predicted to be due to Se rich soil in that area (Bajaj et al. 2011). It has also been reported in groundwater due to weathering, leaching and oxidation of soluble selenium compounds (Virk 2018). Though, selenium has an essential role in human health but at the same time toxicity had been reported at higher concentrations (Dhillon and Dhillon 2003) in Punjab. There was no reason behind occurrence of As contamination in Punjab (Virk 2019). The aluminum was reported naturally as oxides, hydroxides and silicates or as a complex with organic matter. The samples with negligible concentrations of Zn, Cd, Co, Pb, Mo and Ni in both the seasons were observed in the presentstudy.3.3 Statistical analysis
One-way ANOVA is used for assessment of variation during the pre- and post-monsoon season in physico-chemical parameters as well as for the heavy metal content. It is necessary to evaluate the level of difference over change in sampling sites for both the seasons (pre- and post-monsoon) and their interaction. The heavy metals which were below detection limits were not used for statistical analysis. Results had shown statistically significant difference for most of the parameters at p < 0.05 (Tables 2, 3 and 4) (Kumar et al. 2016). The significant difference was observed due to different sampling locations, leaching of agrochemicals, influence of river and tributary water, inputs via industrial and domestic waste in groundwater.
Groundwater quality for drinking purpose
The water samples have been evaluated for suitability for drinking purpose based on the guidelines given by WHO (2011). It was noticed that values of water quality parameter and number of samples exceeding desirable limits (DL) and maximum permissible limits (MPL) set by the WHO (2011) and their health implications are given in Table 5. The suitability of studied water samples for drinking was examined from its TDS, TH and ionic concentration which is explained under following headings:
Total dissolved solids (TDS)
Total dissolved solids are regarded as one of the major determinants for studying suitability of drinking water. As per WHO, TDS value higher than 1000 mg/L along with high pH value resulted in unpleasant taste. Groundwater samples were classified into six classes on the basis of TDS (Singh et al. 2013) as: excellent, good, fair, poor and unacceptable. TDS value (mg/L) ranged from 272.33 to 378.33 with an average value of 316.09 and from 320.00 to 475.00 with an average value of 406.18 for the pre- and post-monsoon samples, respectively. Out of the total 45 samples collected during the each season, 15 samples were under excellent class while remaining 30 samples were under good class for the pre-monsoon while in the post-monsoon season, all samples were under good class (Table 6). Similar findings were observed by Krishan et al. 2014 and Rao et al. 2017.
Total hardness (TH)
Total hardness (mg/L) ranged from 145 to 190 for pre-monsoon and from 180.00 to 211.67 for the post-monsoon season. The TH was of samples were observed as: post-monsoon season > pre-monsoon. Further, the classification of samples based on TH (Sawyer and McCartly 1967) has shown that 4 samples were under moderately hard class and 41 samples were under hard class during pre-monsoon while in the post-monsoon season all the 45 samples fall under hard class (Table 7).
Health risk assessment
Health risk assessment characterizes the adverse health effects caused by exposure of pollutants on children and adults (Adimalla and Qian 2019). Drinking is the most prominent factor of ingestion of the heavy metals through contaminated groundwater (Chen et al. 2016). In the present study, Fe exceeded the desirable limits (DL) (WHO 2011). Fe concentration was more than DL in 23 water samples and 26 samples, collected, respectively, in the pre-monsoon and post-monsoon season. On the other hand, concentration of As and Se was found lower than DL during both seasons. The health risk assessment of these heavy metals was determined by calculating the hazard quotient (HQ). The calculations were done on the basis of standards given by USEPA (2004). The results of the health risk assessment are shown in Table 8. The HQ values for As during the pre-monsoon were ranged from 0 (B3) to 6.12E-02 (O5) for children and 0 (B3) to 6.38E-02 (O5) for adults while in the post-monsoon, it was ranged from 1.39E-03 (B3) to 7.93E-2 (O5) for children and from 1.45E-03 (B3) to 8.26E-02 (O5) for adults. On the other hand, results had shown that the HQ values for Fe during the post-monsoon ranged from 6.36E-03 (B1) to 2.64E-02 (P3) for children and from 6.63E-03 (B1) to 2.75E-02 (P3) for adults while it ranged from 9.53E-03 (B2) to 3.10E-02 (P3) for children and from 9.94E-03 (B2) to 3.23E-02 (P3) for adults during the pre-monsoon. While in case of Se, the HQ values ranged from 3.67E-03 (O1) to 1.15E-02 (B5) for children and from 3.83E-03 (O1) to 1.20E-02 (B5) for adults during the pre-monsoon while during the post-monsoon, it was ranged from 4.09E-03 (O1) to 1.19E-02 (B5) for children and from 4.26E-03 (O1) to 1.24E-02 (B5) for adults. It is a scientific fact that the HQ < 1 indicates no certain non-carcinogenic risk (USEPA 2004; Koki et al. 2015; Vetrimurugan et al. 2017; Victor et al. 2018). It was noticed that the HQ value for As, Fe and Se was found less than 1 in all the samples which showed that the contamination was below risk level in the study area for both the adults and children.
Groundwater quality for irrigation purpose
The quality of the groundwater for the irrigation purposes has been given by the different experts. However, the most important parameters to determine suitability of the water samples were EC, SAR, Na%, RSC, MR, CR, PI and KR (Tripathi et al. 2012). The classification of groundwater samples for their suitability for irrigation purpose is given in Table 9.
In addition, the suitability of the water samples for irrigation purpose was determined by plotting data on various scientific diagrams which included USSL diagram, Wilcox diagram and Piper Diagram which are explained in the following discussion.
Salinity hazard (EC)
Wilcox (1955) had given classification of the groundwater on the basis of salinity hazard (EC) to check its suitability for irrigation purposes. He had classified the groundwater samples into five classes. After analyzing, it was observed that 22 groundwater samples were under good class and 23 samples were in permissible class during the pre-monsoon while during the post-monsoon all the samples collected from the study area were found in permissible class. Thus, results have shown that salinity get increased after the monsoon season which might had been due to the leaching of salts from upper layer of the soil into the groundwater table (Vinod et al. 2009; Ahamed et al. 2015). Value of the EC had been reported to rise with increase in TDS of groundwater (Adimalla and Venkatayogi 2018).
Alkalinity hazard (SAR)
High concentration of Na+ relative to Ca2+ and Mg2+ had been reported to reduce water supply to crop by decreasing soil permeability and the soil texture. SAR values in study the area were observed to be less than 10 in both seasons. So, the water samples collected in the both the seasons were under excellent category. Table 9 represents that the groundwater in study area was suitable for the irrigation purpose in the both the pre-monsoon and the post-monsoon season.
Sodium percentage (Na%)
It was reported that sodium reduces the permeability of soil by reacting with the ions (Ca2+ and Mg2+) present in the soil (Selvakumar et al. 2014). Na+ ion was absorbed by the clay particles and destroyed the structure of the soil (Singh et al. 2015). So, classification of the water on the basis of the sodium percentage is an important parameter to check its suitability for irrigation purposes.
Wilcox (1955) had classified the water samples into five classes on the basis of Na% to evaluate their suitability for the irrigation purpose (Table 9). After analyzing samples, it was observed that all the water samples collected during both the seasons were under good class and suitable for irrigation use.
Magnesium ratio (MR)
Groundwater was classified into suitable (>50%) and unsuitable class (<50%) based on MR value (Khodapanah et al. 2009). Mostly Mg2+ and Ca2+ are present in equilibrium concentration with respect to each other. But if Mg2+ content is higher in water then it was observed that soil becomes alkaline and adversely affected the crop yield (Nagaraju et al. 2014; Kumar et al. 2017). After analysis, it was noticed that all the 45 samples collected from study area during the pre-monsoon were found suitable but during the post-monsoon season, 42 samples fall in the suitable class and remaining 3 samples fall in the unsuitable class (Table 9). The high amounts of Mg2+ ion at few places were due to the high dissolution of dolomite mineral after monsoon as reported in earlier study (Singh et al. 2020).
Kelly’s ratio (KR)
The Kelly’s ratio more than 1 is an indication of alkali hazard (Karanth 1987; Bhat et al. 2018). It was observed that all the samples collected during both the seasons were in the suitable category (Table 9). So, according to classification based on KR, the water of study area was found to be suitable for irrigation. Further it was observed that presence of low sodium content was good for permeability and structure of the soil (Ramesh and Elango 2012; Patel and Vadodaria 2013).
Residual sodium carbonate (RSC)
The presence of RSC in water had resulted darkening of upper soil surface, burning of foliage which had resulted in reduce crop yield (Toumi et al. 2015). It was observed that out of total 45 samples, 29 samples were fit and 16 samples were marginal during the pre-monsoon while during the post-monsoon season, 23 samples were fit and 22 samples fell in marginal class (Table 9). Results have indicated that HCO3− content gets increased with respect to Ca2+ and Mg2+ after the rainy season which could be due to excessive leaching of HCO3− ions from upper layer to lower layers of soil. Nishanthiny et al. (2010) and Bhat et al. (2018) also had evinced similar results in their studies.
Corrosivity ratio (CR)
Water is considered safe if its CR value is less than 1 while the value more than 1 denotes corrosive nature of water as per Tripathi et al. 2012. The CR value indicated non-corrosive nature of all the 45 water samples collected during both the seasons as represented in Table 9. As a result of CR value, groundwater of this area is advised to be safely uplifted and transported by pipes.
US salinity diagram (USSL 1954)
US salinity diagram (USSL) was used to access the suitability of water samples for irrigation use (USSL 1954) in this studied area. It is based on EC and SAR values of water samples. On the basis of this diagram, groundwater was classified into: C1, C2, C3 and C4 types on the basis of salinity hazard (EC) and S1, S2, S3 and S4 types on the basis of sodium hazard (SAR) (Lokhande and Mujawar 2016). The groundwater data of study area were plotted using Grapher software (version: 16.2.354) which illustrates in Fig. 2 that out of total 45 samples collected in the pre-monsoon, 48.9% samples are C2S1 type and remaining 51.1% samples are C3S1 type having medium salinity—low sodium hazards and high salinity—low sodium hazards, respectively, while during the post-monsoon, all the samples were C3S1 type having high salinity—low sodium hazards. So, leaching of salts is required to eliminate salinity hazard so that all crops can be produced efficiently (Bhandari and Joshi 2013; Adimalla et al. 2018, 2020).
Wilcox diagram (Wilcox 1955)
The Wilcox (1955) had given a diagram to evaluate the suitability of water for irrigation purpose by classifying into various classes on the basis of values of EC and Na% (Singh et al. 2020). The samples had been classified into five classes: excellent to good, good to permissible, permissible to doubtful, doubtful to unsuitable and unsuitable. Diagrams software (version- 6.61) was used to plot the Wilcox diagram which illustrates that out of the total 45 samples collected during the pre-monsoon season, 71.1% samples are in excellent to good class and remaining 28.9% samples are in good to permissible class whereas, during the post-monsoon season, only 17.8% samples fall in excellent to good class and the remaining 82.2% samples are in good to permissible class as reported in earlier study (Singh et al. 2020). The results are represented in Fig. 3 illustrating that groundwater of the study area was good to be used for irrigation purposes during both the seasons.
Geochemical classification of groundwater (Piper diagram)
Piper (1944) had given trilinear diagram for geochemical analysis of groundwater. It has two equilateral triangle fields (i.e., cations, anions) and one central diamond-shaped field (Oki and Akana 2016). The major ion data collected was plotted in a Piper diagram using Grapher software (version- 16.4.432) which is represented in Fig. 4a, b. During both the seasons, cation triangle indicates, all the samples fall in no dominant type region but the anion triangle shows that HCO3− was dominant among anions and all the samples are in HCO3− type zone. The resultant diamond-shaped field has shown that all the samples were Ca–Mg–HCO3 type tributaries. Thus, there was no variation in geochemical faces in water samples during both the seasons and Kaur et al. (2017) had also observed similar results. The Ca2+ ion was present in the form of Kankar in this region while Mg2+ and HCO3− were supposed to be present due to the influence of Beas River (Purushothaman et al. 2013). High content of Ca2+ had been reported due to dissolution of agrochemicals during the monsoon (Singh et al. 2013). The main source of Mg2+ ion had been documented due to presence of dolomite. Further, HCO3− ion had been found in excess due to its leaching, bacterial oxidation of organic matter through aerobic and anaerobic complex chemical reactions and dissolution of CO32−-rich minerals (Sharma et al. 2017).
Conclusions
The interpretation of hydro-geochemical analysis of the study area in the proximity of tributaries of Beas River, Punjab has revealed that the quality of groundwater was good. The bulk of samples had lower values than permissible limits prescribed by WHO and BIS. Considering TDS as a parameter, the samples were under suitable class of drinking and irrigation purposes in all the studied locations. The abundance order of major cations was Ca2+>Mg2+>Na+>K+ and Ca2+> Na+>Mg2+>K+, respectively, in the pre-monsoon and the post-monsoon season while abundance of major anions was in the order of HCO3−> SO42−> Cl−> CO32− in both the seasons. Among heavy metals, Fe, As, Al and Se were found abundant in the samples having average concentration 0.30 mg/L, 0.19 μg/L, 0.02 mg/L and 0.78 μg/L, respectively, during the pre-monsoon and 0.33 mg/L, 0.25 μg/L, 0.03 mg/L and 0.84 μg/L, respectively, during the post-monsoon. Health risk assessment of Fe, As and Se show that their concentrations were below risk level in the study area. From this study, it was concluded that the groundwater of the study area was suitable for drinking and irrigation purposes. The continuous monitoring of water in this area is recommended to cope with the rising contamination of groundwater and adverse effects on human health.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adimalla N, Qian H (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, South India. Ecotoxicol Environ Saf 176:153–161. https://doi.org/10.1016/j.ecoenv.2019.03.066
Adimalla N, Qian H, Nandan MJ (2020) Groundwater chemistry integrating the pollution index of groundwater and evaluation of potential human health risk: a case study from hard rock terrain of South India. Ecotoxicol Environ Saf 206:1–10. https://doi.org/10.1016/j.ecoenv.2020.111217
Adimalla N, Venkatayogi S (2018) Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl Water Sci 8:1–14. https://doi.org/10.1007/s13201-018-0682-1
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process 5:1–21. https://doi.org/10.1007/s40710-018-0297-4
Agrawal A, Pandey RS, Sharma B (2010) Water pollution with special reference to pesticide contamination in India. J Water Resour Prot 2:432–448. https://doi.org/10.4236/jwarp.2010.25050
Ahamed AJ, Loganathan K, Jayakumar R (2015) Hydrochemical characteristics and quality assessment of groundwater in Amaravathi river basin of Karur district, Tamil Nadu South India. Sustain Water Resour Manag 1:273–291. https://doi.org/10.1007/s40899-015-0026-3
Ali SA, Ahmad A (2019) Analysing water-borne diseases susceptibility in Kolkata Municipal Corporation using WQI and GIS based Kriging interpolation. GeoJournal 85:1151–1174. https://doi.org/10.1007/s10708-019-10015-3
APHA (2017) Standard methods for the examination of water and waste waters, 23rd edn. American Public Health Association, Washington, DC
Baaij JHF, Hoenderop JGJ, Bindels RJM (2015) Magnesium in man: Implications for health and disease. Physiol Rev 95:1–46. https://doi.org/10.1152/physrev.00012.2014
Bajaj M, Eiche E, Neumann T, Winter J, Gallert C (2011) Hazardous concentrations of selenium in soil and groundwater in North-West India. J Hazard Mater 189:640–646. https://doi.org/10.1016/j.jhazmat.2011.01.086
Bhandari NS, Joshi HK (2013) Quality of spring water used for irrigation in the Almora district of Uttarakhand, India. Chin J Geochem 32:130–136. https://doi.org/10.1007/s11631-013-0615-5
Bhat MA, Wani SA, Singh VK, Sahoo J, Tomar D, Sanswal R (2018) An overview of the assessment of groundwater quality for irrigation. J Agri Sci Food Res 9:209–218
BIS (2012) Specification for drinking water. IS: 10500, pp 1–4. http://cgwb.gov.in/Documents/WQ-standards.pdf
Bondy SC, Campbell A (2017) Water quality and brain function. Int J Environ Res Public Health 15:1–15. https://doi.org/10.3390/ijerph15010002
CGWB (2017) Annual report: central ground water board. NH-IV, Bhujal Bhawan, Faridabad, Haryana, India. http://cgwb.gov.in/GW-Assessment/GWRA-2017-National-Compilation.pdf
Chen J, Wu H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, Northwest China. Expo Health 8:349–359. https://doi.org/10.1007/s12403-016-0208-8
Dev R, Bali M (2018) Evaluation of groundwater quality and its suitability for drinking and agricultural use in district Kangra of Himachal Pradesh India. J Saudi Soc Agric Sci 17:350–358. https://doi.org/10.1016/j.jssas.2018.03.002
Dhillon KS, Dhillon SK (2003) Quality of underground water and its contribution towards selenium enrichment of the soil-plant system for a seleniferous region of Northwest India. J Hydrol 272:120–130. https://doi.org/10.1016/S0022-1694(02)00259-7
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans. In: Karcioglu O (ed) Poisoning in the modern world–new tricks for an old dog. Intechopen, London, pp 1–23
Fowler BA, Selene CH, Chou J, Robert, Jones L, Dexter et al (2015) Chapter 28- Arsenic. In: Nordberg G, Fowler B and Nordberg M (ed) Handbook on the toxicology of metals (4th edn), pp 581–624.
Ganiyu SA, Badmus BS, Olurin OT, Ojekunle ZO (2018) Evaluation of seasonal variation of water quality using multivariate statistical analysis and irrigation parameter indices in Ajakanga area, Ibadan, Nigeria. Appl Water Sci 8:1–15. https://doi.org/10.1007/s13201-018-0677-y
Gholami S, Srikantaswamy S (2009) Analysis of agriculture impact on the Cauvery river water around KRS Dam. World Appl Sci J 6:1157–1169
Gupta R, Srivastava P, Khan AS, Kanaujia A (2018) Ground water pollution in India-a review. IJTAS 10:79–82
Hejaz B, Khatib IA, Mahmoud N (2020) Domestic groundwater quality in the Northern governorates of the West Bank, Palestine. Int J Environ Res Public Health 2020:1–6. https://doi.org/10.1155/2020/6894805
Hem JD (1989) Study and interpretation of chemical characteristics of natural waters book. 3rd edn, US geological survey water supply paper 2254, pp 26–32. https://pubs.usgs.gov/wsp/wsp2254/pdf/wsp2254a.pdf
Humbarde SV, Panaskar DB, Pawar RS (2014) Evaluation of the seasonal variation in the hydrogeochemical parameters and quality assessment of the groundwater in the proximity of Parli thermal power plant, Beed, Maharashtra, India. Adv Appl Sci Res 5:24–34
Jain N, Mujawar HU (2019) A research paper on contamination of water due to fluoride lead and arsenic and its removal techniques. IJITEE 8:160–169
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. https://doi.org/10.2478/intox-2014-0009
Jha MK, Shekhar A, Jenifer MA (2020) Assessing groundwater quality for drinking water supply using hybrid fuzzy-GIS-based water quality index. Water Res 179:1–16. https://doi.org/10.1016/j.watres.2020.115867
Kamble BS, Saxena PR (2016) Environmental impact of municipal dumpsite leachate on ground-water quality in Jawaharnagar, Rangareddy, Telangana, India. Appl Water Sci 7:3333–3343. https://doi.org/10.1007/s13201-016-0480-6
Karanth KR (1987) Ground water assessment development and management. Tata McGraw-Hill, Noida, p 720
Kaur T, Bhardwaj R, Arora S (2017) Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, Southwestern part of Punjab, India. Appl Water Sci 7:3301–3316. https://doi.org/10.1007/s13201-016-0476-2
Kelley WP (1946) Permissible composition and concentration of irrigation waters. Proceed Am Soc Civil Eng 66:607–613
Khodapanah N, Sulaiman WNA, Khodapanah N (2009) Ground water quality for different purpose in Eshtehard district of Tehran. Iran Eur J Sci Res 36:543–553
Koki IB, Bayero AS, Umar A, Yusuf S (2015) Health risk assessment of heavy metals in water, air, soil and fish. Afr J Pure Appl Chem 9:204–210. https://doi.org/10.5897/AJPAC2015.0654
Krishan G, Rao MS, Loyal RS, Lohani AK, Tuli NK, Takshi KS et al (2014) Groundwater level analyses of Punjab, India: a quantitative approach. Octa J Environ Res 2:221–226
Kshetrimayum KS, Hegeu H (2016) The state of toxicity and cause of elevated iron and manganese concentrations in surface water and groundwater around Naga Thrust of Assam-Arakan basin, Northeastern India. Environ Earth Sci 75:1–14. https://doi.org/10.1007/s12665-016-5372-4
Kumar PJS (2016) Influence of water level fluctuation on groundwater solute content in a tropic. Model Earth Syst Environ 2:1–9. https://doi.org/10.1007/s40808-016-0235-2
Kumar MD, Shah T (2006) Groundwater pollution and contamination in India: the emerging challenge. IWMI-TATA Water Policy Research Program, Vallabh Vidyanagar, Gujarat, India. https://publications.iwmi.org/pdf/H043376.pdf
Kumar R, Singh K, Singh B, Aulakh SS (2013) Mapping groundwater quality for irrigation in Punjab, North-West India, using geographical information system. Environ Earth Sci 70:187–191. https://doi.org/10.1007/s12665-013-2421-0
Kumar V, Sharma A, Chawla A, Bhardwaj R, Thukral AK (2016) Water quality assessment of river Beas, India, using multivariate and remote sensing techniques. Environ Monit Assess 188:1–10. https://doi.org/10.1007/s10661-016-5141-6
Kumar V, Sharma A, Thukral AK, Bhardwaj R (2017) Water quality of river Beas of India. Curr Sci 112:1138–1157
Lokhande PB, Mujawar HA (2016) Graphic interpretation and assessment of water quality in the Savitri River Basin. J Sci Eng Res 7:1113–1123
Magesh NS, Chandrasekar N (2013) Evaluation of spatial variations in groundwater quality by WQI and GIS technique: a case study of Virudunagar district, Tamil Nadu, India. Arab J Geosci 6:1883–1898. https://doi.org/10.1007/s12517-011-0496-z
Nagaraju A, Kumar KS, Ejaswi A (2014) Assessment of groundwater quality for irrigation: a case study from Bandalamottu lead mining area, Guntur district, Andhra Pradesh, South India. Appl Water Sci 4:385–396. https://doi.org/10.1007/s13201-014-0154-1
Naseem S, Hamza S, Bashir E (2010) Groundwater geochemistry of Winder agricultural farms, Balochistan, Pakistan and assessment for irrigation water quality. Eur Water 31:21–32
Nishanthiny SC, Thushyanthy M, Barathithasan T, Saravanan S (2010) Irrigation water quality based on hydro chemical analysis, Jaffna, Sri Lanka. Am Eurasian J Agric Environ Sci 7:100–102
Oki AO, Akana TS (2016) Quality assessment of groundwater in Yenagoa, Niger Delta, Nigeria. Geosci J 6:1–12. https://doi.org/10.5923/j.geo.20160601.01
Oyekanmi A, Okibe F, Dauda WP (2018) Toxic elements levels in water and some vegetable crops grown in farms in Bade Local Government area of Yobe state, Nigeria. Asian J Phys Chem Sci 6:1–12. https://doi.org/10.9734/AJOPACS/2018/39560
Palliwal KV (1972) Irrigation with saline water. In International conference of advance research and innovation, New Delhi. Monograph no. 2, pp 198.
Patel Y, Vadodaria GP (2013) Hydro-chemical analysis of groundwater quality for irrigation of Mehsana district, Gujarat state, India. Int J Sci Res 4:2319–7064
Pathak H, Pramanik P, Khanna M, Kumar A (2014) Climate change and water availability in Indian agriculture: impacts and adaptation. Indian J Agric Sci 84:671–679
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–923. https://doi.org/10.1029/TR025i006p00914
Purushothaman P, Rao MS, Kumar R, Rawat YS, Krishan G, Gupta S et al (2012) Drinking and irrigation water quality in Jalandhar and Kapurthala districts, Punjab, India: using hydrochemistry. Int J Earth Sci 5:1599–1608
Purushothaman P, Rao MS, Rawat YS, Krishan G, Kumar CP (2013) A study on surface water and groundwater interactions in Bist-Doab region, India. International conference on Integrated Water, Waste Water and Isotope Hydrology, 25–27 July 2013 at Bangalore University, Bangalore, India. pp 2–6
Ragunath HM (1987) Ground water, hydrogeology, ground water survey and pumping tests, rural water supply and irrigation systems. Wiley Eastern, New Delhi, India
Raman V (1985) Impact of corrosion in the conveyance and distribution of water. J Indian Water Work Assoc 11:115–121
Ramesh K, Elango L (2012) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. Environ Monit Assess 184:3887–3899. https://doi.org/10.1007/s10661-011-2231-3
Rao MS, Krishan G, Kumar CP, Purushothaman P, Kumar S (2017) Observing changes in groundwater resource using hydro-chemical and isotopic parameters: a case study from Bist Doab, Punjab. Environ Earth Sci 76:416–423. https://doi.org/10.1007/s12665-017-6492-1
Rawat KS, Singh SK, Gautam SK (2018) Assessment of groundwater quality for irrigation use: a peninsular case study. Appl Water Sci 8:1–24. https://doi.org/10.1007/s13201-018-0866-8
Richards RL (1954) Agriculture Hand Book No.60. U.S. Salinity Laboratory, U.S.D.A.
Rodriguez J, Mandalunis PM (2018) A review of metal exposure and its effects on bone health. J Toxicol 2018:1–11. https://doi.org/10.1155/2018/4854152
Rouabhia A, Baali F, Fehdi C, Boumezbeur A (2011) Hydrogeochemistry of groundwaters in a semi-arid region, El Ma El Abiod aquifer, Eastern Algeria. Arab J Geosci 4:973–982. https://doi.org/10.1007/s12517-010-0169-3
Saraswat C, Kumar P, Dasgupta R, Avtar R, Bhalani P (2019) Sustainability assessment of the groundwater quality in the Western India to achieve urban water security. Appl Water Sci 9:73–85. https://doi.org/10.1007/s13201-019-0956-2
Sawyer GN, McCartly DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw-Hill, New York
Scheelbeek PFD, Chowdhury MAH, Haines A, Alam DS, Hoque MA, Butler AP et al (2017) Drinking water salinity and raised blood pressure: evidence from a short study in coastal Bangladesh. Environ Health Perspect 125:1–40. https://doi.org/10.1289/EHP659
Selvakumar S, Ramkumar K, Chandrasekar N, Magesh NS, Kaliraj S (2014) Groundwater quality and its suitability for drinking and irrigational use in the Southern Tiruchirappalli district, Tamil Nadu, India. Appl Water Sci 7:411–420. https://doi.org/10.1007/s13201-014-0256-9
Setia R, Dhaliwal SS, Kumar V, Singh R, Kukal SS, Pateriya B (2020) Impact assessment of metal contamination in surface water of Sutlej River, India on human health risks. Environ Pollut 265:1–10. https://doi.org/10.1016/j.envpol.2020.114907
Sharma S, Bhattacharya A (2017) Drinking water contamination and treatment techniques. Appl Water Sci 7:1043–1067. https://doi.org/10.1007/s13201-016-0455-7
Sharma S, Chhipa RC (2016) Seasonal variations of ground water quality and its agglomerates by water quality index. GJESM 2:79–86. https://doi.org/10.7508/gjesm.2016.01.009
Sharma DA, Rishi MS, Keesari T (2017) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in Southwest Punjab, India using hydrochemical approach. Appl Water Sci 7:3137–3150. https://doi.org/10.1007/s13201-016-0456-6
Sharma S, Nagpal AK, Kaur I (2019) Appraisal of heavy metal contents in groundwater and associated health hazards posed to human population of Ropar wetland, Punjab, India and its environs. Chemosphere 227:179–190. https://doi.org/10.1016/j.chemosphere.2019.04.009
Sheen RT, Kahler HL, Ross EM, Betz WH, Betz LD (1935) Turbidimetric determination of sulphate in water. Ind Eng Chem Anal 7:262–265. https://doi.org/10.1021/ac50096a022
Singh K, Singh D, Hundal HS, Khurana MPS (2013) An appraisal of groundwater quality for drinking and irrigation purposes in Southern part of Bathinda district of Punjab, Northwest India. Environ Earth Sci 70:1841–1851. https://doi.org/10.1007/s12665-013-2272-8
Singh S, Bhardwaj A, Verma VK (2020) Remote sensing and GIS based analysis of temporal land use/land cover and water quality changes in Harike wetland ecosystem, Punjab, India. J Environ Manage 262:124–135. https://doi.org/10.1016/j.jenvman.2020.110355
Singh J, Singh Z, Kaur S, Sharma NG, Bath KS (2015) Physico-chemical analysis of drinking water and Hudiara drain water in Amritsar district, Punjab, India. Int J Curr Res Biosci Plant Biol 2:86–91
Srivastava A, Chahar V, Sharma V, Swain KK, Hoyler F, Murthy GS et al (2019) Study of toxic elements in river water and wetland using water hyacinth (Eichhornia crassipes) as pollution monitor. Global Chall 3:87–93. https://doi.org/10.1002/gch2.201800087
Subba Rao N, Marghade D, Dinakar A (2017) Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh. India Environ Earth Sci 76:747. https://doi.org/10.1007/s12665-017-7093-8
Talabi AO, Kayode TJ (2019) Groundwater pollution and remediation. J Water Resour Prot 11:1–19. https://doi.org/10.4236/jwarp
Taylor AA, Tsuji JS, Garry MR, McArdle ME, Goodfellow WL, Adams WJ, Menzie CA (2020) Critical review of exposure and effects, implications for setting regulatory health criteria for ingested copper. Environ Manage 65:131–159. https://doi.org/10.1007/s00267-019-01234-y
Thakur T, Rishi MS, Naik PK, Sharma P (2016) Elucidating hydrochemical properties of groundwater for drinking and agriculture in parts of Punjab, India. Environ Earth Sci 75:1–15. https://doi.org/10.1007/s12665-016-5306-1
Todd DK (1995) Ground water hydrology, 3rd edn. Wiley, New York, USA
Toumi N, Hussein BHM, Rafrafi S, Kassas NE (2015) Groundwater quality and hydrochemical properties of Al-Ula Region, Saudi Arabia. Environ Monit Assess 187:1–16. https://doi.org/10.1007/s10661-014-4241-4
Tripathi AK, Mishra UK, Mishra A, Dubey P (2012) Assessment of groundwater quality of Gurh Tehseel, Rewa district Madhya Pradesh, India. J Sci Eng Res 3:1–12
Trivedy RK, Goel PK, Trisal CL (1987) Practical methods in ecology and environmental science. Enviro Media Publications, Karad
USEPA (2004) Risk assessment guidance for superfund. Volume I, Human health evaluation manual (Part E).
Vetrimurugan E, Brindha K, Elango L, Ndwandwe OM (2017) Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl Water Sci 7:3267–3280. https://doi.org/10.1007/s13201-016-0472-6
Victor O, Chukwnemeka PIK, Oluchi NE (2018) Heavy metals contents and health risk assessment of classroom corner dusts in selected public primary schools in Rivers State, Nigeria. JEPHH 6:138–147
Vinod KG, Suthar S, Singh S, Sheoran A, Garima M, Jai S (2009) Drinking water quality in villages of Southwestern Haryana, India, assessing human health risks associated with hydrochemistry. Environ Geol 58:1329–1340. https://doi.org/10.1007/s00254-008-1636-y
Virk GS (2018) Selenium contamination of groundwater of Majha belt of Punjab, India. J Toxicol 8:1–7
Virk GS (2019) Groundwater contamination due to heavy metals and other pollutants in Amritsar district of Punjab. J Toxicol 9:19–28
WHO (2011) Guidelines for drinking water quality. World Health Organization, Geneva, p 340
Wilcox LV (1955) Classification and use of irrigation waters. U.S. Department of Agriculture, Washington, DC, pp 969.
Acknowledgements
Authors would like to thank Principal Khalsa College, Amritsar for providing necessary resources. Authors would also like to thank Dr. Kultar Singh from post-graduation department of chemistry for analytical determinations.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
JS performed the experiments, analyzed the data, prepared figures and/or tables and approved the final draft. SKS assisted in design the experiment, statistically analyzed the data, prepared figures and/or tables, prepared manuscript and approved the final draft. KS critically reviewed the drafts of the paper and approved the final draft. DS conceived and designed the experiments, authored or reviewed drafts of the paper.
Corresponding author
Ethics declarations
Conflict of interest
Author’s have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, J., Sehgal, S.K., Singh, K. et al. A hydrogeochemical approach to evaluate groundwater quality in the vicinity of three tributaries of the Beas River, North-West India. Appl Water Sci 12, 5 (2022). https://doi.org/10.1007/s13201-021-01541-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01541-y