Abstract
Fifty groundwater samples were obtained pre and post-monsoon seasons in parts of hard rock terrain in Andhra Pradesh, South India, in order to assess the drinking water quality. PIG values of groundwater samples ranged from 0.95–1.53 and 0.83–1.28 during pre and post-monsoon seasons. PIG values are slightly higher in the pre-monsoon season when compared to the post-monsoon season. In the pre monsoon season, 96% of the groundwater samples showed insignificant pollution class (< 1), 4% of the groundwater samples are low pollution (1–1.5). 82% of the groundwater samples showed insignificant pollution status (< 1), 18% of the groundwater samples fall under the low pollution (1–1.5), is noticed in post-monsoon season, respectively. WQI values of groundwater samples ranged from 108.5–204 mg/L and 112.6–170 mg/L during pre and post-monsoon seasons; its shows that 100% are very poor for drinking purpose. Piper diagram reveals that groundwater is majorly mixed Ca2+-Mg2+-Cl−, Ca2+-Mg2+-Cl−-SO42−, Na+-K+-Cl−- SO42− type in this region. The Gibbs plot indicates that groundwater samples fall within the field of rock dominance. Through applying GIS techniques, the spatial distribution of groundwater quality analysis reveals that most of the groundwater samples do not comply drinking water quality standards and water needs to be prior treatment before consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In this environment, water is important for the life of plants, animals and all living things. The quality of groundwater, especially shallow groundwater, is changing due to human activity (Adimalla and Venkatayogi 2018; Sunitha and Muralidhara Reddy 2014; Sunitha et al. 2014). Impairment of water quality is particularly alarming with anthropogenic interventions and climate change which increase health risk in many natural water bodies like rivers, lakes, coastal lagoons, etc. As a consequence, monitoring of water quality becomes crucial in terms of environmental protection and sustainability and anticipated potential environmental changes. Generally groundwater quality mainly depends up on two phenomenon: anthropogenic and geogenic activities. In view of anthropogenic activities, after the time of industrialization and the green revolution, the discharge of untreated effluents from industries and agricultural wastes which enters in the environment, disturbs the biological balance with the growth of technology (Sunitha et al. 2016; Subbarao 2018; Al-Hadithi 2012). Quality of groundwater also depends on various geogenic activities. Highly localized factors like topography and lithology effect the quality to vary within short distances in the area examined (Kadam et al. 2021a, 2021b; Gaikwad et al. 2021; Subba Rao et al. 2012; Raju et al. 2009; Nageswara Rao et al. 2019). The quantity is also subject to weathering, groundwater movement, individual ion content and ion exchange, environment and time variability in the recharge and discharge cycle. The geochemistry of waters is governed by the following factors: (i) the geochemistry of rocks and soils (ii) the semi-arid climate with abundant water in monsoon and scarcity of water in summer (iii) interchange among aquifers due to pressure differentials resulting from continuous withdrawal (iv) contamination of ground water by polluted surface water (v) direct entry of sewage water into wells of poor design and (vi) the extent of use of water. Rock or mineral composition is reflected by its elemental constituents in the form of major elements or trace elements (Karunanidhi et al. 2019). In the case of detrital rocks of sedimentary origin determination of grain size, fabric, roundness and sphericity of grains are resorted to decipher their genetic history, while non-detrital rocks like argillaceous and calcareous sedimentary rocks, chemical analysis is diagnostic value (Subba Rao 2018; Al-Omran et al. 2016). This may reflect the nature of the source material, conditions of transport and environmental conditions of deposition. Though the uniformity of chemical composition is expected over wide areas, because of the uniformity in the environmental conditions of deposition with low amplitude of fluctuation, the conditions are usually far from ideal and ever changing both in time and space affecting considerable change in the composition of different lithological units, either vertically or horizontally (Adams et al. 2001). Groundwater chemistry of a region is usually not homogeneous and it is driven by flow, geochemical processes, evaporation and evapotranspiration and possible sources of pollution (Sunitha et al. 2019; Sreedevi et al. 2018; Sunitha 2012b). Recognition of several related geochemical processes will aid to understand the causes of water quality changes due to contact with aquifer in particular in weathered rock formations. Hydrogeochemical processes can also help to prepare and maintain polluted sites in order to preserve aquifers that are contaminated by natural and anthropogenic phenomena. Therefore knowledge of geochemical processes that govern groundwater chemistry is therefore important for understanding groundwater quality issues (Sreedevi et al. 2019).
Metal ions are necessary for humans at low doses, but in excessive amounts, they are harmful or even cancerous. Many metallic ions can be found in the environment, and they are distinguished not only by their physicochemical forms, but also by their varying toxicities to living organisms. Water contamination caused by new developing toxins is becoming a source of concern around the world, with potentially disastrous environmental repercussions. New developing contaminants have been discovered in a number of water resources. Nano-particles, also known as next generation nano-adsorbents, are utilised to eliminate these pollutants (Imran Ali et al. 2005; Basheer 2017, 2018; Basheer and Ali 2018). However, the chemistry of groundwater is governed by geochemical processes that occur along the flow direction. It is vital to locate the geochemical reactions of the aquifer in order to have access to the distribution of the region's key ion chemistry. Groundwater quality is changing in India due to increased urbanisation, over-withdrawal of groundwater, excessive use of fertilisers, inappropriate waste disposal, geogenic reasons and other factors (Balamurugan et al. 2020).
The pollution index of groundwater (PIG) is an effective technique for evaluating the suitability of drinking water quality in any area and communicating the overall water quality information. In assessing water adaptability for various applications, the Water Quality Index (WQI) method is very useful and offers reliable information on water quality to ordinary citizens and decision-makers in order to monitor water quality. For example, WQI is an efficient tool that can be used to assess the suitability of drinking water quality in any area and to relay information on the overall quality of water. Horton (1965) originally developed WQI in the USA and was primarily used in Asia and Africa (Li et al. 2010; Prasanna et al. 2011). Different researchers are trying to set up a number of water quality metrics for groundwater quality assessment. The index choice is based on the input parameters for groundwater and the desired result (Sunitha et al. 2016; Suvarna et al. 2018; Sudharshan et al. 2020; Prasad et al. 2019; Reddy et al. 2019; Suvarna et al. 2019). It represents the overall water quality with indicator numbers and offers information on water quality with a single value (Aminiyan et al. 2018). The influence of various parameters of water quality is expressed and evaluated by WQI (Chaturvedi et al. 2010; Bouderbala 2017). These indexes are used for the applicability of human use by the most researchers. Therefore, on the basis of some physical and chemical data in the Anantapur area, attempts have been made to calculate WQI, which will provide a database that is very important for water management technology planning and development.
GIS is an important tool for storing huge quantities of data that can be spatially linked and retrieved to generate the necessary output for spatial analysis and integration. In the last few decades, scientists in different fields have developed the Geographic Information System for spatial investigation, study and integration (Burrough et al. 1998). GIS serves as a powerful tool for solving water resource concerns, evaluating water quality, deciding the availability of water, preventing flooding, understanding the natural environment and controlling local or regional water supplies (Tiandra et al. 2003). The main objective of the study is to assess the suitability of drinking water by measuring the methodology of the Water Quality Index (WQI) and the geographic information system (GIS) applications in order to understand the quality distribution, thus determining the concentration areas of high, medium and low chemical elements in this region. The present work also highlights the geochemical classification and hydrochemical processes of groundwater in Anantapur, Andhra Pradesh, parts of hard rock terrain.
Study area
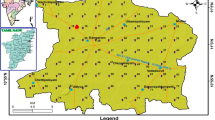
The present study area is located in the southeastern part of the district of Anantapur and forms part of the India Toposheet Survey Nos: 57 F/14, F/15, F/16, 57 J/3, J/4 and lies between the North latitudes 14°0′0″-14035′0″ and East longitudes 77°15′0″-78050′0″. Location map of the present study is depicted in Fig. 1. Prominent lithological formations noted in this area are Archean age peninsular gneisses consisting of pink granites, schists, Dharwar-age composite gneisses, few intrusion of the pegmatite dyke. Denudational hills, dissected pediments, pediplain and alluvium are dominant geomorphic units of this region (Sunitha et al. 2012a, b, c). Chitravathi River of fifth order of streams with dendritic drainage pattern flowing from South to North is noted in this area. There is a minimum temperature of 17 °C in January and a maximum temperature of 42 °C in May. Red soil dominates much of this area and black soil is noted in a few areas and is typically alkaline (with a soil pH of about 9). Black soil is observed in a few areas. This area has a semi-arid climate with a mean annual precipitation of 560 mm. Groundwater occurs in rocks that produce secondary porosity in weathered and eroded areas, such as granites, gneisses and Dharwarian schists. The depth of open wells varies from 6.0 to 25.0 m below ground level (bgl) and from 1.5 to 23 m bgl at water level (CGWB 2012).
Materials and methods
Sampling and analytical procedure
50 groundwater samples were collected from different villages in and around parts of hard rock terrain, Anantapur District, Andhra Pradesh, during pre-monsoon and post-monsoon of 2019 and 2018, respectively. Sampling points were located with GPS to ensure consistency. Samples were collected in pre-cleaned and well-dried polyethylene bottles at a low temperature (putting ice in the box at 4 °C) in the dark and were carried to the laboratory. The time between sampling and analysis was tried to be kept at minimum time. Immediately after sampling, pH, electrical conductivity (EC), and total dissolved solids (TDS) were determined in the field itself. pH and EC are measured by pH metre, conductivity metre, TDS by TDS metre (Raghunath 2003); titrimetric method was employed for determination of Total Hardness, Ca2+, Mg2+, HCO3− and Cl− while Na+ and K+ are measured by flame photometry (Model No.128; Systronic Company), SO42− and NO3− are measured by spectrophotometric method). Fˉ is measured by using ion selective electrode (Model: pH/ISE; Orion 4 star ion metre). All major chemical parameters were determined as per the standard procedures (APHA 2012; Hem 1985; Raghunath 1987) and comprehensive procedure is given in Table 1. All the parameters were analysed according to the bore well depth varies from 250 to 700 feet.
Analytical accuracy
In order to determine the analytical accuracy between the total cation concentration TZ (Na+, K+, Ca2+, Mg2+) and the total anion concentration (HCO3−, Cl−, SO42−, NO3− and F−) TZ denoted in milliequivalent per litre (meq/L) for each sample, ionic equilibrium error (IBE) was tested to ensure analytical accuracy by means of the following equation:
The estimated IBE value is within the ± 5 permissible limit (Domenico and Schwartz 1990).
Pollution index of groundwater (PIG) estimation:
Pollution index of ground water (PIG) was initially proposed by Subba Rao (2012). Pollution index measures the status of relative impact on individual water quality parameters. The index has been computed by considering the water quality variables, namely pH, EC, TDS, TH, Ca2+, Mg2+, Na+, K+, Cl−, HCO3−, SO42−, NO3−, F−.
The Pollution index of groundwater (PIG) was calculated by following successive steps (Subba Rao 2012,2018).
The first step is assigning weight
Individual chemical parameters have been given a weight (Rw) from 1 to 5 according as per relative importance of ground water as denoted in Table 2.
The second step is weight parameter
The weight parameter (Wp) is determined by the equation given below:
where Wp is the weight parameter, Rw is the weight of each constituent. Determined weight parameter (Wp) values of each constituent are shown in Table 2.
The third step is status of concentration
The status of concentration for each parameter is calculated by dividing the individual chemical variable concentration of each water sample by its corresponding drinking water quality standard (WHO 2011).
where Sc is the status of concentration, C is the chemical variable concentration of individual water sample and Ds is the standard drinking water level (WHO 2011) for individual chemical constituent.
The fourth step is overall chemical quality of water
Overall chemical quality of water (Ow) is calculated by multiplying the Wp with the Sc.
where Ow is the overall chemical quality of water, Wp is weight parameter, Sc is the status of concentration.
The fifth step is pollution index of ground water
The pollution index of ground water is calculated by sum of the overall chemical quality of water accounted due to all water quality measures of each water sample.
In assessment of PIG, the relative contribution of concentration of water quality variables of each water sample was taken into account. If the overall quality of water (Ow) is > 0.1, it contributes for 10% of the value of 1.0 of the PIG denoting the significance of pollution on the groundwater quality (Subba Rao 2012). The ground water quality is classified based on PIG classification, as (I) insignificant pollution, when PIG < 1; (II) low pollution, if it falls in between 1 to 1.5; (III) moderate pollution, if it is in between 1.5 to 2; (IV) moderate pollution, ranging from 2 to 2.5; (V) very high pollution, when PIG > 2.5 (Table 6).
Water Quality Index (WQI) estimation
Three consecutive phases consist of the WQI calculations. The first step is to “assign weight” by assigning a weight (wi) to each of the 13 parameters based on its relative importance to the quality of drinking water. By adopting the following equation, the second step is relative weight calculation.
The third stage is a "quality rating (qi)" measured using the equation below.
If Ci is the concentration of each parameter in each water sample, Si is the prescribed WHO value for each parameter (Kouadra and Demdoum 2020). In total, Wi and qi have been used to measure the SIi for individual parameters, so the following equations will determine WQI.
where SIi is represented as sub index of each parameter.
GIS analysis
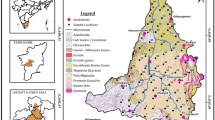
Spatial variation of groundwater quality based on GIS can be calculated with the Arc GIS 10.3 spatial analyst and geostatistical analyst modules after the geo database has been developed. This was achieved by the method of interpolation, such as weighted inverse distance (IDW). For spatially interpolating values, IDW is an algorithm that can be calculated between measurements. Every value represents weighted average of surrounding points. Computation of weights is performed by inverse distance method from location observation to the estimation point. In conjunction with original input data from all several deterministic interpolation procedures, the use of IDW with a squared distance has given good performance (Burrough et al. 1998; Mathes et al. 2006). A comprehensive collection of methods that can be used to imagine, interpret and determine spatial phenomena will be supported by the geostatistical analyst module. This includes prediction of spatial and structural surface analysis and evaluation of effects (Sunitha et al. 2012a, b, c; Reddy et al. 2015, 2020). The study area base map was prepared using Survey of India topographic sheets 57 F/14, F/15, F/16, 57 J/3, J/4 and digitised using Arc GIS 10.3 programme (Fig 1). The spatial analyst programme extension is used to interpolate the inverse distance weighted algorithm (IDW) as an effective tool to prepare spatial distribution maps of this region groundwater physicochemical parameters. For many purposes, the IDW method has been commonly used worldwide to construct spatial distribution charts, significantly distinguishing concentration zones of high, medium and low chemical elements.
Results and discussion
Hydrogeochemical analysis of the suitability of groundwater for drinking purposes
The results of the groundwater study and statistical data of groundwater samples collected during the pre- and post-monsoon seasons are present in Table 2, and the ion concentration is contrasted with that of the World Health Organization (WHO) and the Bureau of Indian Standards (BIS) as shown in Table 3.
pH
The recommended pH limit for drinking water is 6.5–8.5 (WHO 1990). pH in the study region varies from 7.2 to 8.8 during the pre-monsoon era and from 6.8 to 8.8 during the post-monsoon period. The most groundwater samples (92 percent) fall within the ideal limit (6.5–8.5), according to WHO (2004) guidelines, and only a few groundwater samples have a pH value below 7. Though pH does not have a direct effect on human health, all biochemical reactions are vulnerable to pH changes (Rao et al. 1993). The most groundwater has been found to be alkaline in nature, suggesting that bicarbonate is dominant over carbonate ions, influencing the pH of most water (Laar et al. 2011). The presence of hard water minerals and the release of agricultural waste water can lead to factors influencing alkalinity (Dinka 2014).
Electrical Conductivity (EC)
EC depends on the temperature and the ions current concentration and form (Hem 1991). 1,500 μmhos/cm is the optimal limit for EC in drinking water (WHO 2011). EC can be graded as type I if the salt concentration is low (EC < 1500 μS/cm). Type II is when the salt concentration is low (EC < 1500 and 3000 μS/cm); if the salt concentration is high (EC > 3000 μS/cm) is type III. According to the above conductivity description, Type I (low salt enrichment) accounted for 100% of the samples over the two seasons (Table 3). In this area, the EC value is within the permissible limit (1500 μS/cm).
Total dissolved solids (TDS)
TDS contains calcium, magnesium, potassium, sodium, bicarbonate, chloride and sulphate, primarily inorganic salts dissolved in groundwater (WHO 2011; Subba Rao et al. 2017; Adimalla et al. 2018). A general measure of the overall suitability of water for many purposes is the total concentration of dissolved minerals in water. In the region prior the TDS concentration was 262–429 mg/L during pre-monsoon, and after monsoon onset, the TDS concentration was 270–400 mg/L. Both samples are within the optimal limit of total dissolved solids (500 mg/L) before and after, according to WHO guidelines. TDS values of < 600 mg/L are commonly considered beneficial to human health, although WHO finds TDS values of > 1000 mg/L unpleasant (2011). The most groundwater is delectable, according to this classification. The groundwater of this area was graded in accordance with the United States Geological Survey (2000). 100 per cent of the samples were freshwater forms in both seasons (Table 4).
Total Hardness (TH)
In the region during pre-monsoon, the TH concentration was 360–710 mg/L, and during post-monsoon TDS concentration was 310–820 mg/L. As per WHO guidelines, all samples are within the ideal amount of total dissolved solids (500 mg/L) before and after the monsoon. The groundwater in this area has been listed according to the United States Geological Survey (2000). 100 per cent of the samples were freshwater type in both seasons (Table 4). Water hardness denotes the capacity of soap to neutralise water. High hardness can inevitably be due to industrial waste in this study area, which is attributed to the handling of untreated and poorly treated waste. Compared to the post-monsoon season, there is a greater shift in the hardness of the samples before the monsoon due to the leaching of calcium and magnesium bicarbonate in the replenishment (Ritesh Vijay et al. 2011).
Sodium (Na+)
In all natural waters, sodium is present in varying quantities and is pervasive. The pre-monsoon sodium concentration is 65–200 mg/L, and the post-monsoon sodium concentration is 65–180 mg/L. The recommended sodium level in potable water is 200 mg/L (WHO 1990; BIS 2012). Few samples have a greater concentration of sodium. 82 per cent of groundwater samples are within the permissible range before and after the monsoon. There is a higher sodium content in 12 per cent of the samples. Soil structure and permeability may be adversely affected by high sodium concentration, leading to alkaline soils. Water in contact with igneous rocks dissolves sodium by the deposition and decomposition of various minerals and the weathering of clay minerals from their natural sources (Abbas Abbasnia et al. 2018; Sunitha et al. 2019). Sodium and calcium interactions also increase the concentration of sodium in ions and other cations through agricultural waste, urban waste and runoff from distributed sources, sodium can also seep into natural water (Muralidhara Reddy et al. 2019).
Potassium (K+)
Potassium salts are more soluble and therefore the last to crystallise during evaporation than sodium salts (Karanth 1997). Both groundwater samples from this area are within the allowable potassium levels during the pre-monsoon and post-monsoon seasons. While K+ is an important essential nutrient when consumed in excess, laxative effects can occur (Alam et al. 2012). The occurrence of potassium in all water bodies is approximately one-tenth to one hundredth that of sodium, which may be due to its poor migratory ability and resistance to decomposition by weathering (Golditch 1938; Pradhan et al. 2011; Nikanorov et al. 2012).
Calcium (Ca2+) and magnesium (Mg2+)
Calcium is the fifth abundant natural element that is dissolved from soils, rocks and the essential component responsible for the hardness of water. Geological sources, agricultural waste and industrial waste could be used to generate calcium in drinking water (Deshpande 2011). In drinking water, the recommended calcium limit is 200 mg/L (WHO 1990). Ca2+ concentrations range from 30 to 74 mg/L in pre-monsoon and from 20 mg/L to 72 mg/L in post-monsoon. (Table 3). During the pre- and post-monsoon seasons, 12 percent of the groundwater in this area is above the allowable calcium limit. Calcium and magnesium are also important components of the bone and nervous system and also influence the metabolic operations of the body. This area was found to be deficient in calcium, as indicated for drinking purposes by BIS 2012. Osteoporosis, defective teeth, nephrolithiasis (kidney stone), rickets, hypertension and stroke, etc., can result from inadequate calcium intake (Faruqi 2002). The prime sources of magnesium (Mg2+) in the natural water are several rock types, sewage and industrial wastes (Deshpande 2011). Higher magnesium levels in drinking water can trigger unwanted drinking water tastes that cause laxative effects. Mg2+ values range from 30 to 90 mg/L in the pre-monsoon season and from 28 to 90 mg/L in the post-monsoon period. For drinking purposes, the necessary permissible limit of magnesium in groundwater is 150 mg/L (WHO 2011). The bulk of the groundwater is below the allowable magnesium limit.
Bicarbonate (HCO3 −)
Bicarbonate concentration varies from 65 to 130 mg/L during pre-monsoon and post-monsoon (Table 3). The acceptable limit of 300 mg/L of bicarbonate in drinking water is (WHO 1990; Table 3). During the pre- and post-monsoon seasons, 66% of the groundwater in the study region is above the allowable limit. Most of groundwater is below the allowable limit during the pre- and post-monsoon seasons. Major water samples in that area do not contain carbonate ions.
Sulphate (SO4 2−) and chloride (Cl−)
Groundwater sample sulphate concentrations range from 70 to 240 mg/L during the pre-monsoon period (Table 3) and from 70 to 180 mg/L during the post-monsoon period (Table 3). Sulphate occurs naturally in water, namely gypsum and other common minerals, due to leaching from nearby rock bodies, and can also be applied to water by adding fertilisers (Hem 1970). The normal sulphate concentration limit for drinking water is 200 mg/L (WHO 1984). Most of the samples, except for a few samples deviating from the appropriate limit, are below the desirable limit. The possible sources of sulphate in rocks are sulphur minerals, sulphides of heavy metals which are of common occurrence in the igneous and metamorphic rocks, gypsum and anhydrite found in some sedimentary rocks, input from volcanoes and biochemical processes; human economic activities (Hem 1970; (Nikanorov et al. 2012; Herojeet et al. 2013). However, sulphates can be added by the application of fertilisers, apart from these natural sources (Karanth 1997). In the pre-monsoon season, higher sulphate concentrations may be due to the action of leaching and anthropogenic activities in the atmosphere through the release of sulphur gases from factories and urban utilities (Saxena 2004). Over the allowable limits, 58% of groundwater samples contain sulphate. Chloride values range from 90 to 300 mg/L during the pre-monsoon period and 50–110 mg/L during the post-monsoon period. As per WHO (2011) Cl− has standard limit of 200 mg/L (WHO 1990; BIS 2012). Nearly 26% of the groundwater chloride concentration in the study region during the pre-monsoon and 24% during the post-monsoon area is above the permissible level. Higher chloride concentration in certain areas can be derived from different sources such as weathering, rock, soil leaching, domestic, urban, industrial effluents, dry environment (Sarath Prashanth et al. 2012; Subba Rao et al. 2017). Residual water in pores of granites may contain chloride (Shand 1952). The high concentration of chloride gives a salty taste to water, which can cause physiological harm. Usually, water with a high chloride content has an unpleasant taste and may be dangerous for certain agricultural purposes. When ingested in higher concentrations, higher chlorides cause laxative effects in humans (Sunitha et al. 2019).
Fluoride (F−) and nitrate (NO3 −)
In this area, fluoride concentrations range from 1.2 to 5.9 mg/L during the pre-monsoon season to 0.9 to 5.6 mg/L during the post-monsoon season (Table 3). Fluoride in the pre-monsoon season are higher than those in the post-monsoon season. According to WHO (2011) guidelines, 84% and 80% of the samples exceeded the allowable fluoride level (1.5 mg/L) during the pre and post-monsoon seasons, respectively (Table 2). Fluoride reduces dental caries and promotes enamel production at 0.8–1.0 mg/L in children under 8 years of age (Sunitha et al. 2018). Dental mottling, an early indication of dental fluorosis characterised by opaque white patches on the teeth, may result in ingestion of water with a fluoride concentration above 1.5–2.0 mg/L. In advanced phases of dental fluorosis, teeth exhibit brown to black staining, followed by pitting of teeth surfaces. Dental fluorosis has led severe tooth decay and considerable physiological stress for affected people. In children up to the age of 12 years, high manifestations of dental fluorosis are often observed. In different parts of the Aantapur District, dental fluorosis is clearly seen (Sunitha et al. 2008,2012,2018). In this region high fluoride concentration > 3.0 mg/L causing skeletal fluorosis is clearly observed at Ralla ananthapuram village. The dissolution of fluoride bearing minerals is often significantly influenced by the concentration of fluoride in groundwater due to various sources such as rock-water interaction, alkaline nature of water usually low calcium, and high magnesium and bicarbonate. There are three primary sources of fluoride, such as fluorospar or calcium fluoride (CaF2), apatite or rock phosphate (Ca3F (PO4)3) and cryolite (Na3AlF6) (Sunitha et al. 2012a). Groundwater fluoride is typically connected to the broken hard rock zone with pegmatite veins (Ramesam et al. 1985). The primary source of fluoride in groundwater in this area is due to the weathering of granite rocks (Reddy et al. 2019). Due to the potential effects of groundwater use on human health, nitrate accumulation in drinking water is of particular concern. The nitrate concentration of groundwater samples ranges from 36 to 92 mg/L during the pre-monsoon period and from 40 to 89 mg/L during the post-monsoon period in this area. The upper nitrate concentration level in drinking water is stated as 45 mg/L (WHO 1984). 80% of all the nitrogen is added together to the environment through Agriculture and livestock production. Paddy cultivation alone results in 40 percent of India’s fertilizers and almost 50 percent of the nitrogen fertilizers used are washed out and escaped into the atmosphere (Jalali 2011; Sunitha et al. 2012a, b, c). It is noted from the study area that 40% of the groundwater samples during pre- and post-monsoon seasons has desired limit of nitrate concentration and 60% have concentrations above the desired limit of nitrate as per WHO standards during both seasons. The use of fertilizers dependent on nitrogen such as NPK (Nitrogen, Phosphorus and Potassium), improper disposal of human and animal waste, unlined drainage and drainage lines can also result in contamination of nitrate groundwater. Nearly 80% of all the nitrogen added to the atmosphere accounts for the development of agriculture and livestock. Agricultural practices, septic tank leakage, unlined drainage and sewerage pipes, domestic sewage, leaching from indiscriminate disposal of animal waste can result in higher nitrate concentrations (Reddy et al. 2013, Datta et al. 1996; Sunitha et al. 2012a, b, c). The majority of people in this area depend for their livelihood on farming practices, the use of fertilizers for crop yields may be the main contributing factor for high nitrate concentrations. Higher nitrate concentrations in groundwater in this region may also be attributable to the disposal of poultry waste and household/farm animal dung and mainly fertilizer bags that are washed by infiltration and return flow irrigation and drinking practices for entering groundwater in addition to agricultural activities (Ako et al. 2014; Narasimha et al. 2018b; Sunitha et al. 2012a, b, c).
Pollution index of groundwater (PIG)
PIG values of groundwater samples ranged from 0.95–1.53 with an average of 1.19 in pre-monsoon season, whereas PIG values varied from 0.83–1.28 and a mean value of 1.09 in post-monsoon season (Table 5). PIG values are slightly higher in the pre-monsoon season when compared to the post-monsoon season. In the pre-monsoon season, 96% of the groundwater samples showed insignificant pollution class (< 1), 4% of the groundwater samples are low pollution (1–1.5). 82% of the groundwater samples showed insignificant pollution status (< 1), 18% of the groundwater samples fall under the low pollution (1–1.5), is noticed in post monsoon season, respectively (Table 6). This shows a gradual increase in pollution from its low pollution range to very high pollution range by a combination of Ow values of various concentrations of water quality measures. For reference, low pollution zone is chiefly by EC, Mg2+, TDS, HCO3–, TH, Ca2+, which are denoted by higher Ow values, > 0.1 and other parameters pH, Na+, K+, SO42−, Cl−, NO3−, F− their Ow values are less than 0.1 in both the seasons. The TH (0.15), Mg (0.22), NO (0.16) and F(0.14) show the values of Ow more than 0.1 in the insignificant pollution zone, while the pH (0.09), EC (0.08), Na+ (0.03), K+ (0.03), HCO3− (0.04), Cl− (0.08) and SO42− (0.10) have the values of Ow less than 0.1. Thus, they obviously indicate the influence of anthropogenic source rather than the geogenic origin on the groundwater system. In order to verify the role of geogenic and anthropogenic origins as sources of dissolved salts on the aquifer system, it is imperative to consider the difference in the values of Ow between the insignificant pollution zone and the low pollution zone. From Table 6, it is significant to note that there is no much difference in the values of Ow in the cases of all parameters between the insignificant pollution zone and the low pollution zone during the both seasons. This difference could be due to variation in the source of pollution in the groundwater system.
Water Quality Index in groundwater (WQI)
The index of water quality is a rating that reflects the cumulative effect of the parameters of water quality. The Quality Index (WQI) for drinking water is used to assess the quality of groundwater for drinking purposes. The index was defined by assigning weights (w) to water quality parameters (a) on the basis of their perceived threat to water quality. This is achieved by transforming the concentrations of the constituents into a single value that reflects the combined effect of the parameters of water quality. The relative weight water quality index system is used to assess groundwater suitability for drinking purposes in parts of the Anantapur area of Southern India. The choice of physicochemical index calculation parameters depends on a variety of factors, such as the importance of the parameter and the purpose of the index system (Drinking or Irrigation) (Aminiyan et al. 2018).
WQI was given as Class I: Good, Class II: Poor, Class III: Very poor, and Class IV: Unsuitable for drinking purposes. The overall assessment of WQI values (108.5–204 mg/L: pre-monsoon season) (112.6–170 mg/L: post-monsoon season) indicates that 98 percent are poor, and 2 percent of groundwater samples are very poor for drinking in the pre-monsoon season and 100 percent of samples in the post-monsoon season are very poor (Tables 7 and 8). Extensive irrigation practices and extensive groundwater extraction can be attributed to higher WQI in this area, and geogenic activities such as rock weathering, mineral dissolution and even anthropogenic practices are responsible for high WQI values in this region (Sudharshan Reddy et al. 2020a, b).
Spatial distribution of physicochemical parameters
Geographical information system (GIS), together with the hydrogeochemical analysis enables the delineation of groundwater quality applicability for various uses (Narsimha et al. 2013, b; Panaskar et al. 2016; Amiri et al. 2014; Sappa et al. 2015; Sakram and Narsimha 2018). For different physicochemical parameters such as pH, electrical conductivity (EC), total dissolved solids (TDS), total hardness (TH), calcium (Ca2+), magnesium (Mg2+), sodium (Na+) and potassium (K+), bicarbonate (HCO3−), chloride (Cl−), sulphate (SO42−), fluoride (F−), spatial distribution maps are prepared in order to delineate safe and unsafe areas. Spatial maps of the physicochemical parameter distribution is shown in the Fig. 2A(from a to l) & 2B (m to z). The spatial maps of pH distribution in groundwater is shown in Fig. 2A(a & b). It is evident from these maps that groundwater is alkaline in nature. Anthropogenic practices, such as insufficient waste disposal and excess use of nitrogen fertilizers, may contribute to pH changes (Sarath Prasanth et al. 2012). The Electrical conductivity (EC) spatial variation map was prepared and shown in Fig. 2A(c & d). From these maps, it is concluded that EC values in most of the sampling locations were below (1500 μS/cm). In addition, it is also clear that the EC values (1500 μS/cm) were below the permissible maximum. Moreover it is also clear that EC values were below the permissible limit (1500 μS/cm). Soluble salts and nature of rock formation accounts for higher EC (Trivedi and Goel. 1984). High TDS values during the pre-monsoon season were noted in the southern part of this region and lower TDS concentrations were observed during the post-monsoon season in the northern part of the study area, Fig. 2A(e & f). Higher TDS in groundwater can be extracted from leaching during the pre-monsoon season by recharging salts from the ground surface; contact between rock and water; increased agricultural activities (Ballukraya and Ravi 1999; Vijay et al. 2011). Higher TDS applies to excessive treatment of waste and mineral dissolution. Greater TH values were observed from the south-eastern portion of the study area, while low TH values were observed from the north-western portion of the study area, as shown in Fig. 2A(g & h). Groundwater hardness is due to the presence of salts such as CaSO4, MgSO4, CaCl2 and MgCl2 that are removed by the ion exchange process (Nag 2014). Total hardness (TH) is mainly due to the presence of dissolved calcium and magnesium ions, although its concentration contains all other divalent cations (Ikomi and Emuh 2000). The spatial distribution of sodium (Fig. 2A(i & j) shows a higher concentration during the pre- and post-monsoon seasons in the northeastern part of the study region and a low concentration in the southern part of the study area. The exchange of cations and anthropogenic behaviours can be caused by higher Na+. Moreover, due to inadequate domestic sewage, leaching of sodium-rich rocks from industrial and agricultural sites may result in higher sodium concentrations. While higher sodium levels may not be harmful, hypertension, heart failure and problems with kidney stones are often found (Nag and Suchetana 2016). Spatial distribution of potassium (K+) during the pre- and post-monsoon season shows high concentrations in the southeastern portion of the study region and low concentrations in the northern, eastern and western portions of the study area Fig. 2A(k & l). As shown in the maps of spatial distribution, Fig. 2B(m & n) of the pre- and post-monsoon seasons signifies a strong calcium concentration in the southeastern part of the study region and a low calcium concentration in the northern part of the study area. Some southern component packets show a high magnesium concentration and some northern, eastern and western parts of this area show a low concentration of magnesium Fig. 2B(o & p). During pre and post-monsoon seasons, bicarbonate concentration in this area is within the prescribed amount Fig. 2B(q & r). Maps of Sulphate Spatial Distribution Fig. 2B(s & t) during both pre- and post-monsoon, indicates that sulphate is higher than the permissible limits in the southeastern part of the study region.
Higher Cl− values from the southeastern portion of the study area were observed, and low Cl− values from the northwestern portion of the study area were observed, as shown in the spatial distribution maps Fig. 2B(u & v). Distribution maps of nitrate during pre- and post-monsoon seasons were shown in Fig. 2B(w & x). Nitrate of groundwater sample in the pre-monsoon season are higher than the post-monsoon season. The spatial nitrate distribution maps show that higher concentrations are primarily observed during the pre- and post-monsoon seasons in the northeastern part of the study region, which may be due to increased agricultural practices and improper methods of sewage and animal disposal. These maps show that in the southern part of the study area, the greatest concentration is observed (Muralidhara Reddy et al. 2016, 2019). In addition, it is surprising to note that higher concentrations are mostly found as isolated patches in the northeastern portion of this region due to geogenic activities (weathering, mineralization) and small pollution patches are noticed in the northeastern portion of the study area due to anthropogenic behaviour.
Hydrogeochemical facies and water types
The pictorial method of the trilinear diagram of Piper (Piper 1953) is significant for the identification of groundwater based on the constituent ionic concentrations in the basic geochemical characters of groundwater (Fig 3). Most of the natural water is composed of three cationic constituents of magnesium, calcium, sodium and three anionic constituents sulphate, chloride and bicarbonate. Several scientists have identified different types of trilinear plotting. A trilinear diagram of two triangles, one for cations and one for anions and the upper field in the form of a diamond (Piper 1953). The cationic composition is defined by a point plotted on the basis of a percentage of three present in the cation triangle. The anionic composition is expressed in the anion triangle by the location of a similar point. Each point is then projected along the line parallel to the upper margin of the field into the upper filed, and water characteristics are shown by the point where the extension intersects. There are six forms of groundwater in this area based on the Piper diagram: 1) Ca2+-HCO3− 2) Na+-Cl− 3) Mixed Ca2+- Na+-HCO3− 4) Mixed Ca2+-Mg2+-Cl− 5) Ca2+-Cl− 6) Na+-HCO3−. Groundwater in this area is of the majorly calcium-magnesium-chloride form (Mixed Ca2+-Mg2+-Cl−) and Ca2+-Mg2+-Cl−-SO42−, Na+-K+-Cl−SO42− form. The Piper diagram (Fig. 3) also indicates, however, that magnesium and sodium are predominant in groundwater among cations and concentrations of bicarbonate and chloride among anions dominate in groundwater during the pre- and post-monsoon seasons.
Geochemical evaluation (Gibbs diagram)
Gibbs (1970) has proposed for anions (Cl−)/(Cl− + HCO3−) and cations (Na+ + K+)/ (Na+ + K+ + Ca2+) of the groundwater samples were plotted separately against TDS (Fig. 4). Three kinds of different fields controlling groundwater chemistry are shown in the Gibbs diagram. These are the dominance of evaporation, the dominance of precipitation and rock dominance. The most groundwater samples fall during the pre- and post-monsoon seasons.
Mechanism controlling groundwater chemistry plots (Gibbs, 1970) a Cl−/Cl− + HCO3− b (Na+ + K+) / (N+ + K+ + Ca2+) (a & b—pre-monsoon, c & d—post-monsoon)
Conclusions
This work was primarily intended to use PIG, WQI & GIS to evaluate evaluations of groundwater quality for drinking purposes. Groundwater is known to be alkaline and durable. The chemical analysis results were compared to the WHO and BIS requirements.
-
PIG values are slightly higher in the pre-monsoon season when compared to the post-monsoon season. 96% of the groundwater samples showed Insignificant pollution class (< 1), 4% of the groundwater samples are low pollution (1–1.5) in pre monsoon season. 82% of the groundwater samples showed Insignificant pollution status (< 1), 18% of the groundwater samples fall under the low pollution (1–1.5), is noticed in post-monsoon season. This shows a gradual increase in pollution from its low pollution range to very high pollution range by a combination of Ow values of various concentrations of water quality measures.
-
Based on WQI 98% of groundwater samples are poor, and 2% of groundwater samples are very poor for drinking in the pre-monsoon season and 100 percent of samples are very poor in the post-monsoon season.
-
The Piper trilinear geochemical classification diagram indicates that groundwater in this area is of the Ca2+-Mg2+-Cl−, Ca2+-Mg2+-Cl−-SO42−, Na+-K+-Cl−-SO42−. Chloride is dominant in groundwater among cations, magnesium, sodium are the main constituents, and among anions Sulphate, bicarbonate. As per the Gibbs diagram, groundwater samples come under the field of rock dominance.
-
In this area, the spatial distribution of groundwater quality analysis using the GIS technique suggests that the most groundwater samples do not meet the quality requirements for drinking water. The present status of groundwater denotes continuous monitoring and proper strategies for implementation.
References
Abasnia A, Yousefi N, Mahvi AH, Nabizadeh R, Radfard M, Yousefi M, Alimohammadi M (2018) Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: case study of Sistan and Baluchistan province (Iran). Hum Ecol Risk Assess: an Int J. https://doi.org/10.1080/10807039.2018.1458596
Adams SS, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in Western Karoo, South Africa. J Hydrol 241:91–103
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process. https://doi.org/10.1007/s40710-018-0297-4
Ako AA, Eyong GET, Shimada J (2014) Nitrate contamination of groundwater in two areas of the Cameroon Volcanic Line (Banana Plain and Mount Cameroon area). Appl Water Sci 4:99–113
Alam M, Rais S, Aslam M (2012) Hydrochemical investigation and quality assessment of ground water in rural areas of Delhi India. Environ Earth Sci 66:97–100
Al-Hadithi M (2012) Application of Water Quality Index to Assess Suitability of Groundwater Quality for Drinking Purposes in Ratmao-Pathri Rao Watershed, Haridwar District, India. Am J Sci Ind Res 10:5251
Ali I, Gupta VK, Aboul-Enein HY (2005) Metal ion speciation and capillary electrophoresis: application in the new millennium. Electrophoresis 26:3988–4002
Al-Omran AM, Aly AA, Al-Wabel MI, Sallam AS, Al-Shayaa MS (2016) Hydrochemical characterization of groundwater under agricultural land in arid environment: a case study of Al-Kharj, Saudi Arabia. Arab J Geosci 9:68
Aminiyan MM, Aitkenhead-Peterson J, Aminiyan FM (2018) Evaluation of multiple water quality indices for drinking and irrigation purposes for the Karoon river. Iran Environ Geochem Health. https://doi.org/10.1007/s10653-018-0135-7
Amiri V, Rezaei M, Sohrabi N (2014) Groundwater quality assessment using entropy weighted water quality index (EWQI) in Lenjanat. Iran Environ Earth Sci 72:3479–3490
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, New York
Balamurugan P, Kumar PS, Shankar K (2020) Dataset on the suitability of groundwater for drinking and irrigation purposes in the Sarabanga River region, Tamil Nadu. India Data in Brief 29:105255. https://doi.org/10.1016/j.dib.2020.105255
Basheer AA (2017) Chemical chiral pollution: Impact on the society and science and need of the regulations in the 21st century. Chirality. https://doi.org/10.1002/chir.22808
Basheer AA (2018) New generation nano-adsorbents for the removal of emerging contaminants in water. Molliq. https://doi.org/10.1016/j.molliq.2018.04.021
Basheer AA, Ali I (2018) Stereoselective uptake and degradation of (±)-o, p-DDD pesticide stereomers in water-sediment system. Chirality. https://doi.org/10.1002/chir.22989
BIS (2012). Indian standard drinking water specifications IS 10500:2012. Bureau of Indian Standards, New Delhi
Bouderbala A (2017). Assessment of water quality index for the groundwater in the Upper Cheliff Plain, Algeria. J Geol Soc India 90:347–356. https ://doi.org/https://doi.org/10.1007/s1259 4–017–0723–7
Bullukraya PN, Ravi R (1999) Characterization of groundwater in the unconfined aquifers of Chennai City, India. Journal Geological Society of India 54:13–22
Burrough PA, McDonnell RA (1998) Principles of geographical information systems. Oxford University, New York
Central Groundwater Board (CGWB) (2012). Groundwater brochure Anantapur district, Andhra Pradesh. http://www.cgwb.gov.in/District_Profile/AP/ Anantapur-2012.pdf
Chaturvedi MK, Bassin JK (2010) Assessing the water quality index of water treatment plant and bore wells, in Delhi India. Environ Monit Assess 163:449–453. https://doi.org/10.1007/s10661-009-0848-2
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
Deshpande SM, Aher KR (2011) Quality of Groundwater from Tribakeswar-Peth area of Nashik District and its Suitability for Domestic and Irrigation Purpose. Gondwana Geological Magazine 26(2):157–162
Dinka MO, Loisjkandl W, Ndambuki JM (2014) Hydrochemical modelling for Lake Basaka: development and application of a conceptual water budget model. Environ Monit Assess 186:5363–5379
Domenico PA, Schwartz FW (1990) Physical and Chemical Hydrogeology. Wiley, New York p, p 824
Faruqi NH (2002) Final Report on Geoenvironmental Appraisal of Unnao District, Uttar Pradesh, Geological Survey of India, Northern Region, Lucknow, 226024
Gaikwad S, Pawar NJ, Bedse P, Wagh V, Kadam A (2021) Delineation of groundwater potential zones using vertical electrical sounding (VES) in a complex bedrock geological setting of the West Coast of India. Model Earth Sci Sys. https://doi.org/10.1007/s40808-021-01223-3
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Golditch SS (1938) A study in rocks weathering. J Geol 46:17
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water, 3rd edn. Scientific Publishers, Jodhpur, p 2254
Hem JD (1970). Study and Interpretation of the Chemical Characteristics of Natural Water. Second Edition - Geological Survey Water Supply Paper 1473 - United States Government Printing Office Washington
Hem JD (1985). The study and interpretation of the chemical characteristics of natural water, 3rd edition, U.S. Geological Survey Water-Supply Paper 2254, University of Virginia, Charlottesville 263 p
Herojeet RK, Rishi MS, Sidhu N (2013) Hydrochemical Characterization, Classification and Evaluation of Groundwater Regimein Sirsa Watershed, Nalagarh Valley, Himachal Pradesh India. Civil Environ Res 3(7):47–57
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37:300–306
Ikomi RB, Emuh CT (2000) The status of the physico-chemical hydrology of upper Warri river Nigeria. J Sci Environ 2:75–86
Jalali M (2011) Nitrate pollution of groundwater in Toyserkan, western Iran. Environ Earth Sci 62:907–913. https://doi.org/10.1007/s12665-010-0576-5
Kadam A, Wagh V, Patil S, Umrikar B, Sankhua R (2021a) Seasonal assessment of groundwater contamination, health risk and chemometric investigation for a hard rock terrain of western India. Environ Earth Sci 80:172. https://doi.org/10.1007/s12665-021-09414-y
Kadam A, Wagh V, Jacobs J, Patil S, Pawar N, Umrikar B, Sankhua R, Kumar S (2021b) Integrated approach for the evaluation of groundwater quality through hydro geochemistry and human health risk from Shivganga river basin Pune, Maharashtra, India. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15554-2
Karanth KR (1997) Groundwater Assessment, Development and Management. Tata McGraw-Hill Publishing Company Limited, New Delhi, pp 720
Karunaidhi D, Aravinthaswamy P, Subramani T, Jianhu W, Srinivasamoorthy K (2019) Potential health risk assessment for fluoride and nitrate contamination in hard rock aquifers of Shanmuganadhi River basin, South India. Hum Health Risk Assess an Int J. https://doi.org/10.1080/10807039.2019.1568859
Kouadra R, Demdoum A (2020) Hydrogeochemical characteristics of groundwater and quality assessment for the purposes of drinking and irrigation in Bougaa area Northeastern Algeria. Acta Geochim. https://doi.org/10.1007/s11631-019-00393-3
Laar C, Akiti TT, Brimah AK, Fianko JR, Osae S, Osei J (2011) Hydrochemistry and isotopic composition of the Sakumo Ramsar Site. Res J Environ Earth Sci 3(2):146–152
Li P, Qian H, Wu J (2010) Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia. Northwest China E-J Chem 7:209–216
Mathes ES, Rasmussen TC (2006) Combining multivariate statistical analysis with Geographic Information Systems mapping: a tool for delineating groundwater contamination. Hydrogeology. https://doi.org/10.1007/S10040-006-0041-4
Muralidhara Reddy B, Sunitha V (2020) Geochemical and health risk assessment of fluoride and nitrate toxicity in semi-arid region of Anantapur District South India. Environ Chem Ecotoxicol 2:150–161. https://doi.org/10.1016/j.enceco.2020.09.002
Muralidhara Reddy B, Sunitha V, Ramakrishna Reddy M (2013) Fluoride and nitrate geochemistry of groundwater from Kadiri, Mudigubba and Nallamada Mandals of Anantapur District, Andhra Pradesh India. J Agricul Eng Biotech 1(2):37–42
Muralidhara Reddy B, Sunitha V, Ramakrishna Reddy M (2015) Geospatial analysis of fluoride contamination in groundwater of southeastern Part of Anantapur District, Andhra Pradesh. In: Raju N, Gossel W, Sudhakar M (eds) Management of natural resources in a changing environment. Springer, Cham
Muralidhara Reddy B, Sunitha V, Ramakrishna Reddy M (2016) Prevalence of dental fluorosis in southeastern part of Anantapur District, Andhra Pradesh. J Chem Pharm Res 8(4):179–185
Muralidhara Reddy B, Sunitha V, Prasad M, Sudarshan Reddy Y, Ramakrishna Reddy M (2019) Evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Anantapur, Andhra Pradesh State, South India. Groundwater Sustain Develop 9:100262. https://doi.org/10.1016/j.gsd.2019.100262
Nag SK (2014) Evaluation of hydrochemical parameters and quality assessment of the groundwater in Gangajalghati Block, Bankura District, West Bengal, India. Arab J Sci Eng 39:5715–5727
Nag SK, Suchetana B (2016) Groundwater quality and its suitability for irrigation and domestic purposes: a case study in Rajnagar block, Birbhum district, West Bengal India. J Earth Sci Clim Change 7(2):337
Nageswara Rao K, Swarna Latha P (2019) Groundwater quality assessment using water quality index with a special focus on vulnerable tribal region of Eastern ghats hard rock terrain. Southern India Ara J Geosci 12:267
Narasimha A, Venkatayogi S (2018) Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl Water Sci 8:44. https://doi.org/10.1007/s13201-018-0682-1
Narsimha A, Sudarshan V (2013) Hydrogeochemistry of groundwater in Basara area, Adilabad District Andhra Pradesh. India J Appl Geochem 15(2):224–237
Narsimha A, Sudarshan V, Srinivasulu P, Anitha N, Parameshwar V (2013) An integrated approach to assess the quality of groundwater in part of Cheralapally area, Rangareddy District, Andhra Pradesh India. Adv ApplSci Res 4(1):244–253
Nikanorov AM, LV Brazhnikova (2012). Types and Properties of Water II: Water Chemical Composition of Rivers, Lakes and Wetlands. Types and Properties of Water - Vol. II
Panaskar DB, Wagh VM, Muley AA, Mukate SV, Pawar RS, Aamal-awar ML (2016) Evaluating groundwater suitability for the domestic, irrigation, and industrial purposes in Nanded Tehsil, Maharashtra, India, using GIS and statistics. Arabian J Geosci 9(13):615
Piper AM (1953) A Graphic procedure in the geochemical interpretation of water analysis. US Geological Survey Ground Water Note 12: 63
Pradhan B, Pirasteh S (2011) Hydro-chemical analysis of the ground water of the basaltic catchments: upper Bhatsai region. Maharastra Open Hydrol J 5:51–57
Prasad M, Sunitha V, Sudharsan Reddy Y, Suvarna B, Muralidhara Reddy B, Ramakrishna Reddy M (2019) Data on Water quality index development for groundwater quality assessment from Obulavaripalli Mandal, YSR district, A.P India. Data in brief 24:103846 https://doi.org/10.1016/j.dib.2019.103846
Prasanna MV, Chidambaram S, Gireesh TV, Jabir Ali TV (2011) A study on hydrochemical characteristics of surface and sub-surface water in and around Perumal Lake, Cuddalore District, Tamil Nadu South India. Environ Earth Sci 64(5):1419–1431
Raghunath HM (1987). Groundwater. Wiley Eastern Ltd. Delhi, India. pp. 563
Raghunath HM (2003). Groundwater, New Age International (P) Ltd., New Delhi, pp 344–369
Raju NJ, Ram P, Dey S (2009) Groundwater quality in the lower Varuna river basin, Varanasi district. Uttar Pradesh J Geol Soc India 73:178
Ramesam V, Rajagopalan K (1985) Fluoride ingestion into the natural water of hardrock areas. Peninsular India J Geol Soc India 26:25–132
Rao NVR, Rao N, Rao KSP, Schuiling RD (1993) Fluorine distribution in waters of Nalgonda District, AP India. Environ Geol 21:89
Sakram G, Adimalla N (2018) Hydrogeochemical characterization and assessment of water suitability for drinking and irrigation in crystalline rocks of Mothkur region, Telangana State South India. Appl Water Sci 8:143
Sappa G, Ergul S, Ferranti F, Sweya LN, Luciani G (2015) Effects of seasonal change and seawater intrusion on water quality for drinking and irrigation purposes, in coastal aquifers of Dares Salaam, Tanzania. J Afr Earth Sc 105:64–84
Sarath Prasanth SV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal Stretch of Alappuzha District, Kerala, India. Appl Water Sci 2:165–175. https://doi.org/10.1007/s13201-012-0042-5
Sawyer CN, Mccarty PL, Parkin GF (2003) Chemistry for environmental engineering and science, 5th edn. McGraw-Hill, New York, p 752
Saxena VK, Mondal NC, Singh VS (2004) Identification of seawater ingress usingSr and B in Krishna delta. Curr Sci India 86(4):586–590
Shand SJ (1952) Rocks for chemistry. Patmann Publishing Company, New York, p 146
Sreedevi PD, Sreekanth PD, Ahmed S, Reddy DV (2018) Appraisal of groundwater quality in crystalline aquifer; a chemometric approach. Arab J Geosci 11(9):211
Sreedevi PD, Sreekanth PD, Ahmed S, Reddy DV (2019) Evaluation of groundwater quality for irrigation in a semi-arid region of South India. Sustain Water Res Manage 5(3):1043–1056
Subba Rao N (2018) Groundwater quality from a part of Prakasam district, Andhra Pradesh. India. Appl Water Sci 8:30
Subba Rao N, Surya Rao P, Rami V, Reddy G (2012) Chemical Characteristics of groundwater and assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh India. Environ Monit Assess 184:5189–5214
Subba Rao N, Marghade D, Dinakar A (2017) Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh. India Environ Earth Sci 76:747
Sudharshan Reddy Y, Vangala S, Badri S (2020a) Monitoring of groundwater quality for drinking purposes using the WQI method and its health implications around inactive mines in Vemula-Vempalli region, Kadapa District. South India Applied Water Science 10:202. https://doi.org/10.1007/s13201-020-01284-2
Sudharshan Reddy Y, Sunitha V, Suvarna B, Prasad M, Muralidhara Reddy B (2020b) Data on physical impacts and hydrogeochemical assessment of inactive/abandoned mines in and around Southwestern parts of the Cuddapah basin using a conceptual site model (CSM). Data Brief. https://doi.org/10.1016/j.dib.2020.105187
Sunitha V, Muralidhara Reddy B (2014) Determination of fluoride concentration in ground water by ion selective electrode. Int J Curr Res Aca Rev 2(8):159–166
Sunitha V, Muralidhara Reddy B (2018) Defluoridation of water using menthe longifolia (Mint) as Bioadsorbent. J Geophy Union 22(2):207–211
Sunitha V, Reddy, MRK (2008). Fluoride in groundwater and fluorosis http://www.earthscienceindia.info/Sunitha.htm. 31–10–2008
Sunitha V, Reddy YS (2019) Hydrogeochemical evalution of groundwater in and around Lakkireddipalli and Ramapuram, YSR District, Andhra Pradesh India. Hydro Res 2:85–96
Sunitha V, Muralidhara Reddy B, Ramakrishna Reddy M (2012a) Groundwater contamination from agro-chemicals in irrigated environment: field trials. Int J Adv AppSci Res 3(5):3382–3386
Sunitha V, Rajeswara RB, Reddy MR (2012b) Ground Water Quality Evaluation with special reference to Fluoride and Nitrate Pollution in Uravakonda, Anantapur District, Andhra Pradesh- a case Study. Int J Res Chem Environ 2(1):88–96
Sunitha V, MuralidharaReddy B, RamakrishnaReddy M (2012c) Assessment of nitrate contamination due to groundwater pollution in southeastern part of Anantapur District, Andhra Pradesh. Int J Earth Sci Eng 5(1):113–119
Sunitha V, Abdullah Khan J, Muralidhara Reddy B, Prasad M, Ramakrishna Reddy M (2014) Assessment of groundwater quality in parts of Kadapa and Anantapur Districts, Andhra Pradesh, India. Indian J Adv Chem Sci 3:96–101
Sunitha V, Muralidhara Reddy B, Sumithra S (2016) Assessment of groundwater quality index in the Kadapa municipal city, YSR District, Andhra Pradesh. Ind J Appl Res 6(2):545–548
Suvarna B, Sudharshan Reddy Y, Sunitha V, Prasad M (2018) Water Quality Index groundwater in and around Lakkireddy Palli, Ramapuram, YSR District, AP. J Emerg Tech Innova Res 5(11):786–794
Suvarna B, Sudharsan Reddy Y, Sunitha V, Muralidharreddy B, Prasad M, Ramakrishna Reddy M (2019). Data on application of water quality index method for appraisal of water quality in around cement industrial corridor Yerraguntla Mandal YSR District AP South India. https://doi.org/10.1016/j.dib.2019.104872.
FL Tiandra, A Kondhoh, Mohammed Aslam MA (2003) A conceptual database design for hydrology using GIS. Proceedings of Asia Pacific Association of Hydrology and Water Resources 13–15
Trivedi RK and Goel PK (1984). Chemical and Biological methods for water pollution studies. Env Pub Karad India, 215 p
US Geological Survey (2000). Classification of natural ponds and lakes. US Department of the Interior, US Geological Survey, Washington DC
Vijay R, Puja Khobragade PK, Mohapatra, (2011) Assessment of groundwater quality in Puri City, India: an impact of anthropogenic activities. Environmental Monitoring Assessment 177:409–418
WHO (1984) The Guidelines for Drinking Water Quality Recommendations. World Health Organization, Geneva
WHO (1990) Environmental health criteria 81: vanadium [R]. World Health Organization, Geneva, pp 1–35
WHO (2011). Guidelines for drinking water quality, 4th edn WHO press p.564
Acknowledgements
This study was funded by the Department of Science and Technology (DST) under the fast track young scientist initiative. Dr.V.Sunitha, the first author, thanked DST in the form of the Fast Track Young Scientist Scheme for providing financial assistance. The authors sincerely thank the anonymous reviewers for their useful and detailed reviews.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sunitha, V., Reddy, B.M. Geochemical characterization, deciphering groundwater quality using pollution index of groundwater (PIG), water quality index (WQI) and geographical information system (GIS) in hard rock aquifer, South India. Appl Water Sci 12, 41 (2022). https://doi.org/10.1007/s13201-021-01527-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01527-w