Abstract
Mobil Composition of Matter (MCM)-41 molecular sieve was successfully synthesized. Power X-ray diffraction, scanning electron microscopy and transmission electron microscopy (TEM) were used to characterize the sample. The sample showed spherical particles, and the morphology was regular. Its average particle diameter was 110 nm. TEM images showed that the sample structure presented a honeycomb pore structure and the average pore diameter was 3.5 nm. Pb2+ was adsorbed from water body by the MCM-41. The effects of acidity, contact time, temperature, adsorbent material amount and initial Pb2+ concentration on the adsorption rate were studied, and the optimum conditions of adsorption were obtained. The results show that when acidity pH value was 4.5, temperature was 25 °C, contact time was 40 min and the adsorbent material MCM-41/Pb2+ = 7.5, the adsorptive effect was the best, the adsorption rate reached 98.78% and the adsorptive capacity was 131.71 mg/g. Kinetic and thermodynamic properties of the adsorptive process were studied. Experimental results show that the adsorption of Pb2+ by MCM-41 belongs to the pseudo-second-order adsorption. According to thermodynamic equation, ΔG = ΔH − TΔS, ΔH = − 24.86 kJ/mol and ΔS = − 72.34 J/(mol K) were obtained by calculation, indicating that the adsorption was exothermic and an entropy reduction process. At the temperature of 298, 308 and 318 K, the free energy change was, respectively, ΔG1 = − 3.286 kJ/mol, ΔG2 = − 2.586 kJ/mol and ΔG3 = − 1.86 kJ/mol, illustrating that at the above temperature the reaction can be carried on spontaneously. Nitric acid, hydrochloric acid and acetic acid were, respectively, used to investigate their effect on desorption. The results show that the desorption effect using nitric acid as a desorption agent was better than those of hydrochloric acid and acetic acid. When desorption was 5 h and the nitric acid concentration was 0.5 mol/l, the desorption rate was the highest that was reached 70.01%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead is a harmful heavy metal, and pollution caused by lead from various ways to the environment has become a pressing problem. The lead inside body and its compounds content exceed the standard, which will cause very high toxic effects on the human body many systems. Lead poisoning can cause colic, hepatitis, hypertension, peripheral neuritis, toxic encephalitis, anemia, neurasthenia and other. After many chemicals stop for a little time in the environment, they may be degraded and become harmless final compounds. But lead cannot to be degraded, and once it is discharged into the environment, it remains its availability for a long time. Lead has long been persistent in the environment and is potentially toxic to many living tissues, so lead has been classified as a strong pollutant range (Feng and Zhang 2004; Tian 2000; Wu et al. 1998). The methods of treating heavy metals pollution are chemical precipitation method, oxidation reduction method, solvent extraction separation method, membrane separation technology method, ion exchange method (Feng and Zhang 2004) and biological treatment technique method (Tian 2000; Wu et al. 1998). All these methods have their some advantages, but all kinds of methods have their shortcomings, which are given as follows: (1) Chemical precipitation method is easy to produce secondary pollution. (2) In oxidation reduction treatment methods, electrolysis is the main method, but is not suitable for dealing with wastewater with lower concentration of heavy metal ions. High-voltage pulse electrocoagulation method equipment has high cost, the power consumption is high, etc. (3) In solvent extraction separation method, the loss of solvent in the extraction process and the large energy consumption in the regeneration process are some limitations, and its application is greatly limited. (4) For membrane separation technology, as complete sets of equipment are used, the cost is high and the energy consumption is great; this makes its use be limited. (5) In ion exchange treatment method, the manufacture of exchange agent is complex, the cost is high, and the amount of recycling agent consumed is large, which greatly limits its wide application. (6) In biological treatment technology, because the required biology needs domestication, the treatment cycle is relatively long. Adsorption method is one of the most important wastewater treatment methods and has been widely used in various wastewater treatments. The adsorption method processes wastewater and mainly depends on some materials that have a large specific surface area and high surface energy and have a strong adsorption capacity toward pollutants, to separate, remove them from wastewater in order to achieve the goal of purification for wastewater (Feng and Zhang 2004). It is difficult to remove trace lead in water body by the usual method. There have been some materials for the removal of heavy metals, such as activated carbon (Clifford et al. 1986) and agricultural waste (Al-Asheh and Duvnjak 1997; Wafwoyo et al. 1999). However, these materials have many disadvantages, such as low load capacity, weak interaction with metal ions, etc.
Molecular sieve is a kind of new, highly adsorptive selective adsorbent, which can, according to molecular size and configuration, carry on selective adsorption and has a strong adsorption action to unsaturated molecules, polar molecules and polarizable molecules. The open framework structure of mesoporous molecular sieve material and large internal and outside specific surface area makes it have unique adsorption function. At the same time, the good physical and chemical stable performance of molecular sieve makes it have excellent regeneration function (Liu et al. 2012). Mesoporous molecular sieve MCM-41 is a new type of the synthesized molecular sieve with one-dimensional pore structure of the hexagonal regular arrangement and pore size uniformity. Also, by adjusting the synthesis conditions, the pore size is adjusted. The very good adsorption selectivity and stability can be guaranteed. The pore channels of MCM-41 are rich and have very good adsorption ability. These advantages make it have wide applied prospect (Kresge et al. 1992; Beck et al. 1992). Nanoscale MCM-41 (Cai et al. 2001) has larger specific surface area; it is expected that nano-MCM-41 should have better adsorption performance. In this study, cetyltrimethylammonium bromide is used as the template agent with liquid phase method to synthesize nano-MCM-41. With the MCM-41, Pb2+ was adsorbed and the influence of experimental conditions on the adsorption effect was studied. The kinetic and thermodynamic properties of the adsorption have been investigated, and desorption conditions have been studied. This work is of great significance and potential applied value for the removal of water body lead(II) pollution and the study of sewage treatment. Compared with the commonly used activated carbon method, the present method has the advantages of MCM-41 low cost and strong regeneration of the adsorbent and has higher applied value.
Experimental
Reagents
-
1.
Materials for the synthesis of nano-mesoporous MCM-41
Cetyltrimethylammonium bromide (CTMAB), tetraethyl orthosilicate (TEOS) and sodium hydroxide were used.
- 2.
Reagents for the determination of lead
Pb2+ standard solution (1 mg/ml): 0.1599 g of Pb(NO3)2 [Chinese Pharmaceutical (Group) Shanghai Chemical Reagent Company] was weighed and placed in a beaker and dissolved in deionized water. Then, 1 ml of concentrated HNO3 was added; the solution was transferred into a 100-ml volumetric flask, diluted with 100 ml of deionized water and shaken well. Xylenol orange (XO) solution (0.1%, w/v): 0.050 g of XO (Xiya Reagent, China) was weighed and dissolved in a small amount of deionized water. After dissolution, 1 ml of concentrated hydrochloric acid was added and filtered. The solution was diluted with 50 ml of deionized water for use. XO solution is prepared when it is in use and it should not be stored for a long time. Buffer solution (pH 6.0): 0.054 g of sodium acetate (NaOAc) was weighed and dissolved in water. 0.56 ml glacial acetic acid (HOAc) was added, and 100 ml of deionized water was added and shaken well. 0.25% (w/v) of o-phenanthroline solution: 0.25 g of o-phenanthroline was dissolved in 7.5 ml of acetone and diluted with 100 ml of deionized water.
Nitric acid and sodium hydroxide were used for adjusting the acidity of adsorption.
The reagents used in the experiments were of analytical purity. Unless stated, the reagents were purchased from Beijing Chemical Plan, China. The water used was deionized water.
Characterization technique
The determination of lead content was spectrophotometrically made with a 722S spectrophotometer (Shanghai Lengguang Technology Co. Ltd., China). D5005 X-ray diffractometer (XRD, SIEMENS, Germany) was used for powder X-ray diffraction experiments. Through the spectrum of sample to X-ray diffraction, the crystalline phase structure, periodic arrangement, etc., information about sample was determined with Cu-Kα target, λ = 1.5418 Å, operating voltage (tube voltage) 30 kV and operation current (tube current 20 mA). The scanning range was 0° to 10°, and the step length was 0.02°. Netherlands Philips XL30-type field emission scanning electron microscope (SEM) was used to determine and obtain the SEM images in order to observe the particle size and morphology of the samples. The operating voltage was 20 kV. The sample was prepared by ethanol, and the sample was dropped on the slide glass to conduct the conductive layer. Transmission electron microscopy (TEM) photograph was obtained on FEI Tecnai, G2 F20-type field emission transmission electron microscope in order to observe the structural morphology of the sample with a working voltage of 200 kV.
Experimental procedure
Synthesis of MCM-41
In this study, MCM-41 was synthesized by the hydrothermal method, and the specific method is as follows:
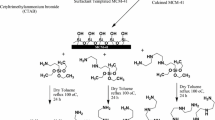
Nanometer MCM-41 sample was prepared according to the literature procedure (Cai et al. 2001) with CTMAB as template under basic conditions. A 1.0 g of CTMAB was dissolved in 480 ml of deionized water at 80 °C under vigorous stirring until the solution became homogeneous. To this solution, 3.5 ml of NaOH (2 mol/l) was added with stirring. After the solution became homogeneous, 5 ml of TEOS was slowly dropped, giving rise to a white slurry. Then, the reaction mixture was kept at 80 °C for 2 h with stirring, and then the solid products were recovered by filtering and dried overnight at room temperature. The surfactant was removed by calcination at 550 °C for 4 h in air, yielding the final white parent powder, nanometer MCM-41.
Drawing of working curve of lead
In a 25-ml volumetric flask, in order 0.02, 0.04, 0.06, 0.08 and 0.10 mg of Pb(II) working solution, 3.75 ml of 0.25% (w/v) o-phenanthroline solution, 5 ml of HOAc–NaOAc buffer solution of pH = 6.0 and 1.0 ml of 0.1% (w/v) XO solution were added, respectively, diluted with deionized water to the scale and shaken well for measurement. The absorbance value was measured with 1-cm cells at 535 nm by using the corresponding reagent blank as reference. The working curve for the determination of lead was drawn by absorbance against Pb(II) solution concentration. A linear regression equation was calculated according to the data experimentally obtained (Du et al. 2009).
Study of adsorption of Pb2+ by MCM-41 molecular sieve
Twenty milliliters of 2 mg/l Pb2+ working solution was taken and placed into a 100-ml beaker. The pH value was adjusted to 4.5 using 6 mol/l HNO3 or 6 mol/l NaOH solutions. The final solution volume was adjusted with water to be 40 ml. 0.30 g of MCM-41 molecular sieve was accurately weighed, added to the above-stated sample, stirred at room temperature (25 ± 1 °C) for 40 min and filtered. The Pb2+ content in the filtrate was determined by the above-stated spectrophotometric method, and the adsorption rate and adsorption capacity were calculated.
When the effects of experimental conditions were studied, the experimental parameters variables values used were: the initial pH (1.0–10.0), the contact time (0–60 min), the amount of adsorbent MCM-41 (0–1.000 g), the initial concentration of Pb2+ (0–10 mg/ml) and the temperature (15–55 °C).
Lead desorption in (MCM-41)–Pb
Desorption of the lead from (MCM-41)–Pb was studied with three kinds of desorption agents HNO3, HCl and HOAc. The experimental material of desorption (MCM-41)–Pb was prepared by the method in “Study of adsorption of Pb2+ by MCM-41 molecular sieve”, denoted as sample A. The sample A was placed in a 100-ml beaker containing 30 ml of 0.1 mol/l HNO3 (or HCl, HOAc), soaked for 0, 1, 3, 5, 7, 8 and 10 h and filtered. The content of lead in the filtrate was determined by spectrophotometry, the desorption rate was calculated, and the desorption time–desorption ratio curve was plotted.
Experimental method of influence of HNO3 concentration on the desorption of lead in (MCM-41)–Pb
The preparation method of desorption (MCM-41)–Pb experimental material was the same as the experiment procedure of “Study of adsorption of Pb2+ by MCM-41 molecular sieve”. It was denoted as sample A. The sample was placed in a 100-ml beaker containing 30 ml of 0.1, 0.3, 0.5, 0.7 and 1.0 mol/l HNO3, soaked for 5 h and then filtered. The content of lead in the filtrate was determined by the spectrophotometric method, the desorption rate was calculated, and the desorption time–desorption ratio curve was plotted.
Results and discussion
Powder X-ray diffraction (XRD)
The analysis results of sample XRD are shown in Fig. S1 (see Supporting Materials), indicating that for the molecular sieve obtained four peaks emerged, which can be assigned to the peaks obtained by (100), (110), (200) and (210) crystal plane diffraction. The diffraction peak locations are basically the same as those reported by the literature (Cai et al. 2001), proving that the synthesized material was MCM-41.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) study
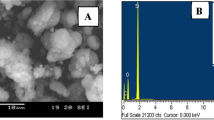
SEM can well reflect the morphology and size of sample particles. From Fig. S2 (see Supporting Materials), the scanning electron micrograph of sample, it can be seen that the morphology of the sample is mainly a spherical grain structure with regular morphology, and the average particle diameter is 110 nm. Figure S3 (see Supporting Materials) is the TEM diagram of nanometer MCM-41. The sample hole presents a honeycombed structure, and the average pore diameter is 3.5 nm.
Working curve for the determination of lead
The test results show that Beer’s law is obeyed over the concentration range of 0–4.0 µg/ml for Pb2+. The linear regression equation of method is A = 0.0288C + 0.0035 (C: µg/ml), A is the absorbance, C is the lead concentration (µg/ml), and the correlation coefficient R = 0.9998. The apparent molar absorptivity of the method for the determination of lead is 2.01 × 104 l mol−1 cm−1, expressing the ability of lead complex to absorb light at a wavelength of 535 nm.
By formula (1), the Pb2+ concentration in the solution to be determined can be calculated.
Influence of the adsorption conditions on adsorption impact
Impact of pH value
Acidity of the solution is one of the most important parameters to control the removal of heavy metals from wastewater. It affects the surface charge of the adsorbent and the degree of ionization of the lead ion. According to the standard work curve of Pb2+ obtained, the adsorption rate of lead at different pH (1.0–10.0) was calculated, and the effect results of pH on adsorption impact obtained are shown in Fig. S4 (see Supporting Materials). It can be seen that when the initial pH of solution is in the range of 1.0–4.5, the adsorption rate of MCM-41 to Pb2+ increases with the increase in pH value. This is because the adsorption of Pb2+ by MCM-41 is always accompanied by the release of H+. After pH increases, neutralization to H+ can reduce the competitive role of H+, which is beneficial to the adsorption of Pb2+ by adsorbent. At the time of pH 4.5, the adsorption rate of Pb2+ by MCM-41 is the highest. After that, with the further increase in pH, the adsorption of Pb2+ on the MCM-41 is suppressed due to the hydrolysis of Pb2+, so the adsorption rate of Pb2+ decreases and so pH 4.5 is chosen as the optimum adsorption acidity.
Effect of contact time
The effect of contact time on the removal of Pb2+ under the condition of MCM-41/Pb2+ = 7.5 is shown in Fig. S5 (see Supporting Materials). It can be observed that the initial adsorption rate is very high. As time goes on, the adsorption rate of Pb2+ shows an upward trend. With the further increase in contact time, when 40 min is reached, the adsorption rate almost reached a constant value, the adsorption reached an equilibrium and the adsorption rate no longer increased with the increase in time. The adsorption material reached the maximum saturation capacity, and Pb2+ reached the maximum adsorption rate. The optimum adsorption time is 40 min. At the beginning time, all the hydroxyl groups on the MCM-41 can be contacted with Pb2+ for ion exchange. As time goes on, these hydroxyl groups are gradually consumed. When the adsorption rate is equal to the desorption rate, the adsorption reaches equilibrium and the adsorption reaches the maximum value.
Effect of Pb2+ initial concentration and MCM-41 dosage
The effect of the initial concentration of Pb2+ on the adsorption results is shown in Fig. S6 (see Supporting Materials). The adsorption rate increases with increasing initial concentration of Pb2+ (0–1.0 mg/ml). The initial Pb2+ concentration provides the driving force to overcome the Pb2+ mass transfer resistance between aqueous and solid phase; increasing the initial concentration of Pb2+ can increase the interaction between adsorbent and Pb2+. The adsorption rate reached the maximum 1.0 mg/ml, i.e., the optimum initial concentration of Pb2+ was 1.0 mg/ml. Figure S7 (see Supporting Materials) shows the influence of MCM-41 dosage on the Pb2+ adsorption rate. It can be seen that when the adsorbent dosage is between 0 and 0.05 g, the adsorption rate of Pb2+ increases with the increase in adsorbent amount. This is because with the increase in adsorbent dosage greater adsorption surface area and more adsorption sites were resulted. When MCM-41 is 0.3 g, the adsorption rate of Pb2+ reaches the maximum, so the optimum amount of molecular sieve was set at 0.3 g.
Influence of temperature
Figure S8 (see Supporting Materials) shows the influence of temperature on the adsorption of Pb2+ by the adsorption system. The adsorption rate increases with the increase in temperature over the range of 15–25 °C. The maximum is reached when the temperature reaches 25 °C. After that, the adsorption rate decreases with the increase in temperature, indicating that the adsorption process is exothermic. But the change trend is not obvious, and the temperature has little influence on the MCM-41 adsorption performance. The reason may be that the influence of temperature on adsorption has two sides. First of all, when the temperature rises, the thermal motion of Pb2+ increases rapidly and the vibration in the solution accelerates, which makes it easier to diffuse into the adsorption material channels. The adsorption rate increases with the increase in temperature, and thus a positive effect is produced. However, the exchange adsorption of Pb2+ and the hydroxyl radical in MCM-41 are carried on, which is exothermic. Therefore, the increase in temperature is not conducive to ion exchange adsorption, which results in the decrease in adsorption rate with the increase in temperature and causes a negative effect. Because of the relative cancellation of the two kinds of effects, variation of the adsorption rate with the temperature is not big. Considering that at the high-temperature a large amount of energy is consumed, the adsorption is carried out at room temperature of 25 ± 1 °C.
The optimized adsorption condition of Pb2+ on MCM-41 molecular sieve was as follows: 0.3 g of the molecular sieve is used at 25 °C to adsorb Pb2+ with 1 mg/ml solution at pH 4.5 for 40 min. The adsorption rate at this time is 98.71%, and the adsorption capacity is 131.71 mg/g.
Kinetic study results of adsorption process
Adsorption kinetics curves of pseudo-first-order model
The kinetic study of an adsorption process is mainly to describe the rate of an adsorbate adsorbed by adsorbent, and the adsorption rate controls the retention time of the adsorbate on the solid–liquid interface. It is necessary to do research on the treatment of wastewater by the adsorption method.
Pseudo-first-order model:
The adsorption kinetic model can be described by Lagrange first-order rate equation (Lagergren 1898):
Where qe is the equilibrium adsorption quantity, mg/g; q is the adsorption amount at a certain time, mg/g; and k1 is the adsorption kinetic rate constant (min−1). For formula (1), integration is made from t = 0 to t > 0 (q = 0 to q > 0), written in the linear form as follows:
Pseudo-second-order model of adsorption kinetics
The pseudo-second-order model of adsorption kinetics can be described by using Ho and McKay equation (Celekli et al. 2011; Ho and Mckay 1998; Ho and McKay 1999). It is established based on the rate-controlling step and is a second-order kinetic equation on the basis of chemical adsorption through chemical reaction or through sharing of electrons or through getting or losing the electrons. The expression formula of second-order kinetic equation is:
Formula (4) is integrated from t = 0 to t > 0 (q = 0 to q > 0); its linear form is written as follows:
Where t is the time (min); qt is the adsorption amount at a certain time (mg/g); k2 is the adsorption kinetic rate constant (g mg−1 min−1); and qe is the equilibrium adsorption quantity (mg/g).
The experiments were carried out to make adsorption studies at the same stirring speed, the temperature 25 °C and the solution pH 4.5 by using 1 and 4 mg/ml Pb2+ solutions. Figures 1a, b and 2a, b show, respectively, pseudo-first-order and pseudo-second-order kinetic simulation curves made by different concentrations of Pb2+. The kinetic parameters obtained by correlation calculations are shown in Table 1. After calculation, the correlation coefficient of the 1 mg/ml Pb2+ pseudo-first-order kinetic equation is R = 0.8924 (R2 = 0.7964), and the coefficient of pseudo-second-order kinetic equation is R = 0.9995 (R2 = 0.9991). The correlation coefficients of 4 mg/ml Pb2+ pseudo-first-order kinetic equations are R = 0.9248 (R2 = 0.8553), and the correlation coefficients of the pseudo-second-order kinetic equations are R = 0.9999 (R2 = 0.9998). It can be seen that when the kinetic data were fitted by the pseudo-first-order kinetic equation, the R21 is smaller, the calculated adsorption equilibrium capacity of different concentrations deviated larger from the values of the experimental measurements, and the differences are larger. When the pseudo-second-order kinetic equations were used to fit, all the correlation coefficients R22 are greater than 0.999 and the calculation values of equilibrium adsorption capacities for different concentrations are basically consistent with the actual measured value, so the dynamics of the adsorption system more conform to pseudo-second-order kinetic equation. Therefore, it can be concluded that the process of adsorption toward Pb2+ by MCM-41 conforms to the pseudo-second-order kinetic equation, and the pseudo-second-order model can be utilized and better predict the adsorption behavior of Pb2+ on mesoporous MCM-41.
Adsorption equilibrium isotherm
The adsorption isotherm is a mathematical formula expressing the relationship between the amount of adsorption and the concentration of the solution under the condition of constant temperature fixing. At present, different kinds of mathematical formulas have been put forward, each of which has its applicable range. There are two kinds of commonly used ones: Langmuir adsorption formula and Freundlich adsorption formula. Langmuir and Freundlich equation is commonly used to describe the adsorption isothermal model of adsorption behavior and parameters. In this study, two models were used to test the adsorption properties and adsorption process of adsorbent. The Langmuir adsorption isotherm model assumed that adsorption takes place at specific homogeneous sites within the adsorbent, and it has been used successfully for many adsorption processes of monolayer adsorption. The Langmuir equation is expressed by the following relation (Langmuir 1918).
Where qe (mg/g) is the equilibrium load capacity of adsorbent, Qm (mg/g) is the maximum adsorption capacity of monolayer coverage, Ce (mg/l) is the equilibrium concentration of adsorbent and KL (l/g) is the adsorption equilibrium constant.
The linearized form of Langmuir isotherm can be written according to the following equation.
The slope and intercept of the plot between Ce/qe versus Ce will give Qm and KL, respectively.
The Freundlich adsorption isotherm model considers a heterogeneous adsorption surface that has unequal available sites with different energies of adsorption. The Freundlich adsorption isotherm model is represented as follows (Freundlich 1906):
The linearized form of Freundlich can be expressed as:
where qe is the amount of adsorbate metal ion adsorbed at equilibrium time (mg/g), Ce is the equilibrium concentration of adsorbate in solution (mg/l), KF is the capacity of the adsorbent and n is the intensity of adsorption constant for Freundlich. The plot of lnqe versus lgCe is employed to determine the KF and n from intercept and slope, respectively. Generally, the value of the linear regression correlation coefficient R2 gives an indication as to which model can be chosen to give the best fit.
In this study, the adsorption isotherms of Langmuir and Freundlich obtained by the adsorption of Pb2+ by MCM-41 are shown in Figs. 3 and 4. The corresponding parameters of its adsorption isotherm equation are shown in Tables 2 and 3. By the linear fitting results of Langmuir and Freundlich isothermal adsorption equations, the fitting effect of Langmuir adsorption isotherm equation is not good, and the maximum saturated adsorption capacity of theory is largely different from the experimental value. The fitting effect of Freundlich adsorption isotherm equation is better, and all the fitted linear correlation coefficients R are greater than 0.99, showing that the Freundlich adsorption isotherm established according to the multi-molecular layer adsorption model can better express this adsorption process. The process of MCM-41 adsorption toward lead(II) conforms to the Freundlich adsorption model and is multi-molecular layer adsorption. The n value of the Freundlich index of this adsorption system is greater than 1. At the time of n < 1, the adsorption is non-preferential adsorption. At the time of n = 1, the adsorption is linear adsorption. At the time of n > 1, the adsorption is preferential adsorption, meaning that molecular affinity between adsorbate molecules and solid adsorbent drops with the increase in temperature and the isotherm slope drops with the increase in the solute concentration in solution phase. Therefore, the adsorption of lead(II) by MCM-41 can be well represented by the Freundlich isotherm adsorption equation established by basing on the multi-molecular layer adsorption model, and the adsorption process is preferential adsorption.
Thermodynamic properties of adsorption
The thermodynamics of an adsorption process may be obtained from a study of the influence of temperature on the process. The mechanism of adsorption can be deduced from thermodynamic parameters: Gibbs free energy change (∆G°), enthalpy change (∆H°), and entropy change (∆S°). The linear equation is listed as follows:
Where KL is the adsorption equilibrium constant (KL is the parameter in the Langmuir equation), Qe is the equilibrium adsorption capacity (mg/g), Ce is the equilibrium concentration (mg/l), T is the thermodynamic absolute temperature and R is a physical constant regarded as a universal gas constant (8.314 J/mol K). ∆G° is the Gibbs free energy change.
The standard enthalpy change and standard entropy change of adsorption process are calculated according to the Van’t Hoff Eq. (12)
Where ΔH0 is the enthalpy change, ΔS0 is the entropy change and ∆G0 is the Gibbs free energy change.
In this study, the thermodynamic properties of nano-MCM-41 adsorption lead(II) process were calculated. Figure 5 shows the Van’t Hoff equation curve of this adsorption; the curve equation is y = 2.6024x − 10.0744 with a correlation coefficient of R = 0.9977. The calculated results of related thermodynamic parameters are listed in Table 4. ΔH0 = − 24.86 kJ/mol < 0 indicates that the process of MCM-41 adsorption Pb2+ is an exothermic reaction. ΔS0 = − 72.34 J mol−1 K−1 < 0 shows that the adsorption is a process of entropy reduction. After MCM-41 adsorbed Pb2+, the degree of order for material arrangement increased. It has been known (Table 4) that ΔG0 is less than zero, showing that the MCM-41 adsorption Pb2+ process has a spontaneous property. With the increase in temperature, the absolute value of ΔG0 decreased, showing that the higher the temperature, the smaller the spontaneous trend of adsorption process.
Desorption results
The desorption results of (MCM-41)–Pb by nitric acid are shown in Fig. S9. The desorption efficiency of Pb2+ increases gradually with the increase in time. This is mainly due to the fact that the Pb2+ electron density is too concentrated, there is an empty orbital in the outer layer, and the complexes formed by Pb2+ and the hydroxyl groups in MCM-41 are not stable enough. With the increase in time, Pb2+ in the solution is gradually liberated, the Pb2+ concentration in solution increases gradually and the desorption rate increases. When desorption time reached 5 h, the desorption rate reached the maximum value of 63.38%. But as time increased, Pb2+ desorption rate decreased. This is probably because as time elapsed, the Pb2+ desorbed in the solution was adsorbed in MCM-41 in the form of physical adsorption, resulting in a decrease in the Pb2+ concentration in the solution, thereby reducing the adsorption rate of Pb2+.
The effect results of nitric acid concentration on desorption of Pb2+ (Fig. S10) show that the desorption rate of Pb2+ increases gradually with the increase in nitric acid concentration. When the concentration of nitric acid is 0.5 mol/l, the desorption rate is the maximum, which is 70.01%. After that, when the concentration of nitric acid was continued to increase, the adsorption efficiency of Pb2+ was decreased. This is mainly because the H+ and Pb2+ in the solution have competitive adsorption action. When the acidity of solution is smaller, the concentration of H+ solution is smaller. The hydroxyl in the modified MCM-41 molecular sieve mainly combines with Pb2+. The Pb2+ content in solution was decreased, and the desorption rate was low. With the increase in acidity, the Pb2+ content in solution increased and the desorption rate also changed to be high. When the acidity continues to grow, the H+ concentration in solution is excessively large, which has an impact on the internal structure arrangement of molecular sieve and thus makes desorption rate be decreased.
To sum up, the optimum desorption acidity is 0.5 mol/l HNO3, If the acidity is too high or too low, this is not beneficial to the desorption. The reason is that the acidity is low, H+ number is small, the capacity of replacement for Pb2+ is low, and therefore, the desorption rate is low.If acid concentration is too high, which causes the change of mechanical strength and the physicochemical properties of adsorbent. This is not conductive to the desorption neither. In addition, the desorption effect of HCl on Pb2+ showed that when the maximum desorption rate time 5 h was reached, the desorption rate was 54.97%. When acetic acid was used as desorbent and the time reached 3 h, the desorption rate reached 52.95%. The effects of both these two desorbents are lower than that of nitric acid.
Conclusions
In this study, mesoporous MCM-41 molecular sieve was successfully prepared. The morphology of the sample was mainly spherical crystal particle structure, with regular morphology and average particle diameter of 110 nm. The optimum conditions for the adsorption of Pb2+ by MCM-41 molecular sieve are MCM-41/Pb2+ (m/m) = 7.5, starting pH = 4.5, at room temperature of 25 ± 1 °C and stirring 40 min. In the kinetic study, it was found that the adsorption of Pb2+ by nanomaterial MCM-41 conforms to the quasi-second-order equation. The adsorption agrees with the Freundlich adsorption isotherm and belongs to a heterogeneous adsorption. The thermodynamic results showed ΔH0 = − 24.86 kJ/mol and ΔS0 = − 72.34 J/(mol K). At the temperatures of 298, 308 and 318 K, ΔG0 < 0, indicating that the adsorption belongs to endothermic entropy reduction spontaneous reaction process. After MCM-41 adsorbed Pb2+, the ordered degree of material arrangement increased. The desorption effect of using nitric acid as desorption agent is better than those of hydrochloric acid and acetic acid. When desorption is 5 h and the concentration of nitric acid is 0.5 mol/l, the desorption rate is the highest, reaching 70.01%.
References
Al-Asheh S, Duvnjak Z (1997) Sorption of cadmium and other heavy metals by pine bark. J Hazard Mater 56(1–2):35–51
Beck JS, Vartuli JC, RothWJ Leonowicz ME, Kresge CT, Schmitt KD, Chu TW (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114(27):10834–10843
Cai Q, Luo ZS, Pang WQ, Fan YW, Chen XH, Cui FZ (2001) Dilute solution routes to various controllable morphologies of MCM-41 silica with a basic medium. Chem Mater 13(1):258–263
Celekli A, Tanriverdi B, Bozkurt H (2011) Predictive modeling of removal of lanaset red G on chara contraria: kinetic, equilibrium, and thermodynamic studies. Chem Eng J 169:166–172
Clifford D, Subramonian S, Sorg T (1986) Removing dissolved inorganic contaminants from water. J Environ Sci Technol 20(11):1072–1080
Du HY, Zhang ZQ, Zhang YN, Li MQ (2009) Color reaction of complex between xylenol orange and lead(II) and application. J Baoji Coll Arts Sci (Nat Sci Ed) 29(1):35–40
Feng B, Zhang LM (2004) Current situation and prospect of research of treatment technologies for electroplating heavy metal wastewater. Jiangsu Environ Sci Technol 17(3):38–40
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:385–387
Ho YS, Mckay G (1998) A comparison of chemisorption kinetics models applied to pollutant removal on various sorbent. Process Saf Environ Prot 76:332–340
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieve synthesized by a liquied-crystal template mechanism. Nature 359:710–712
Lagergren S (1898) About theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Langmuir I (1918) Adsorption of gases on plain surfaces of glass mica platinum. J Am Chem Soc 40:136–403
Liu Y, Pan JM, Chen J, Xie JM, Li CX, Yan YS (2012) Research progress of modified silica-based micro/nano materials and their application in separation chemistry. Metall Anal 30(9):37–46
Tian JM (2000) Application of bioadsorption method in the treatment of wastewater containing heavy metals. J Taiyuan Univ Technol 31(1):74–78
Wafwoyo W, Seo CW, Marshall WE (1999) Utilization of peanut shell as adsorbents for selected metals. J Chem Technol Biotechnol 74(11):1117–1121
Wu J, Li QB, Deng X, Lu YH (1998) Research advances in biosorption of heavy metals. Ion Exchange Adsorpt 14(2):180–187
Acknowledgements
This study was funded by the Natural Science Foundation of Jilin Provincial Science and Technology Department from the Science and Technology Development Program of Jilin Province, China (20180101180JC, 222180102051, KYC-JC-XM-2018-051) as well as Science Research Project of Education Department, Jilin Province, from the 13th Five-Year Plan, China (JJKH20200265KJ). The authors express their thanks.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by all the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, XD., Zhai, QZ. Use of nanometer mesoporous MCM-41 for the removal of Pb(II) from aqueous solution. Appl Water Sci 10, 122 (2020). https://doi.org/10.1007/s13201-020-01203-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01203-5