Abstract
As the surface water in northern India is the main water resource for regional economic and also supply for drinking and irrigation purposes. However, deficiency of water quality leads to serious water pollution in the Pandoh river basin (PRB). Therefore, the main objective of the present study is to evaluate the quality of surface water. With this objective, surface water samples were collected from the PRB of northern India, and analyzed for pH, EC, turbidity, alkalinity, total dissolved solids, and total hardness. Moreover, geographical information system (GIS) tools were used to prepare the geology, drainage pattern, and location maps of the study region. Surface water quality observed from the PRB has an alkaline nature with a moderately hard type. Further studies are encouraged to better understand the water quality in northern India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Access to fresh and clean water is most important and beneficial for drinking, irrigation, and industrial purposes all over the world. Predominantly, groundwater and surface water play a vital role in arid and semi-arid regions (Adimalla 2021; Adimalla and Qian 2021a; Cao et al. 2019). It is distinguished that higher than 2 billion people largely rely on groundwater and also surface water for various uses, especially drinking purposes. Therefore, it is equally important to comprehensively understand the quality of both groundwater and surface water (Adimalla et al. 2020; Adimalla and Qian 2021b; Ahmed et al. 2020; Moussa et al. 2020). About the evaluation of water quality, the great number of previous studies have been conducted in the various regions, primarily emphasized on hydrochemical characteristics of water quality for drinking and irrigations, contamination process, distribution patterns of ions (Chen et al. 2020; Zhao et al. 2020; Subba Rao et al. 2020; Adimalla and Taloor 2020). Most recently, Ahmed et al. (2020) studied the both groundwater and surface water quality for drinking purposes in the south-western coastal region of Bangladesh, and found the surface water quality is better as compared to groundwater. Cao et al. (2019) conducted a study on hydrogeochemistry and water quality in an urban region of Tianjin, China and reported that the quality of surface water is ominously influenced by human activities. Sajeev et al. (2020) focused on Vembanad lake water quality using GIS techniques and concluded that both point and non-point sources of pollution which greatly influenced the lake water quality. Chen et al. (2020) meticulously studied surface water quality prediction performance and remarked as surface water is a kind of non-renewable resource and also impacts the human health especially in some developing countries.
Pandoh river basin (PRB) is a typical snow-trout impacted by extreme man-made/anthropogenic pollution in Himachal Pradesh, India. The PRB is located along the national highway hence the dust gets dumped into the river along with various types of dumps. The low flows lead to the creation of silts in the river causing the spawning of the fish as they breed in the same location (Mainali and Chang 2021). This leads to the dumping of fish’s wastes and it gets stagnant causing foul smell and color changes of the surface water. Due to the decreasing dissolved oxygen level, nutrients and chemical reactions take place and metals are disintegrated from the sediments in turn release downstream. Therefore, PRB is also subjected to the breeding of mosquitoes and various other insects which makes it unfit for any usage. The basic river flow is one of the key sources of purification of the surface water, but in the case of PRB, the water is stagnant thus it leads to various harms such as an increase in density gradients and thermal stratification. The low flow in the surface water may make way for a decrease in the aeration level converting ammonia to nitrate which is harmful in terms of the water ecosystem. Thus, PRB has been facing disproportionate surface water pollution. Therefore, in this study we mainly emphasized on hydrochemical variation in the surface water quality in dry and wet seasons. The main objectives of the present study were to the following: (1) determine the physicochemical parameters and (2) comprehensively assess the water quality of surface for drinking purposes.
Materials and methods
Study area
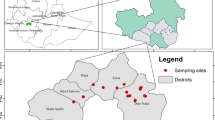
The study region is located in Mandi district, Himachal Pradesh, India and covering of 134 hectares (Fig. 1). Most of the investigated region is found in the hilly region, and only 14.43% of the region is the plain area which is located along with the riverside. The soils such as gray micaceous sandstones, sandstones, and shales of sub-Himalayan are found in this region. These soils are loam to silty loam and medium to high in organic matter (Fig. 2). Generally, these soils are poor in available nutrients. The catchment area is largely comprised of precipitous slopes and the rocks are mainly bare. There are high peaks in the east as well as in the north of the river valley. The altitude varies from 802 m near Pandoh Dam up to 6600 m on the northeast border of the Parbati sub-catchment. About 43.76%, 13.90%, and 0.97% of the study region are majorly occupied by fine loamy, coarse loamy, and loamy skeletal soil type, respectively. Soil characteristics like soil color, texture, depth, gravelliness, calcareousness, etc. up to a depth of 1.2 m and external land features like slope, existing land use, natural vegetation, erosion hazards, etc. were recorded during the field trip. However, the highest temperature (40ºC) records in May and the lowest (−3 °C) in December. The drainage pattern of the study region is depicted in Fig. 3.
Sample collection and analysis
For this research work, the water samples were collected from three sampling sites in the study region. The sites were chosen based on the initial survey taken along the surface water where the pollution has been created more by anthropogenic activities. Before sampling, the all sample bottles were thoroughly sterilized and cleaned with double distilled water, and finally sample bottles were rinsed five-six times with the sampling water before a final sample was taken. The pH and EC were measured using their digital meters (pH: digital pH meter, Naina-NIG 333; EC: digital conductivity meter, Naina–NDC 736; TDS: digital TDS meter –EI 651). Turbidity is measured using the Turbidity Nephelometer; alkalinity by titrating with the acid; and total hardness (TH) by titrating against EDTA, observing the standard procedures proposed by American Public Health Association (APHA 1985). The time-series data of BOD, DO, and total Coliform was collected from the Himachal State Pollution Control Board for the past years (Table S1).
The rainfall–runoff modeling has been carried out with the rainfall data obtained from the IMD Pune for 39 years (1980–2018). The lean season in the study area is from November to February. The average flow for the PRB has been calculated for each year and 15% of the average lean season flow has been taken as the required environmental flow (Fig. 4). The 95% exceedance discharge value of 7Q10 and 7Q100 Flow Duration Curve (FDC) provides the necessary environmental flow value for water quality management and in the decision-making process for the development of new hydropower projects in the river basin (Yulianti et. al. 1998). Failure in maintaining the environmental flow or the high-frequency maintenance of low flow in the river leads to poor water quality.
Results and discussion
Natural water dissolves CO2 to form carbonic acid, H2CO3 (Eq. 1). Also, CO2 is present in the soil zone due to the decay of organic matter and root respiration. This CO2 in the soil zone combines with rainwater to form H2CO3 (Rao and Rao 2010). The dissolution of H2CO3 in water forms H+ and HCO3− ions (Eq. 2).
Typically, HCO3− dissociates to H+ and CO32− (Eq. 3). The pH range of groundwater determines the stability of HCO3− and CO32− ions. Over the pH range of 6–9, HCO3− is the dominant carbonate species (Fetter 2001; Hiscock 2005).
The pH is the hydrogen ion concentration, which expresses the scale of intensity of acidity or alkalinity of water. According to WHO guidelines (WHO 2017), the range of pH values prescribed for drinking purposes is 6.5–8.5. The typical range of pH in the surface water system is from 6.5 to 8.5. However, in the present study, the pH values vary from 8.1 to 8.3, indicating the water is slightly alkaline in nature. It is observed that all collected water samples fall within the permissible, thus the study region water is more suitable for drinking purposes. According to WHO, pH less than 6.5 or greater than 9.2 would markedly impair the consumption of drinking water. pH usually has no direct impact on human health; however, the higher value of pH can increase the scale formation in water pipes and also reduce the disinfection potential of chloride (WHO 2017). More alkaline water requires a longer contact time or a higher free residual chlorine level at the end of the contact time for adequate disinfection (WHO 2017). For example, at pH 6.0–8.0 the free residual chlorine must be 0.4–0.5 mg/L, at pH 8.0–9.0, it is rising to 0.6 mg/L, chlorination become ineffective above pH 9.0 (WHO 2017). However, the high and low frequency of pH is obviously depicted in Fig. 5.
The concentration of turbidity in surface water ranged from 3.15 to 3.17 NTU, with an average value of 3.15 NTU. The turbidity values of all collected water samples exceeded the standard for drinking water of 1 NTU (BIS 2012), in the investigated region. Importantly, the PRB is one of the tourism region. The water quality deterioration due to the high turbidity affects the tourism industry as the aesthetic view of the lake is spoiled. Moreover, alkalinity is important for the fish ecosystem since it secures or supports against quick pH variations. The water with a higher concentration of alkalinity averts pH changes that are destructive to amphibian life. The alkalinity of the water sample from the downstream side study area was 83 mg/L under the acceptable limit of 500 mg/L as per BIS 2012.
The increase in the BOD concentration makes the water sample more noteworthy the measure of natural issue or "nourishment" accessible for oxygen devouring microorganisms. Further, a sudden decrease might speed up hypoxia or anoxic conditions. According to the Central Pollution Control Board (CGWB 2009), the BOD value must be less than the 3 mg/L. It is noted from the present study the BOD is under the acceptable limits (Fig. 6). The DO content is more than 5 mg/L which was the standard set for the surface water (Fig. 7). The Total Coliform should be nil in general, but the board allows a limit of up to 500 MPN/100 ml or less. The total Coliform value stays within the limit but during the study period, it peaks up to 2500 MPN/100 ml.
Electrical conductivity (EC) shows the concentration of ionized substances in water, and also is a good measure of salinity (WHO 2017). The maximum permissible concentration of EC for drinking water is 1400 μS/cm (WHO 2017). Excess salinity reduces the osmotic activity of plants and thus interferes with the absorption of water and nutrients from the soil (Thakur et al. 2018; Cao et al. 2019). In this PRB, the EC values of the water samples were in the range of 142–144 μS/cm, indicating the higher EC values than that of the prescribed limit for drinking water (Table 1).
TDS refers to all solid materials present in solution either in the ionized state or in non-ionized state and excludes the suspended colloids or dissolved gases. TDS indicates the general nature of water quality. Therefore, the level of TDS is one of the characteristics which decide the quality of drinking water. High TDS indicates that the water is highly mineralized, and this water is not suitable either for drinking or for industrial purposes. The concentration of TDS above 2000 mg/L produces laxative effects. TDS classified as four categories such as freshwater (TDS < 1000 mg/L), brackish water (TDS 1000–10,000 mg/L), saline water (TDS 10,000–100,000 mg/L) and brine water (> 100,000 mg/L) respectively (Davis and De Wiest 1966; Fetter 1990). In this study region, TDS vary from 300 to 303 mg/L. It is noted that all collected water samples were the most suitable for drinking purposes in this study region (Table 1). The TH as CaCO3 ranges from 92 to 96 mg/L (Table 1). TH classified as four categories such as soft (TH < 75 mg/L), moderately (TH 75–150 mg/L), hard (TH 150–300 mg/L) and very hard (TH > 300 mg/L) respectively (Freeze and Cherry 1979). Most of the collected water samples are under the moderately hard category in the investigated region. The present investigation is most useful to the local people and other organizations for taking necessary steps to improve the understand of water chemistry. The full-scale investigation is of utmost importance in the study region to improve the water quality and also arrest the pollution activities in the study region.
Conclusions
The present study offers a better understanding of the surface water quality in northern India. The surface water of the study region does not have any undesirable odor taste, color or high amount of turbidity. Particularly, study region surface water quality shows alkaline nature with low electric conductivity. The water of the present investigated region is fresh with low TDS, soft and has a low concentration of dissolved species. Thus, the water of the study region is most suitable for drinking and irrigation purposes. However, future studies also need to take up the role of the geochemistry of surface water quality, mechanism of contamination process characterization of sources of pollution and mitigation of contamination by applying the various profound geochemical methods.
References
Adimalla N (2021) Application of the entropy weighted water quality index (EWQI) and the pollution index of groundwater (PIG) to assess groundwater quality for drinking purposes: a case study in a rural area of Telangana State, India. Arch Environ Contam Toxicol 80(1):31–40
Adimalla N, Qian H (2021a) Geospatial distribution and potential noncarcinogenic health risk assessment of nitrate contaminated groundwater in southern India: a case study. Arch Environ Contam Toxicol 80(1):107–119
Adimalla N, Qian H (2021b) Groundwater chemistry, distribution and potential health risk appraisal of nitrate enriched groundwater: a case study from the semi-urban region of South India. Ecotox Environ Safe 207:111277. https://doi.org/10.1016/j.ecoenv.2020.111277
Adimalla N, Taloor AK (2020) Hydrogeochemical investigation of groundwater quality in the hard rock terrain of South India using geographic information system (GIS) and groundwater quality index (GWQI) techniques. Groundw Sustain Dev 10:100288. https://doi.org/10.1016/j.gsd.2019.100288
Adimalla N, Qian H, Nandan MJ (2020) Groundwater chemistry integrating the pollution index of groundwater and evaluation of potential human health risk: a case study from hard rock terrain of south India. Ecotoxicol Environ Saf 206:111217. https://doi.org/10.1016/j.ecoenv.2020.111217
Ahmed A, Ghosh PK, Hasan M, Rahman A (2020) Surface and groundwater quality assessment and identification of hydrochemical characteristics of a south-western coastal area of Bangladesh. Environ Monit Assess 192(4):258
BIS I (2012) 10500 Indian standard drinking water–specification, second revision. Bureau of Indian Standards, New Delhi
Cao X, Lu Y, Wang C, Zhang M, Yuan J, Zhang A, Song S, Baninla Y, Khan K, Wang Y (2019) Hydrogeochemistry and quality of surface water and groundwater in the drinking water source area of an urbanizing region. Ecotox Environ Safe 186:109628
Chen K, Chen H, Zhou C, Huang Y, Qi X, Shen R, Liu F, Zuo M, Zou X, Wang J, Zhang Y, Chen D, Chen X, Deng Y, Ren H (2020) Comparative analysis of surface water quality prediction performance and identification of key water parameters using different machine learning models based on big data. Water Res 171:115454
Davis SN, Dewiest RJM (1966) Hydrogeology. John Wiley and Sons Inc, NY
Fetter CW (1990) Applied hydrogeology. CBS Publishers and Distribu- tors, New Delhi
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall Inc, New Jersey
Mainali J, Chang H (2021) Environmental and spatial factors affecting surface water quality in a Himalayan watershed. Central Nepal. Environ Sustain Indicators 9:100096
Moussa AB, Chandoul S, Mzali H, Salem SBH, Elmejri H, Zouari K, Hafiane A, Mrabet H (2021) Hydrogeochemistry and evaluation of groundwater suitability for irrigation purpose in the Mornag region, northeastern Tunisia. Environ Dev Sustain: A multidiscip Approach Theory Pract Sustain Dev 23(2):2698–2718
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers, 2nd edn. McGraw-Hill, New York
Subba Rao N, Sunitha B, Adimalla N, Chaudhary M (2020) Quality criteria for groundwater use from a rural part of Wanaparthy District, Telangana State, India, through ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). Environ Geochem Health 42(2):579–599. https://doi.org/10.1007/s10653-019-00393-5
Thakur N, Rishi M, Sharma DA et al (2018) Quality of water resources in Kullu Valley in Himachal Himalayas, India: perspective and prognosis. Appl Water Sci 8:20. https://doi.org/10.1007/s13201-018-0668-z
UNRCO and CEEW (2018) The Perfect storm-Pathways to Managing India’s Water Sector Sustainably. 207-285
WHO (2017) Guidelines for drinking-water quality. World Health Organization, Geneva
Yulianti JS, Burn DH (1998) Investigating links between climatic warming and low streamflow in the Prairies region of Canada. Can Water Resour J 23(1):45–60
Zhao Y, Han J, Zhang B, Gong J (2021) Impact of transferred water on the hydrochemistry and water quality of surface water and groundwater in Baiyangdian Lake. North China. Geosci Front 12(3):101086
Acknowledgements
The research work done is a part of the DOES & T, Himachal Pradesh, India funded research project. We would like to express our sincerest gratitude to DOES & T, Himachal Pradesh, India for funding this research project. We express our sincere thanks to the anonymous reviewers and Editor for their significant comments which greatly helped us to improve the quality of the manuscript.
Funding
The author recieved no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prakasam, C., Saravanan, R., Sharma, M.K. et al. Assessment and distribution of water quality of Pandoh river basin (PRB), Himachal Pradesh, North India. Appl Water Sci 11, 137 (2021). https://doi.org/10.1007/s13201-021-01468-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01468-4