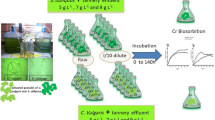
Abstract
Recently, mass production of lipid along with heavy metal reduction is gaining momentum due to their cost-effective and greener approach towards waste water treatment. The purpose of this study is to investigate the small scale photo bioreactor treatment of tannery effluent using Chlorella sp. isolated form Yercaud lake, Tamil Nadu, India. The results showed a significant decrease in the heavy metals content in the tannery effluent after the treatment. Maximum reduction of the heavy metal Chromium (Cr) of 10.92 mg L−1 was recorded, followed by Cobalt (Co)-7.37 mg L−1, Nickel (Ni)-9.15 mg L−1, Cadmium (Cd)-8.48 mg L−1, Lead (Pb)-12.54 mg L−1, Zinc (Zn)-11.56 mg L−1 and Copper (Cu)-10.71 mg L−1 at the end of the 20th day of treatment. The microalgae, Chlorella sp. was analyzed for their biosorption ability and the maximum biosorption capacity (qmax) rate against heavy metals was 81.36, 70.53, 82.15, 63.29, 58.92, 83.43, 64.83 µg L−1 for Cr, Pb, Ni, Cd, Co, Zn, and Cu respectively. It matched with the Langmuir and Freundlich kinetics models. The maximum CO2 utilization was found to be 60.50% and maximum concentration of lipid, carbohydrate and protein was found to be 0.95 g L−1, 250 µg mL−1 and 160 µg mL−1, respectively. The presence of various groups such as hydroxyl, alkyl, carbonyl and carboxylic acids was confirmed using Fourier transform infrared analysis. Thus, the isolated microalgae showed good biosorption ability towards the various heavy metal pollutants from tannery waste water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is one of the most crucial natural resources. Owing to the increasing population, urbanization, industrialization and worldwide mobility, the quality of water is deteriorating, leading to an inadequate supply of uncontaminated water, especially in developing countries. Most of the wastewater generated from domestic, agricultural and industrial sources is contaminated with both organic and inorganic pollutants comprising of a variety of heavy meals, plastic based components and high concentration nitrates, sulfates, phosphates, etc. Such pollutants can disturb the food chain and also endanger lives (Muñoz et al. 2009; Chowdhury et al. 2016; Sousa et al. 2018; Eerkes-Medrano et al. 2019). For this reason, immediate attention needs to be directed towards waste water treatment technologies in order to eliminate pollutants from the contaminated water. All these pollutants cannot be removed with the help of a single technology as the contaminants may vary based on their types, indigenous conditions and concentrations (Wollmann et al. 2019). Additionally, precipitation and coagulation procedures implemented during removal of metals lead to sludge formation imposing supplementary treatment for harmless clearance (Sharma et al. 2017). Several studies reported by various researchers have utilized different forms of microalgae for the treatment of wastewaters for the removal of heavy metals pollutants (Zhao et al. 2015; Yang et al. 2015), CO2 sequestration (Eloka-Eboka et al. 2017; Khan et al. 2018; Fu et al. 2018), and biomass production (Zhao et al. 2015).
Findings from the earlier studies show that the utilization of microalgae for the treatment of wastewaters is effective, safe and also aids in removal of various toxic chemicals at a reduced cost. Additionally, Yadavalli et al. (2014) reported the cultivation of algae using wastewater from dairy effluents and stated that the biomass generated during the treatment process could be effectively used for biofuel production. Similar research involving removal of heavy metals and toxic pollutants has been studied by different researchers. Their effluent treatment using microalgae offers good results, and can be used as an alternative treatment technology.
(Luo et al. 2017; Selvan et al. 2019). The utilization of microalgae for the treatment of wastewater seems to be promising due to their extreme metabolic flexibility in the presence of various pollutants (Hu et al. 2018; Tamil Selvan et al. 2020).
Another important feature of microalgae bioremediation is the production of huge biomass during the process. The utilization of industrial waste water for the micro algal biomass production puts forward the opportunity to recycle industrial residues for energy and material generation. The algal biomass produced can be further utilized for the generation of various sustainable bio products like, fatty acids, proteins, animal feeds, bio-fuels etc., (Mohan et al. 2016; Yu et al. 2017; Madeira et al. 2017). The waste water discharged from the tannery industries possesses bio toxic substances such as heavy metals. These toxins when released into the ecosystem can serve as major threats to the environment and also cause health issues in humans. The cultivation of microalgae on tannery wastewater (TWW) has been explored in certain research studies as it is considered to have a potential biological function in trimming down the contaminant load accompanied by safe discharge of effluents (Nagi et al. 2020).
The present study aims to investigate the production of biomass from the potential microalgae, Chlorella sp. along with its capacity to sequestrate CO2 and remove heavy metals from tannery effluent with 90% of concentration adaptation.
Materials and methods
Isolation and microscopic identification of microalgae
For the present study, freshwater associated with microalgae was collected from the Yercaud lake, Tamil Nadu, India, and brought to the laboratory. The samples collected were inoculated in Bold Basal Medium (BBM) and BG11 medium in a 100 ml sterile bottle. Different microalgae present in the fresh water were further isolated by spread plate technique using bold basal medium (BBM). At the end of incubation, pure forms of microalgal colonies were isolated and identified based on microscopic observations. The isolation medium was supplemented with antibiotics such as, ampicillin, chloramphenicol and gentamycin for the prevention of unwanted bacterial growth. The isolated pure culture of microalgae was further grown in sterile bold basal medium (BBM) and allowed to incubate at light intensity of 110 µmol m−2 s−1, with a temperature of 23 °C, in 12/12 light and dark cycle (Andersen 2005). The pure colony of the microalgal strain was observed under light microscopy for further identification.
Morphological identification of Microalgae
The microalgal cells inoculated on BBM agar plates were screened for the presence of unialgal strains. The purity of the algal forms was confirmed using microscopic analysis. Further inoculation in fresh medium was carried out for maintaining their monoculture state.
Collection and Physicochemical analysis of Tannery Effluent
In the present study, the tannery effluent samples were collected from Melvisaram, Vellore district Tamil Nadu. The effluent samples were collected in a sterile container, brought to the laboratory and stored at 4 °C till further processing. The various physical and chemical parameters of the effluent such as pH, total dissolved solids (TDS), total suspended solids (TSS), total solids (TS), alkalinity, ammonia, total hardness, chloride, sulphate, chromium, calcium, iron, nitrate, nitrite, phosphate, BOD (biological oxygen demand), and COD (chemical oxygen demand) as per the standard procedures of APHA (APHA 2005) were checked. The reduction of the organic and inorganic chemical pollutants in the effluent samples was calculated in terms of percentages (%) as follows.
where, IC- Initial concentration and CV- Concentration value.
Determination of heavy metals
The concentration of heavy metals in the tannery effluent was determined based on the methodology followed by Wolf et al. (1979). Briefly, the effluent sample was centrifuged at 10,000 rpm for 15 min, the sediment was collected, air dried and further subjected to various heavy metal analysis (Cd, Cr, As, Cu, Fe, Mn, Ni, Pb and Zn) using Atomic Absorption Spectrometer (Perkin Elmer, USA).
Laboratory scale treatment of effluent
For the laboratory scale, 50 L photo bioreactor was used with a total working volume of 40 L. Prior to the bioremediation study, 5 L of selected microalgal species was grown in pure culture form. Then, 3 L of pure culture of microalgal species was transferred to 50 L photo bioreactor containing 37 L of tannery effluent. The plastic tank was enabled with continuous (pure) CO2 gas supply with 50 ml/min and the setup was incubated for 20 days in batch operation under laboratory condition. During the period of incubation, various physiochemical properties, presence of heavy metals, CO2 biosorption rate, algal growth rate, total biomass and lipid content were determined from the samples collected every day and recorded (Scheme 1).
Determination of algal growth rate kinetics
The algal growth rate was determined by measuring the growth of microalgae using spectrophotometer. Breifly, samples were withdrawn and the algal growth was measured by recording the absorbance at 680 nm. For the determination of biomass, the algal suspension was centrifuged at 15,000 rpm for 10 min, dried at 55 °C for 60 min in hot air oven. The relation between the growth rate and the biomass was estimated using liner regression equation (eq:2) and specific growth rate (eq:3) (Tamil Selvan et al. 2020):
where, X—optical density at 680 nm and N—dry biomass weight (gmL−1)
Were, µ—Specific growth rate and Ln—Liner regression.
Determination of CO2 utilization kinetics
The CO2 biosorption ability and the biofixation efficiency rate (BCO2) of the selected microalgal algal species was calculated using modified methodology of De Morais and Costa (2007). The biofixation efficiency rate, percentage of CO2 removal and consumption rate was calculated as mentioned below:
The BCO2 (Eq:4) and CO2 removal (%)(Eq:5) were estimated based on the equations, given as the determination of biofixation efficiency rate:
where, Xc % of carbon content from the given microalgal cell, P is the biomass productivity expressed in terms of mg mL−1d−1, ZC is the molecular weight Carbon (C) and ZCO2 is the molecular weight carbon dioxide (CO2).
The determination of CO2 removal (%):
where, RCO2 is carbon dioxide (CO2) removal in terms of percentage and V is the volume of CO2.
Determination of heavy metals biosorption capacity
To study the biosorption ability of heavy metals using microalgae, 50 mL of the effluent treated with algae was filtered using nylon millipore membrane filter. The filtered microalgal cells were collected and utilized for biosorption capacity studies using atomic adsorption spectroscopy. The kinetics studies of heavy metals biosorption using microalgae was evaluated by kinetic models such as Langmuir and Freundlich model (Tamil Selvan et al. 2020) as mentioned below.
For Langmuir model, the biosorption ability was calculated using the following equation:
where,qe is Algal biosorption capacity at equilibrium (mg g−1),Ce is Concentration of metals at equilibrium (mg L−1),qmax is Maximum biosorption capacity (mg g−1) andb is Langmuir constant (L g−1).
For Freundlich model, the biosorption ability was calculated using the following equation:
where,KF is Freundlich constant,1/n is Adsorption intensity,qe is Algal biosorption capacity at equilibrium (mg g−1) andCe is Concentration of metals at equilibrium (mg L−1).
Scanning Electron Microscopic (SEM) Studies
The interaction between the microalgal cells and the heavy metals during biosorption process was analyzed by studying the cell surface of both treated and untreated microalgal cells using a scanning electron microscope (SEM).
Estimation of total lipid content
The concentration of lipids in the microalgal cells was determined following biomass extraction using methanol, chloroform, and hexane (2:1.5:1.5 v/v) by modified Floch’s method (Folch et al. 1957).
Estimation of total carbohydrate
The total carbohydrate content of the microalgal cells was determined by Anthrone method using glucose as a standard (Pons et al. 1981). Five gram of algal biomass was homogenized with 10 mL of conc. H2SO4 and the preparation was centrifuged at 10,000 rpm for 15 min. The supernatant was collected, incubated further for 10 min at room temperature and used for carbohydrate content determination by recording the absorbance at 490 nm. The carbohydrate content was calculated using the standard equation as mentioned below:
where, X1 is the total biomass and Y1 is the carbon content.
Estimation of total microalgal biomass
For determining the total microalgal biomass, the microalgae was harvested, filtered using 15 µm size of fiberglass filter cloth at the end of the 15th day and air dried under direct sunlight for 2 days. The total biomass weighed was calculated using following equation:
where, Y1 is concentration of biomass at time t1 and Y0 is concentration initial biomass at time t0.
Determination of Protein by Lowry’s Method
The concentration of intracellular protein was estimated using Lowry's method with BSA as a standard.
Determination of Chlorophyll content
The chlorophyll content (Chlorophyll a, b and c) of the microalgae was analyzed using spectrophotometric method.
FTIR Fourier Transform Infrared Spectrum analysis lipid extracts
The extracted lipid was analyzed using FTIR spectroscopy in order to investigate the functional groups present on cell surface of the microalgae.
Results
Isolation of microalgae
The algal growth was monitored based on the observation of green color of the selected culture and identification by light microscopic analysis.
Morphological identification of Microalgae
The pure form of microalgal strain was identified as Chlorella sp. based on the morphological characteristic features such as spherical shape with size ranging from 2 to 10 μm in diameter, green photosynthetic pigments without flagella (Supplementary 1).
Physicochemical analysis and lab scale treatment of tannery effluent using Chlorella sp.
The physicochemical characteristics of the tannery effluent were studied, recorded and tabulated (Table 1). The results from the tests confirmed the potential bioremediation ability of Chlorella sp. against tannery effluent in larger scale treatment as a clear reduction in turbidity was visualized by the 20th day (Supplementary 2).
Determination of algal growth rate kinetics
In the present study, the growth of the microalgal culture was determined by measuring the absorbance at 680 nm. The lag phase of the microalgal growth was measured both in the treated and untreated sample which was recorded and calculated as 0.57 × 109 cells mL−1. The growth kinetics of the microalgae during the treatment with wastewater was studied at 6 different time periods and tabulated (Table 2).
Determination of CO2 utilization kinetics
The maximum utilization (61.60%) of CO2 was found when tannery effluent was treated with microalgal cells supplied with different concentrations of CO2 gas ranging from 10 to 50 mL min−1 (Table 3a, b).
Determination of heavy metals biosorption capacity
To determine the heavy metal biosorption ability of micro algal cells in tannery effluent, the heavy metal composition and quantity was analyzed using atomic adsorption spectroscopy, before and after the heavy metal biosorption treatment. The microalgal cells were successful in absorbing a greater part of the heavy metal Chromium (Cr),10.92 mg L−1 (95.59%), followed by Cobalt (Co)-7.37 mg L−1 (94.12%), Nickel (Ni)-9.15 mg L−1, (93.94%), Cadmium (Cd)-8.48 mg L−1 (93.98%), Lead (Pb)-12.54 mg L−1 (93.43%), Zinc (Zn)-11.56 mg L−1 (93.84%) and Copper (Cu)-10.71 mg L−1, (89.38%) (Table 4).
The biosorption ability of the different metals using microalgae in tannery effluents was studied and analyzed using Langmuir isotherm and Freundlich kinetics model (Fig. 1 and Table 4). The Langumiur model biosorption capacities (qmax) were noted to be 81.36 µgL−1,70.53 µgL−1, 82.15 µg L−1, 63.29 µg L−1, 58.92 µg L−1, 83.43 µg L−1, 64.83 µg L−1, for Cr, Pb, Ni, Cd, Co, Zn, and Cu respectively (Table 5).
SEM Studies
The morphological changes in the microalgae during the biosorption of heavy metals were investigated using SEM studies. The biomass after the treatment of tannery effluent was observed under high resolution. The scanning electron microscopic image during the treatment with tannery effluent great structural changes (Fig. 2).
Estimation of total lipid content
In the present study, the lipid concentration was estimated using modified Floch’s method. The Lipid content present in tannery effluent Control culture (C) and Melvisharam Untreated (MSU) was found to be 0.63 g L−1 and 0.95 g L−1 respectively (Fig. 3). The extracted lipid was further analyzed using thin layer chromatography (TLC) and FTIR.
Estimation of Total Carbohydrate
The intracellular carbohydrate content present in tannery effluent control culture (C) and Melvisharam Untreated (MSU) was found to be 165 µg mL−1 and 250 µg mL−1 respectively (Fig. 4).
Determination of Protein by Lowry’s Method
The protein content present in tannery effluent Control culture (C), and Melvisharam Untreated (MSU) was found to be 120 µg mL−1 and 160 µg mL−1 respectively (Fig. 5).
Determination of Chlorophyll content
The Chlorophyll a content present in tannery effluent Control culture (C) and Melvisharam Untreated (MSU) was found to be 5.6 µgmL−1 and 7.1 µgmL−1, respectively. Similarly, the Chlorophyll b content was found to be 7.4 µgmL−1 and 9.6 µgmL−1 respectively. Among the tested samples, the Melvisharam untreated (MSU) showed rich content of Chlorophyll a and b when compared to effluent treated biomass, whereas Chlorophyll c content was found to be 5.3 µg, and 6.5 µg respectively (Fig. 6).
FTIR Fourier Transform Infrared Spectrum analysis of the lipid extracts
The extracted lipids analyzed using FTIR spectroscopy is depicted in the Fig. 7. FT-IR analysis for lipid extracts was performed to find out the chemical composition. Both control and treated biomass were analyzed using FTIR spectroscopy to investigate the functional groups present on cell surface of the microalgae. The results revealed that the untreated control biomass showed characteristic absorption peaks corresponding to hydroxyl groups (–OH), alkyl (–C–C), carboxylic acids (–COOH), carbonyl (–C O), and esters (–COOR) and lipid absorption ranges up to (2000–3000)cm−1. The FTIR spectral analysis of MSU showed the presence of characteristic peaks corresponding to hydroxyl groups, alkyl, carbonyl, carboxylic acids and esters, however slight changes in the peak values of functional groups was detected. These changes observed in the treated biomass correspond to the presence of toxic compounds (Table 6).
Discussion
The present study focuses on evaluating the efficacy of the microalgae Chlorella sp. in reducing the toxic components present in tannery effluents. Initially, the pure culture of microalgae was isolated and primary identification was confirmed based on shape, size and color intensity of the strain.
During the remediation process by microalgae, the pH level of the effluent turns neutral or roughly neutral since the dissolved CO2 is reduced by the microalgae during the process of photosynthesis which in turn elevates the pH level. The chemical oxygen demand (COD) and the biochemical oxygen demand (BOD) of the tannery wastewater were reduced to 95.46% and 95.17% respectively after treatment using Chlorella sp. This can be attributed to the effectiveness of the strain to improve the quality of the wastewater. Almost similar results were obtained by Das et al. (2017) in a similar study with Chlorella vulgaris, where the COD and BOD values came down to 94.74 and 95.93%, respectively, after 21 days of treatment. In another study, Das et al. (2018) examined the bioremediation potential of two different marine microalgae strains individually and in consortium for the reduction of pollutants in tannery wastewater. It was found that Chlorella sp. and Phormidium sp, were able to reduce BOD, COD, chromium, total nitrogen, total phosphorous upon incubation for 20 days.
The results from the algal growth rate kinetics studies clearly indicate that the presence of tannery waste water supported the microalgal growth while also increasing its metabolic activity. Thus, effluent treatment using microalgal cultures attests to be a good choice for the treatment process. The biosorption kinetics evaluation results obtained closely integrated with both the Langmuir and Freundlich isotherms which are also in accordance to the study by Pradhan et al. (2019) who investigated the biosorption ability of microalgal biomass from Scenedesmus sp. Maximum utilization of CO2 was observed when tannery effluent was treated with microalgal cells supplied with different concentrations of CO2 gas ranging from 10 to 50 mL min−1. Tannery wastes are attributed to contain high levels of BOD, COD, suspended solids, total dissolved solids, chromium and sulfides. The efficiency of the isolated Chlorella sp. to remove the heavy metals contaminants, especially Chromium ((95.59%) correlated with the findings obtained by Das et al. (2017) who stated that microalga Chlorella vulgaris notably removed 100% of the chromium for 12 days of culture along with the removal of phosphates and sulphates.
Total dissolved solids (TDS) is a significant chemical factor of water, which specifies the occurrence of a range of minerals including nitrate, nitrite, phosphate, sulphates, metallic ions, alkalis and acids in both colloidal and dissolved forms. According to Das et al. (2017) a very high concentration of TDS in untreated wastewater was condensed by C. vulgaris by day 21 with an overall TDS removal of 41% which is contrary to the results obtained in the current study as the percentage of the treatment given by the isolated strain is 90.11% which is indicative of its high potency. The results from the study by Ajayan et al. (2015) revealed that the algal biomass during the growth period reduced the pollution load of heavy metals like (Cr-81.2–96%, Cu-73.2–98%, Pb-75–98% and Zn-65–98%) which are almost comparative to the current study results as given- (Cr)-10.92 mg L−1 (95.59%), (Cu)-10.71 mg L−1, (89.38%), (Pb)-12.54 mg L−1 (93.43%), and Zinc (Zn)-11.56 mg L−1 (93.84%). The control biomass showed low lipid content when compared to the effluent treated biomass. While fascinatingly, the effluent treated biomass showed high rich carbohydrate content when compared to the control biomass. The protein content present of MSU was higher (160 µg mL−1) than the control (120 µg mL−1). The chlorophyll ‘b’ content of the biomass treated effluent was found to be highest followed by concentration of chlorophyll ‘a’ and ‘c’. Analogous findings of Santhosh et al. (2020) were reported with using Chlorella sp. strain, which highlighted that the chlorophyll a and c content were higher in 50% concentration but chlorophyll c was highest in 60% concentration.
FTIR spectral analysis of MSU confirmed the presence of characteristic peaks corresponding to hydroxyl groups, alkyl, carbonyl, carboxylic acids and esters. Vidyadharani et al. (2013) studied the FT-IR spectrum for the analysis of lipid from Chlorella vulgaris. The results from this study were compared to those values in order to confirm the stretching pattern of Chlorella sp. The peak at 3007.02 cm−1 corresponds to olefinic C–H stretching due to the presence of unsaturated fatty raw materials and a similar peak at 2920 cm−1 was identified in literature studies. The peaks for C-H bending vibration were visualized around 1425.40 cm−1. FTIR based lipid analysis was much more superior to the other methods like nile red fluorescence microscopy analysis and thin layer chromatography (TLC) due to its technical advantages such as sensitivity and high throughput means to assess carbon allocation changes.
Conclusion
The results of the present study offer possible applications of microalgae, Chlorella sp. for the removal of different heavy metals from the tannery effluents. The biosorption capacity of Chlorella sp. was confirmed against different heavy metals such as Cr, Pb, Ni, Cd, Co, Zn, and Cu during tannery effluent treatment with a maximum efficiency in removing chromium (95.59%). The pseudo order kinetics was well fitted with Langmuir and Freundlich kinetics model. The ability of CO2 sequestration during the treatment was also found to be high which aids in the production of elevated level of carbohydrate, lipid and protein. In conclusion, the isolated microalgae, Chlorella sp, is found to be a versatile microbe which assists in the removal of heavy metals and also to fix CO2 from the environment, thus providing a promising alternative technology for the treatment of effluent waste water.
References
Ajayan KV, Selvaraju M, Unnikannan P, Sruthi P (2015) Phycoremediation of tannery wastewater using microalgae Scenedesmus species. Int J Phytoremediation 17:907–916. https://doi.org/10.1080/15226514.2014.989313
American Public Health Association (2005) Standard methods for the examination of water and wastewater. APHA, Washington (DC)
Andersen RA (ed) (2005) Algal culturing techniques. Elsevier, Netherland
Chowdhury S, Mazumder MAJ, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ 569–570:476–488
Das C, Naseera K, Ram A et al (2017) Bioremediation of tannery wastewater by a salt-tolerant strain of Chlorella vulgaris. J Appl Phycol 29:235–243. https://doi.org/10.1007/s10811-016-0910-8
Das C, Ramaiah N, Pereira E, Naseera K (2018) Efficient bioremediation of tannery wastewater by monostrains and consortium of marine Chlorella sp. and Phormidium sp. Int J Phytoremediation 20:284–292. https://doi.org/10.1080/15226514.2017.1374338
De Morais MG, Costa JA (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechn 129(3):439–45
Eerkes-Medrano D, Leslie HA, Quinn B (2019) Microplastics in drinking water: a review and assessment. Curr Opin Environ Sci Heal 7:69–75
Eloka-Eboka AC, Inambao FL (2017) Effects of CO2 sequestration on lipid and biomass productivity in microalgal biomass production. Appl Energy 195:1100–1111. https://doi.org/10.1016/j.apenergy.2017.03.071
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Boil Chem 226(1):497–509
Fu W, Gudmundsson S, Wichuk K et al (2018) Sugar-stimulated CO2 sequestration by the green microalga Chlorella vulgaris. Sci Total Environ 654:275–283. https://doi.org/10.1016/j.scitotenv.2018.11.120
Hu J, Nagarajan D, Zhang Q et al (2018) Heterotrophic cultivation of microalgae for pigment production: a review. Biotechnol Adv 36:54–67
Khan SA, Malla FA, Rashmi, et al (2018) Potential of wastewater treating Chlorella minutissima for methane enrichment and CO2 sequestration of biogas and producing lipids. Energy 150:153–163. https://doi.org/10.1016/j.energy.2018.02.126
Luo Y, Le-Clech P, Henderson RK (2017) Simultaneous microalgae cultivation and wastewater treatment in submerged membrane photobioreactors: a review. Algal Res 24:425–437. https://doi.org/10.1016/j.algal.2016.10.026
Madeira MS, Cardoso C, Lopes PA (2017) Microalgae as feed ingredients for livestock production and meat quality: a review. Livest Sci 205:111–121
Mohan SV, Nikhil GN, Chiranjeevi P, Reddy CN, Rohit MV, Kumar AN, Sarkar O (2016) Waste biorefinery models towards sustainable circular bioeconomy: critical review and future perspectives. Bioresour Technol 215:2–12
Muñoz I, Gómez-Ramos MJ, Agüera A et al (2009) Chemical evaluation of contaminants in wastewater effluents and the environmental risk of reusing effluents in agriculture. TrAC Trends Anal Chem 28:676–694. https://doi.org/10.1016/j.trac.2009.03.007
Nagi M, He M, Li D, Gebreluel T, Cheng B, Wang C (2020) Utilization of tannery wastewater for biofuel production: new insights on microalgae growth and biomass production. Sci Rep 10(1):1–14
Nithiya EM, Tamilmani J, Vasumathi KK, Premalatha M (2017) Improved CO2 fixation with Oscillatoria sp. in response to various supply frequencies of CO2 supply. J CO2 Util. 18:198–205. https://doi.org/10.1016/j.jcou.2017.01.025
Pons A, Roca P, Aguiló C, Garcia FJ, Alemany M, Palou A (1981) A method for the simultaneous determinations of total carbohydrate and glycerol in biological samples with the anthrone reagent. J Biochem Bioph Meth 4(3–4):227–231
Pradhan D, Sukla LB, Mishra BB, Devi N (2019) Biosorption for removal of hexavalent chromium using microalgae Scenedesmus sp. J Clean Prod 209:617–629. https://doi.org/10.1016/j.jclepro.2018.10.288
Santhosh S, Rajalakshmi AM, Navaneethakrishnan M, Angel SJ, Dhandapani R (2020) Lab-scale degradation of leather industry effluent and its reduction by Chlorella sp. SRD3 and Oscillatoria sp. SRD2: a bioremediation approach. Appl Water Sci 10(5):1–11
Selvan ST, Govindasamy B, Muthusamy S, Ramamurthy D (2019) Exploration of green integrated approach for effluent treatment through mass culture and biofuel production from unicellular alga, Acutodesmus obliquus RDS01. Int J Phytoremediation 21(13):1305–1322
Sharma M, Hazra S, Basu S (2017) Kinetic and isotherm studies on adsorption of toxic pollutants using porous ZnOSiO2 monolith. J Colloid Interface Sci 504:669–679
Skrupski B, Wilson KE, Goff KL, Zou J (2013) Effect of pH on neutral lipid and biomass accumulation in microalgal strains native to the Canadian prairies and the Athabasca oil sands. J Appl Phycol 25:937–949
Sousa JCG, Ribeiro AR, Barbosa MO et al (2018) A review on environmental monitoring of water organic pollutants identified by EU guidelines. J Hazard Mater 344:146–162
Tamil Selvan S, Velramar B, Ramamurthy D, Balasundaram S, Sivamani K (2020) Pilot scale wastewater treatment, CO2 sequestration and lipid production using microalga, Neochloris aquatica RDS02. Int J Phytoremediation 22(14):1462–1479
Vidyadharani G, Dhandapani R (2013) Fourier transform infrared (FTIR) spectroscopy for the analysis of lipid from Chlorella vulgaris. Elixir Appl Biol 61:16753–16756
Wolf WR, Stewart KK (1979) Automated multiple flow injection analysis for flame atomic absorption spectrometry. Anal Chem 51:1201–1205. https://doi.org/10.1021/ac50044a024
Wollmann F, Dietze S, Ackermann JU, Bley T, Walther T, Steingroewer J, Krujatz F (2019) Microalgae wastewater treatment: biological and technological approaches. Eng Life Sci 19(12):860–871
Yadavalli R, Rao CS, Rao RS, Potumarthi R (2014) Dairy effluent treatment and lipids production by Chlorella pyrenoidosa and Euglena gracilis : study on open and closed systems. Asia-Pacific J Chem Eng 9:368–373. https://doi.org/10.1002/apj.1805
Yang JS, Cao J, Xing GL, Yuan HL (2015) Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour Technol 175:537–544. https://doi.org/10.1016/j.biortech.2014.10.124
Yu KL, Show PL, Ong HC (2017) Microalgae from wastewater treatment to biochar: feedstock preparation and conversion technologies. Energy Convers Manag 150:1–13
Zhao X, Moates GK, Elliston A (2015) Simultaneous saccharification and fermentation of steam exploded duckweed: improvement of the ethanol yield by increasing yeast titre. Bioresour Technol 194:263–269. https://doi.org/10.1016/j.biortech.2015.06.131
Acknowledgements
The authors thank the Fermentation Technology Laboratory, Department of Microbiology (Supported by DST-FIST), Periyar University for providing us with the laboratory, instrumentation facilities to carry out this research work.
Funding
Tamil Nadu State Council for Science and Technology (TNSCST/ S&T Project/VR/ES/01/2016-2017) for finical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests to disclose.
Ethical approval
This research work does not contain any studies related with human or animals.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rajalakshmi, A.M., Silambarasan, T. & Dhandapani, R. Small scale photo bioreactor treatment of tannery wastewater, heavy metal biosorption and CO2 sequestration using microalga Chlorella sp.: a biodegradation approach. Appl Water Sci 11, 108 (2021). https://doi.org/10.1007/s13201-021-01438-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01438-w