Abstract
Caffeine is a well-known central nervous system stimulant, which can cause anxiety, insomnia and nervousness. Domestic wastes of caffeinated drinks, beverages and chocolates are the major sources for entry of caffeine in the environmental system. Caffeine has been widely detected in natural water resources. The current study describes a method for efficient removal of caffeine from aqueous solution by a laboratory scale dielectric barrier discharge (DBD) in open air. Caffeine concentrations in various sample solutions were monitored by high-performance liquid chromatography, and the degradation products were identified by directly injecting the sample to mass spectrometer. The consequences of varied parameters such as input power, initial concentration and initial pH of the solution on the degradation of caffeine were investigated. Removal efficiency of caffeine from aqueous solution was 72.6% and 96.6% for the initial concentrations of 100 and 1 µg/mL, respectively, at initial pH 7 after 4 min treatment in DBD plasma system with 60 W input powers. Caffeine removal efficiency was less in acidic solutions (initial pH 4), and insignificant degradation was observed in alkaline solutions (initial pH 10). Furthermore, the degradation of caffeine was also enhanced by increasing the input power in DBD system. The DBD system used in this study has been considered to be fast, effective and economical. It was operated at atmospheric condition in open air without using catalyst, expensive gases or organic solvents, and significant degradation of caffeine was achieved in a short (4 min) treatment time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ever-increasing usage and disposal of pharmaceutically active substances as well as personal-care products are becoming a matter of concern worldwide. The chemical pollutants present in these products are progressively entering into various natural water resources such as lakes, rivers and groundwater. The dewatered municipal biosolids are frequently used in agricultural manure in order to improve physicochemical properties of soil and enrich soil fertility (Liu and Wong 2013; Moldovan 2006; Usman et al 2012). However, it could also lead to the release of persistent pharmaceutical pollutants in the ecosystem, including groundwater (Sabourin et al. 2011). The solicitation of dewatered municipal biosolids in soil profile has remarkable influence on depravity rate of various biodegradable substances and decreases their possible ecological hazard (Al‐Rajab et al. 2015). Since the recognition of small quantities of active pharmaceutical substances in the groundwater has been noticed, the alertness about their existence and hazards has increased (Ternes 1998). In the recent decades, these compounds have been detected in wastewater and subsequently reach into surface, ground and drinking water in North America, Australia and Europe (White et al. 2006).
Caffeine (1,3,7-trimethylpurine-2,6-dione) is a well-known central nervous system stimulant and excessive consumption for long time can produce adverse health effects such as anxiety, insomnia, nervousness and hypertension; may affect the quality and quantity of sleep, even at low concentration (Gokulakrishnan et al. 2005; Shilo et al. 2002; Waring et al. 2003). It can also produce harmful effects in patients with cardiac complications (James 1997). Caffeine is considered to be addictive compound which has more prominent effects in women than men, its withdrawal symptoms are reported to be fatigue, headache, drowsiness and apathy (Greenberg et al. 1999; Daly and Fredholm 1998; Landolt et al. 1995; Nehlig 1999). Moreover, its exposure to plants and animals have harmful impact (Pincheira et al. 2003; Meyer et al. 2004). The domestic wastes of caffeinated drinks, beverages and chocolates are the major sources of entry of caffeine in the environmental system. About 90% of the world adult population consume caffeine in one way or another with the highest average consumption of 440 mg/day/person in USA, followed by 300, 210 and 171 mg/day/person in Switzerland, UK and Brazil, respectively (Frary et al. 2005; Buerge et al. 2003; Standley et al. 2002; Monica and Camargo 1999). The global average consumption of caffeine varies in the range of 80–400 mg/day/person (Gokulakrishnan et al. 2005). Although caffeine is present naturally in coffee, tea, cocoa, kola nuts and added to beverages, its presence in environmental water is mainly attributed to the discharge of domestic wastewater or mediation moving through inefficient onsite wastewater treatment (Buerge et al. 2003; Seiler et al. 1999; Siegener and Chen 2002). Because of its high solubility (13.5 g/L), caffeine is largely expected to persist in water (Buerge et al. 2006; Chen et al. 2002). High concentrations of caffeine in wastewater were attributed from densely populated areas (Peeler et al. 2006; Rodriguez del Rey et al. 2012). It ranges from traces to enormously excessive concentrations (up to 192 µg L−1) in wastewaters (Gomez et al. 2007). Caffeine concentration reported in various water resources include, surface water (112–781 ng L−1) (Daneshvar et al. 2012), groundwater (0.23 µg L−1) (Seiler et al. 1999), effluent wastewater (0.07–126 µg L−1) (Weigel et al. 2004), coastal water (15–185 ng L−1) (Cantwell et al. 2016) and well water (1.7 µg L−1) (Reddy et al. 2006).
Caffeine is comprehensively found in nature because of its consistent disposal into the sewage framework by the transfer of unconsumed coffee, tea or beverages down the channels, and the flushing of coffee pots and mugs (Seiler et al. 1999). Several techniques, such as photo-electrolysis, ozonation, activated charcoal adsorption, electro-chemical oxidation, photo-oxidation, biodegradation and dielectric barrier discharge (DBD) plasma, have been investigated for caffeine removal from wastewater (Kim et al. 2008; Lin et al. 2010; Yu et al. 2014). In recent years, DBD plasma technique has been utilized as an effective system for removal of pharmaceutical contaminants in wastewater treatment plants (Magureanu, et al. 2015). In DBD plasma technique, discharge electrode plates separated by dielectric barrier, generally made up of quartz are used (Reddy et al. 2014a). Electric discharge in water leads in the production of UV radiation, high electric fields and overpressure shock waves and generation of active species such as hydroxyl radicals(˙OH), ozone (O3), atomic oxygen (O), hydrogen peroxide (H2O2), and hydroperoxyl radicals (˙HO2) resulting in oxidative degradation of organic contaminants in water (Al-Rajab et al. 2018; Lin et al. 2010; Reddy et al. 2014a, b; Wang et al. 2017). Buerge et al. (2003) have reported 81–99.9% removal of caffeine from wastewater containing low concentrations of caffeine (7–73 µg/L) at Swiss wastewater processing plants, they have also analyzed the suitability of caffeine as chemical marker for surface water pollution from domestic discharge. Sui et al. (2010) have investigated the removal of 13 pharmaceuticals including caffeine from wastewater at four different wastewater treatment plants in Beijing, China, and reported about 12–100% elimination rate of micro-pollutants using ozonation and microfiltration/reverse osmosis techniques. In another publication, 94% caffeine removal from aqueous solution (50 mg/L) by using a DBD plasma treatment technique coupled with goethite catalysis was reported in 24 min treatment (Wang et al. 2017). In addition, several methods based on biodegradation of caffeine by different bacterial and fungal strains have also been described (Edwards et al. 2015; Gokulakrishnan et al. 2005). However, most of the available methods for caffeine removal from wastewater suffered one or more drawbacks of being expensive, time consuming, low caffeine removal rate, incomplete degradation, high-energy consumption, usage of catalysts, expensive gases and toxic organic solvents and complicated procedure such as solvent and supercritical fluid extraction. Therefore, to remove the potential organic contaminants from water resources, and hence to improve the quality of life, a technically and economically balanced method is still needed. As a consequence, in the present work, we attempted to develop a cost-effective method based on DBD plasma technique with faster caffeine removal from water system. Furthermore, the DBD system utilized in this investigation was operated at atmospheric pressure in open air without application of any catalyst, expensive gases and organic solvents. The concentration of caffeine in treated water samples was monitored by validated high-performance liquid chromatography (HPLC) method.
Materials and methods
Materials
Caffeine (99.0%) was purchased from Sigma Aldrich, Germany, and used without further purification. Ammonium acetate, acetic acid, ammonium hydroxide and acetonitrile (HPLC grade) were procured from Sigma Aldrich, Germany. All the reagents used in this experiment were of analytical grade or were specified. HPLC grade water was produced in-house using Milli-Q water purification system (Millipore, Molsheim, France).
Instrumentation and optimization of experimental parameters
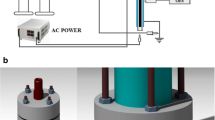
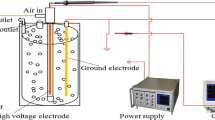
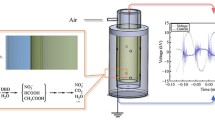
A laboratory scale dielectric barrier discharge (DBD) system, as described in our previous experiment, used for the removal of acetaminophen from water samples (A-Rajab et al. 2018) with little modification was utilized in this study. The major component of the plasma system consisted of plasma reactor; a high voltage current generator developed by Nanjing Suman Electronics, China and an oscilloscope (Lecroy, WaveSurfer 24x-A) to measure the discharge voltage and current generated. A schematic diagram of plasma DBD system used in this experiment is represented in Fig. 1. The plasma reactor comprised of two stainless steel electrodes organized in a parallel plate arrangement. 20 mL of sample solution was put in a glass petri-dish on the lower electrode, and the upper electrode was enclosed by means of 1 mm thick quartz plate. The distance between the quartz plate and petri-dish (two dielectric) was 7 mm, while the gap between sample surface and quartz plate was approximately 5 mm. In the current study, all the solutions were subjected to 60 W power, 10 kHz frequency at atmospheric pressure and room temperature (20 °C) for 4 min. The total input plasma power (P), averaged over the alternating voltage period (T) in the DBD system was calculated by using the following Eq. (1):
where V(t) and i(t) represent measured instantaneous voltage and current at time t, respectively (Pipa and Brandenburg 2019; Wang et al. 2016). The samples were analyzed at a time interval of 30 s. The content of caffeine and solution pH was determined after each plasma treatment. The experiment was performed in triplicate and the energy density (ED) was calculated using the following formula:
The experimental parameters including, initial concentration of caffeine solution, initial pH of the solutions and treatment time were investigated to optimize the degradation process. In order to study the effect of initial concentration on degradation process, different concentrations viz., 1, 25, 50, 75 and 100 ppm were processed, whereas, to optimize the treatment time, the DBD process was carried out at room temperature (20° C) and the samples were evaluated from the reactor at different time intervals viz., 0, 0.5, 1, 2, 3 and 4 min. To find out the optimum pH of the initial caffeine sample, several trials were processed at acidic, neutral and basic pH values.
Caffeine analysis
The concentration of caffeine was measured by high-performance liquid chromatographic system (Waters Breeze 1525, Netherland). The system was equipped with auto-sampler (Waters 2707), binary pump (Waters 1525) and UV–Visible detector (Waters 2489). Data were monitored by using Waters Breeze 2 LC solution software. The caffeine solutions were injected into Symmetry C-18 analytical column (75 mm × 4.6 mm id., particle size 3.5 µm). Ammonium acetate buffer (10 mM, pH 4.0) and acetonitrile in the ratio of 90:10 v/v were used as mobile phase, which was monitored at a flow rate of 1 mL/min, throughout the experiment. The injection volume was 20 µL and UV detection wavelength was 220 nm. Linearity of the method was evaluated by analyzing a range of caffeine working standard solutions at concentrations between 1 to 128 µg/mL. Calibration curve was constructed by plotting peak area against respective concertation. The accuracy of the method was demonstrated by estimating the recovery of caffeine at three concentration levels (2, 64 and 128 µg/mL).
Caffeine and its degradation products were identified by direct injection of samples to the mass spectrometry system containing LCQ fleet Max ion trap (Thermo Fischer Scientific, Inc., USA) equipped with electrospray interface (ESI) source operated in positive ion mode with optimum operating parameters viz., spray voltage 5 kV, ion-transfer capillary temperature 275 °C, capillary voltage 10 V. The collision gas pressure was 0.25 by argon and nebulizer and sheath gas flow rate were maintained at 35 arbitrary unit loaded by nitrogen gas. The mass spectra in the m/z range from 100 to 500 were recorded in ion scan mode. The sample solutions were injected directly into the ion trap MS without chromatographic separation at a flow rate of 10 µL/min by using 500 µL syringe.
Preparation of solutions
Standard stock solution for plasma treatment was prepared by dissolving 100 mg of caffeine into 100 mL Milli Q water. The stock solution was diluted to obtain a working solution of 100 µg/mL concentration, which was used as initial caffeine concentration. Caffeine working standard solutions of concentrations, 1, 2, 4, 8, 16, 32, 64 and 128 µg/mL were prepared by diluting the standard stock solution with mobile phase to obtain calibration curve.
Results and discussion
Increasing usage and disposal of pharmaceuticals as well as personal-care products are becoming a matter of concern as pollutants, which progressively contaminating various water resources including lakes, rivers and groundwater. Caffeine is generally found in considerably high concentrations in water streams, which can be easily estimated. Hence, it can be proposed as a probable wastewater tracer which will indicate the occurrence and level of pollution in water samples, in addition, to differentiate between wastewater and water from other environmental resources. In the present experiment, efficiency of a laboratory scale dielectric barrier discharge plasma technology has been investigated with regards to the removal of caffeine, a potential pollutant, from water samples. The aqueous caffeine solution (20 mL) was exposed to plasma treatments and the degradation of caffeine was assessed at different times. The DBD system used in this study has shown fast and effective removal efficiency, as a degradation of 72.55% of caffeine in water (100 µg/mL initial concentration, pH 7) was achieved in a short time period (4 min) of plasma treatment at atmospheric pressure in open air. The energy efficiency of the system was excellent. Moreover, the method was also considered to be cost-effective and safe, as no gas, catalyst and organic solvents were utilized. Concentration of caffeine in the sample solution before and after plasma treatment was measured by HPLC analysis.
In the HPLC method development process, chromatographic parameters were optimized by various trials and symmetric peak of caffeine was obtained at a mobile phase composition of 90:10 v/v of ammonium acetate buffer (10 mM, pH 4) and acetonitrile. The analytical elution of caffeine was achieved by using symmetry C-18 analytical column at mobile phase flow rate of 1 mL/min, and the retention time of the analyte peak was observed at 4.96 min. The method was linear in the concentration range of 1–128 µg/mL (R2 ˃0.999). Precision of the method was demonstrated by analyzing quality control sample at three concentrations, 2, 64 and 128 µg/mL (LQC, MQC and HQC, respectively), and relative standard deviation (RSD) of peak area of caffeine for six replicate injections was ˂1%. Recovery of the analyte at all three concentration levels was within 100 ± 2%. The representative chromatogram of caffeine working standard solution is shown in Fig. 2.
In this study, the DBD system was operated in the ambient air at room temperature (20ºC), active species ˙OH, O3 and H2O2 may be produced, which resulted in the degradation of caffeine. The O2 was bombarded by high-energy electrons in the electric field and O˙ was generated through direct electron impact dissociation (Eq. 3) (Kogelschatz et al. 1988). This was followed by generation of ozone (O3) due to interaction of O˙ with molecular oxygen (O2) (Eq. 4) (Reddy et al. 2014a, b). The dissolved O˙ and O3 in the water may produce hydroxyl radical (˙OH), which is stronger oxidant than O3 itself and reported to participate in the degradation of organic compounds, in this case, caffeine (Eq. 5–8) (Kim et al., 2015; Wang et al 2016). In addition to that, in the system, strong oxidant, H2O2 was also produced, which was believed to be responsible for the degradation of the target molecule to some extent (Eq. 9–10) (Wang et al., 2017). The generation of active species in the present DBD conditions may be represented by the following series of reaction equations:
After DBD treatment, the caffeine degradation products were characterized by directly injecting the sample solution in the mass spectrometer. The full scan spectrum was recorded in the range of m/z 100–500 and major degradation products were identified as molecular ion m/z = 194.9 and fragment ions at m/z 168.8, 179.8, 142.8, 126.8, 101.8 and 59.8 (Fig. 3). The fragment ions at m/z 168.8 and 179.8 were due to removal of –C = N and –CH3 ions, respectively, whereas, the fragment ions at m/z 142.8 and 126.8 were formed by cleavage of one more –C = N ion and consequently a methyl group from m/z 168.8 fragment. Successive cleavage of the pyridine ring yielded fragment ions at m/z 101.8 and 59.8 by removal of –C = CH and –CON ions, respectively.
Effect of time and initial concentration on caffeine degradation
The degradation rate of caffeine at 100 µg/mL initial concentration was found to be significantly increased with time. When the solution was treated by applying 60 W DBD plasma power, about 7.07% of caffeine was degraded in 30 s, followed by 13.4, 26.7, 34.48, and 72.55% in 1, 2, 3 and 4 min, respectively. This degradation pattern was considered to be significant. The removal efficiency of caffeine from water at 100 µg/mL initial concentration is represented in Fig. 4. To optimize the initial sample concentration, caffeine removal efficiency by DBD plasma was investigated by using samples solutions of 1, 25, 50, 75 and 100 µg/mL caffeine concentration. Results showed that the caffeine removal efficiency from water was significantly greater at lower initial concentrations, whereas at higher concentrations, lesser caffeine degradation was observed. This greater degradation of caffeine at lower concentration may be due to fast dissipation of caffeine from less saturated solution, whereas, at higher concentrations, the molecular caffeine, intermediate and fragment ions are more to compete with reactive species of DBD reactor which are constant for a particular experimental condition leading to reduced removal efficiency. The caffeine degradation rates in water were 19.31, 16.79, 9.12, 8.23 and 7.07, when the sample solution was treated by using 60 W plasma power for 30 s at 1, 25, 50, 75 and 100 µg/mL initial concentrations, respectively. The degradation rates were significantly enhanced to 96.59, 94.38, 81.6, 76.18 and 72.59%, respectively, when the treatment time was increased to 4 min, while keeping the other experimental parameters same. The removal efficiency of caffeine from water samples of above mentioned initial concentrations at different treatment times is depicted in Fig. 5.
Effects of initial pH of sample solution
Usually, pH values of analytical solutions play an important role in the degradation of the pharmaceutical substances. It can alter the rate and pathway of a degradation reaction. The pH of aqueous solution might be vital factor in the generation and stability of oxidizing species such as ozone, hydrogen peroxide, hydroxyl and oxygen free radicals in water interphase. Consequently, in this study, caffeine degradation in aqueous solution (100 µg/mL initial concentration) at acidic, neutral and basic pH values, 4, 7 and 10, respectively, was evaluated and degradation efficiency was recorded after 0.5, 1, 2, 3 and 4 min DBD treatment at 60 W DBD plasma input power. The results showed that the initial pH value of caffeine aqueous solution had significant influence on the degradation of caffeine. Maximum degradation was recorded at pH 7, while no significant degradation of caffeine was observed in solution with pH 10. The degradation rate of caffeine at pH 7 were 7.07, 13.4, 26.7, 35.48 and 72.55% of initial caffeine content at 0.5, 1, 2, 3 and 4 min treatment time, respectively, whereas these values decreased to 1.42, 3.76, 8.48, 20.29 and 21.8%, respectively, in the same treatment times at pH 4. Only 3.15% of caffeine was degraded in 4 min DBD treatment in sample solution with initial pH 10. The Caffeine removal efficiency at pH 4, 7 and 10 with respect to time of DBD treatment is depicted in Fig. 6. DBD plasma treatment of the caffeine solution (100 µg/mL) has also resulted in drop of initial solution pH which was proportional to the time of treatment. The initial pH 7 was decreased to 3.3 in 30 s treatment, which then gradually declined to 2.5 in 3 min and reached 2.0 in 4 min treatment. The sample solution with pH 4 was found to be relatively less affected as in first 30 s treatment the pH reduced to 3.5, which remained unaffected up to 1 min, and finally reached to 3.0 in 4 min of DBD treatment. No significant reduction in pH values of the solution with pH 10 was observed as a result of DBD plasma treatment for similar time periods. The variation in initial pH values as a function of time DBD plasma treatment is shown in Fig. 7. Similar pattern of reduction in pH values after DBD plasma treatment of caffeine solution (initial pH 7) was observed at 1, 25, 50 and 75 µg/mL initial concentrations (Fig. 8).
Effect of input power
The influence of input power on the removal efficiency of caffeine from aqueous solution was also investigated. The initial concentration of the test solution was 100 µg/mL and the initial pH was 7. It was observed that an increase in input power has increased the caffeine removal from solution. The caffeine degradation was 26.59% in 4 min after treatment at 40 W DBD plasma power, which was increased to 72.55%, when plasma power was increased to 60 W. The caffeine removal efficiency was reached to 77.36% and 87.55% in 4 min treatment at input power of 80 W and 100 W, respectively, in the DBD system (Fig. 9). Furthermore, the intensity of input power displayed similar effect to the pH of the caffeine solution after DBD treatment. The initial pH 7 was decreased to 3.3, 3.3, 2.9 and 2.7 in 0.5 min of treatment in the DBD system at 40, 60, 80 and 100 W input powers, respectively. The solution pH gradually decreased and finally reached to 2.5, 2.0, 1.9 and 1.8, respectively, after 4 min treatment. The relationship between input power and pH of the caffeine solution with treatment time is depicted in Fig. 10. Improved removal efficiency of caffeine from aqueous solution can be explained by generation of more active species at higher input power. The higher input power enhances the rate of electron participation, which generated stronger electric field and increased the intensity of particle collision in the system (Wang et al., 2017; Avramenko, 2013).
Energy efficiency
In this study, approximately 72.55% of caffeine from aqueous solution (100 µg/mL initial concentration) was removed in DBD plasma reactor operated in open air condition with an energy density of 7.2E05 JL−1. The energy efficiency in this study was better than that observed in our previous work (energy density 9.6E05 JL−1), in which removal of acetaminophen was investigated using similar DBD plasma system (Al-Rajab, et al, 2018). In another study, Liu et al., (3013) have reported more energy-efficient DBD system (1.5E05 JL−1) used in the removal of iopromide from water samples. The energy density may vary from one DBD system to another depending on the operating conditions and samples to be investigated. However, DBD plasma reactor used in this study is more cost-effective and safer because no carrier gas was used and the system was operated at atmospheric condition without using any organic solvent.
Conclusion
Caffeine has been considered to be one of the concerning contaminants in various surface and groundwater resources, and discharge of domestic wastes is the main source of contamination. In this study, a dielectric barrier discharge plasma system was investigated for removal of caffeine from water. After DBD plasma treatment, the caffeine concentration in the samples was estimated by HPLC and the degradation products were identified by directly injecting the sample to mass spectrometer. The influence of several experimental parameters on degradation efficiency of caffeine was evaluated. About 72.55% degradation of caffeine was achieved in a neutral sample (pH 7) after 4 min plasma treatment with an input power of 60 W. The caffeine removal was remarkably reduced at acidic (pH 4) and basic (pH 10) conditions. The removal efficiency was greater at lower initial concentrations and caffeine degradation was decreased from 96.59% to 72.55%, when the sample concentration was increased from 1 µg/mL to 100 µg/mL. Moreover, the caffeine (100 µg/mL) removal was increased from 26.59% to 87.88% after 4 min treatment, when the input power in the DBD system was enhanced from 40 to 100 W, respectively. The present DBD system was operated at atmospheric pressure in open air without use of any catalyst, expensive gases and organic solvent; therefore, it can be considered as efficient and economical. However, further efforts would be required to implement the method at large scale as well as to investigate the effluent toxicity.
References
Al-Rajab AJ, Mujahid ZI, Hakami O, Albratty M, Alhazmi HA, Hala AM (2018) Degradation of acetaminophen in water by using dielectric barrier discharge. Desalin Water Treat 109:337–343. https://doi.org/10.5004/dwt.2018.22114
Al-Rajab AJ, Sabourin L, Lapen DR, Topp E (2015) Dissipation of triclosan, triclocarban, carbamazepine and naproxen in agricultural soil following surface or sub-surface application of dewatered municipal biosolids. Sci Total Environ 512:480–488. https://doi.org/10.1016/j.scitotenv.2015.01.075
Avramenko VB (2013) Some particularities of the formation and flow of the plasma jet of an ablation pulse plasma accelerator with cylindrical geometry. High Temp 51:753–758. https://doi.org/10.1134/S0018151X13060047
Buerge IJ, Poiger T, Muller MD, Buser HR (2003) Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environ Sci Technol 37(4):691–700. https://doi.org/10.1021/es020125z
Buerge IJ, Poigner T, Müller MD, Muser HR (2006) Combined sewer overflow to surface waters detected by the anthropogenic marker caffeine. Environ Sci Technol 40(13):4096–4102. https://doi.org/10.1021/es052553l
Cantwell MG, Katz DR, Sullivan JC, Borci T, Chen RF (2016) Caffeine in Boston Harbor past and present, assessing its utility as a tracer of wastewater contamination in an urban estuary. Mar Pollut Bull 108(1–2):321–324. https://doi.org/10.1016/j.marpolbul.2016.04.006
Chen Z, Pavelic P, Dillon P, Naidu R (2002) Determination of caffeine as a tracer of sewage effluent in natural waters by on-line solid-phase extraction and liquid chromatography with diode-array detection. Water Res 36:4830–4838. https://doi.org/10.1016/s0043-1354(02)00221-x
Daly JW, Fredholm BB (1998) Caffeine-an atypical drug of dependence. Drug Alcohol Depend 51:199–206. https://doi.org/10.1016/s0376-8716(98)00077-5
Daneshvar A, Aboulfadl K, Viglino L, Broseus R, Sauve S, Madoux-Humery AS (2012) Evaluating pharmaceuticals and caffeine as indicator of fecal contamination in drinking water sources of the Greater Montreal region. Chemosphere 88(1):131–139. https://doi.org/10.1016/j.chemosphere.2012.03.016
Edwards QA, Kulikov SM, Garner-O Neale LD (2015) Caffeine in surface and wastewaters in Barbados. West Indies. Springerplus 4:57. https://doi.org/10.1186/s40064-015-0809-x
Frary CD, Johnson RK, Wang MQ (2005) Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc 105:110–113. https://doi.org/10.1016/j.jada.2004.10.027
Gokulakrishnan S, Chandraraj K, Gummadi SN (2005) Microbial and enzymatic methods for the removal of caffeine. Enz Micro Technol 37(2):225–232. https://doi.org/10.1016/j.enzmictec.2005.03.004
Gomez MJ, Martinez Bueno MJ, Lacorte S, Fernandez-Alba AR, Aguera A (2007) Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 66(6):993–1002. https://doi.org/10.1016/j.chemosphere.2006.07.051
Greenberg JL, Lewis SE, Dodd DK (1999) Overlapping addictions and self-esteem among college men and women. Addict Behav 24:565–571. https://doi.org/10.1016/s0306-4603(98)00080-x
James JE (1997) Is habitual caffeine use a preventable cardiovascular risk factor? Lancet 349:279–281. https://doi.org/10.1016/S0140-6736(96)04253-5
Kim KS, Kam SK, Mok YS (2015) Elucidation of the degradation pathways of sulfonamide antibiotics in a dielectric barrier discharge plasma system. Chem Eng J 271:31–42
Kim WJ, Kim JD, Kim J, Oh SG, Lee YW (2008) Selective caffeine removal from green tea using supercritical carbon dioxide extraction. J Food Eng 89:303–309. https://doi.org/10.1016/j.jfoodeng.2008.05.018
Kogelschatz U, Eliasson B, Hirth M (1988) Ozone generation from oxygen and air discharge physics and reaction-mechanisms. Ozone Sci Eng 10:367–377
Landolt HP, Dijk D-J, Gauss SE, Borb´ely AA, (1995) Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacol 12:229–238. https://doi.org/10.1016/0893-133X(94)00079-F
Lin AY, Lin CA, Tung HH, Chary NS (2010) Potential for biodegradation and sorption of acetaminophen, caffeine, propranolol and acebutolol in lab-scale aqueous environments. J Hazard Mater 183:242–250. https://doi.org/10.1016/j.jhazmat.2010.07.017
Liu JL, Wong MH (2013) Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China. Environ Int 59:208–224. https://doi.org/10.1016/j.envint.2013.06.012
Liu Y, Sun Y, Hu J, He J, Mei S, Xue G, Ognier S (2013) Removal of iopromide from an aqueous solution using dielectric barrier discharge. J Chem Technol Biotechnol 88:468–473. https://doi.org/10.1002/jctb.3851
Magureanu M, Mandache NB, ParvulescuVI, (2015) Degradation of pharmaceutical compounds in water by non-thermal plasma treatment. Water Res 81:124–136. https://doi.org/10.1016/j.watres.2015.05.037
Meyer L, Caston J, Lieberman HR (2004) Stress alters caffeine action on investigatory behaviour and behavioural inhibition in the mouse. Behav Brain Res 149:87–93. https://doi.org/10.1016/S0166-4328(03)00213-4
Moldovan Z (2006) Occurrences of pharmaceutical and personal care products as micropollutants in rivers from Romania. Chemosphere 64(11):1808–1817. https://doi.org/10.1016/j.chemosphere.2006.02.003
Monica C, Camargo R (1999) Caffeine daily intake from dietary sources in Brazil. Food Addit Contam 16(2):79–87. https://doi.org/10.1080/026520399284244
Nehlig A (1999) Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev 23:563–576. https://doi.org/10.1016/s0149-7634(98)00050-5
Peeler KA, Opsahl SP, Chanton JP (2006) Tracking anthropogenic inputs using caffeine, indicator bacteria, and nutrients in rural freshwater and urban marine systems. Environ Sci Technol 40(24):7616–7622. https://doi.org/10.1021/es061213c
Pincheira J, L´opez-S´aez JF, Carrera P, Navarreteb MH, Torre CDL, (2003) Effect of caffeine on in vivo processing of alkylated bases in proliferating plant cells. Cell Biol Int 27:837–843. https://doi.org/10.1016/s1065-6995(03)00169-0
Pipa AV, Brandenburg R (2019) The equivalent circuit approach for the electrical diagnostics of dielectric barrier discharges: The classical theory and recent developments. Atoms 7:14–32. https://doi.org/10.3390/atoms7010014
Reddy PMK, Krushnamurty K, Mahammadunnisa SK, Dayamani A, Subrahmanyam C (2014a) Preparation of activated carbons from bio-waste: effect of surface functional groups on methylene blue adsorption. Int J Environ Sci Technol 12:1363–1372. https://doi.org/10.1007/s13762-014-0506-2
Reddy PMK, Mahammadunnisa S, Subrahmanyam C (2014b) Catalytic non-thermal plasma reactor for mineralization of endosulfan in aqueous medium: a green approach for the treatment of pesticide contaminated water. Chem Eng J 238:157–163. https://doi.org/10.1016/j.cej.2013.08.087
Reddy S, Benotti MJ, Yin HF, Barber LB, Brownawell BJ (2006) Steroid estrogens, nonylphenolethoxylate metabolites, and other wastewater contaminants in groundwater affected by a residential septic system on Cape Cod. MA. Environ Sci Technol 40(16):4894–4902. https://doi.org/10.1021/es052595
Rodriguez del Rey Z, Granek EF, Sylvester S (2012) Occurrence and concentration of caffeine in Oregon coastal waters. Mar Pollut Bull 64(7):1417–1424. https://doi.org/10.1016/j.marpolbul.2012.04.015
Sabourin L, Al-Rajab AJ, Chapman R, Lapen DR, Topp E (2011) Fate of the antifungal drug clotrimazole in agricultural soil. Environ Toxicol Chem 30:582–587. https://doi.org/10.1002/etc.432
Seiler RL, Zaugg SD, Thomas JM, Howcroft DL (1999) Caffeine and pharmaceuticals as indicators of wastewater contamination in wells. Groundwater 37:405–410. https://doi.org/10.1111/j.1745-6584.1999.tb01118.x
Shilo L et al (2002) (2002) The effects of coffee consumption on sleep and melatonin secretion. Sleep Med. 3:271–273. https://doi.org/10.1016/s1389-9457(02)00015-1
Siegener R, Chen RF (2002) Caffeine in Boston Harbor seawater. Mar Pollut Bull 44:383–387. https://doi.org/10.1016/s0025-326x(00)00176-4
Standley LJ, Kaplan LA, Smith D (2002) Molecular tracers of organic matter sources to surface water resources. Environ Sci Technol 34(15):3124–3130. https://doi.org/10.1021/es991381n
Sui Q, Huang J, Deng S, Yu G, Fan Q (2010) Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing. China. Water Res 44(2):417–426. https://doi.org/10.1016/j.watres.2009.07.010
Ternes TA (1998) (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32:3245–3260. https://doi.org/10.1016/S0043-1354(98)00099-2
Usman K, Khan S, Ghulam S, Khan MU, Khan N, Khan MA, Khalil SK (2012) Sewage sludge: an important biological resource for sustainable agriculture and its environmental implications. Am J Plant Sci 3:1708–1721. https://doi.org/10.4236/ajps.2012.312209
Wang J, Sun Y, Feng J, Xin L, Ma J (2016) Degradation of triclocarban in water by dielectric barrier discharge plasma combined with TiO2/activated carbon fibers: effect of operating parameters and byproducts identification. Chem Eng J 300:36–46. https://doi.org/10.1016/j.cej.2016.04.041
Wang J, Sun Y, Jiang H, Feng J (2017) Removal of caffeine from water by combining dielectric barrier discharge (DBD) plasma with goethite. J Saudi Chem Soc 21(5):545–557. https://doi.org/10.1016/j.jscs.2016.08.002
Waring WS, Goudsmit J, Marwick J, Webb DJ, Maxwell SRJ (2003) Acute caffeine intake influences central more than peripheral blood pressure in young adults. Am J Hypertens 16:919–924. https://doi.org/10.1016/s0895-7061(03)01014-8
Weigel S, Berger U, Jensen E, Kallenborn R, Thoresen H, Hühnerfuss H (2004) Determination of selected pharmaceuticals and caffeine in sewage and seawater from Tromsø/Norway with emphasis on ibuprofen and its metabolites. Chemosphere 56(6):583–592. https://doi.org/10.1016/j.chemosphere.2004.04.015
White JR, Belmont MA, Metcalfe CD (2006) Pharmaceutical compounds in wastewater: wetland treatment as a potential solution. Sci World J 6:1731–1736. https://doi.org/10.1100/tsw.2006.287
Yu JG et al (2014) (2014) Aqueous adsorption and removal of organic contaminants by carbon nanotubes. Sci Total Environ 482–483:241–251. https://doi.org/10.1016/j.scitotenv.2014.02.129
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al Bratty, M., Al-Rajab, A.J., Rehman, Z. et al. Fast and efficient removal of caffeine from water using dielectric barrier discharge. Appl Water Sci 11, 97 (2021). https://doi.org/10.1007/s13201-021-01413-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01413-5