Abstract
With an increasing global usage of dyes and colorants in various industries, the increase in dye contamination level in water has frequently reported. Developments of appropriate techniques for the degradation of dyes in industrial wastewater are critical and urgently required. Utilization of low-cost materials for the treatment of various dyes using an alternative driving energy could be promising in near future. In the study, activated carbon (AC) was prepared using agricultural waste (coconut shell) and utilized for designing the CeO2/AC composites using soft chemical route. The effect of AC on the composite was studied by varying the AC and CeO2 weight ratios, and as-prepared photocatalytic composites were characterized using suitable analytical techniques. Characterization results clearly indicated a remarkable impact of AC on porosity and surface area, surface morphology, structural and textural properties and photocatalytic activities of the composites. Complete removal of industrial dyes used in aqueous media was observed and 91.3% of degradation efficiency of organic pollutants in real-time textile industrial wastewater within 5 h under LED light source. Reusability study clearly indicated that CeO2/AC composites could be reused up to three cycles for the removal of organic pollutants and dyes in aqueous media under visible light source as an alternative driving energy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All around the world, water is becoming an increasingly scant resource and people are forced to consider any source of water, which might be used economically and effectively to promote further development. Many industries are producing large amount of dyes and use them in textile, papers, plastics, pharmaceuticals, food industries, etc. Dye and coloring materials are labeled as important sources of environmental contamination, due to their high toxicity and non-biodegradability effects on aquatic creatures and humans (Foo and Hameed 2010). Some amount of dye molecules is highly visible and affects water quality to a larger extent. Most of the industrial dyes are very difficult to degrade, as they are stable to heat, light and oxidizing agents. Initially, chlorination methods were employed to decolor the water that was contaminated by various dyes, and later, it was confirmed that chlorination processes caused greater danger than the color itself by producing chlorinated hydrocarbon as by-products (Gosetti et al. 2004). Physicochemical and biological removal techniques like adsorption, coagulation, flocculation, membrane filtration, ozonation, electrochemical, radiolysis, bacterial, algal, fungal and advanced oxidation processes have been known to decolorize the industrial wastewater (Ince and Tezcanh 1999; Kannan and Sundaram 2001; Rai et al. 2005; Wojnarovits and Takacs 2008; Solmaz et al. 2009; Chaudhari et al. 2011). Unfortunately, these wastewater treatment techniques have their own drawbacks to complete treatment of dye molecules from various industrial wastewaters. Wastewater being released from various industries comprise of different dyes and colorants. Hence, it becomes essential to promote the prevailing techniques as well as to look for new techniques that decolorize the mixture of dyes rather than a single dye solution. It is now a popular perception that the solutions to the existing and future water challenges will hinge upon further developments of advanced materials and methods. Designing the novel and affordable materials for wastewater treatment has experienced a significant growth in recent days. It is poised to make its contribution in creating advanced next-generation water treatment technologies. Nanocomposite materials have emerged as suitable alternatives to overcome limitations of microcomposites and monolithics, while posing preparation challenges related to the control of elemental compositions and stoichiometry in the nanocluster phases (Roy et al. 1986; Schmidt et al. 2002). In the view of possessing design uniqueness and property combinations, general understanding of these properties is influenced by various compositions and ratios yet to be studied. Nowadays, designing the novel photocatalytic composites with desired properties offers new technologies and opportunities for advanced and eco-friendly water treatment. In the present research work, the preparation of low-cost composite materials were carried out using indigenously prepared activated carbon (AC) with CeO2 through soft chemical processes. As-prepared carbon CeO2/AC photocatalytic composites were demonstrated for the treatment of various industrial dyes in aqueous media. Organic pollutants with complex mixtures of various dyes in real-time textile wastewater were effectively removed CeO2/AC photocatalytic composites within short duration using LED source as an alternative driving energy.
Materials and methods
Preparation of activated carbon (AC) using coconut shell
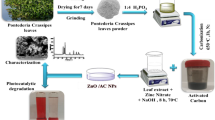
Activated carbon was prepared indigenously using coconut shell as raw material. Coconut shell was collected, cleaned and shredded into small pieces. It was then subjected to pyrolysis in a muffle furnace at 800 °C followed by soaking and drying at 60 °C. The preparation of AC using coconut shell in detail can be obtained from our previous publication (Shivaraju et al. 2018). As-prepared AC material was grinded, sieved and stored in airtight containers. Schematic of indigenous preparation of AC is shown in Fig. 1.
Preparation and characterization of CeO2/AC composites
The CeO2/AC composites were prepared using soft chemical and hydrothermal treatment techniques using alkaline solution of pH 10. Cerium oxide (N. R. Chemicals, India) and activated carbon (obtained from coconut shell) were mixed under alkaline condition with continuous stirring using magnetic stirrer for 10 h. As-obtained homogeneous solution was kept in dark for 24 h for aging and treated under hydrothermal conditions at 120 °C for 6 h (Shivaraju 2011). After hydrothermal treatment, the final product was carefully washed using deionized water and dried using dust-free hot air oven. Same procedure was used to prepare CeO2/AC composites by varying the weight ratios of AC from 0 to 80%. The CeO2/AC composites prepared were characterized by analytical techniques like UV–Vis spectroscopy, powder X-ray spectroscopy, scanning electron microscopy, Fourier infrared spectroscopy, dynamic light scattering and photocatalytic activity under different photon sources (Shivaraju et al. 2017).
Photocatalytic degradation of industrial dyes
Photocatalytic degradation of the selected model industrial dyes such as brilliant green (HiMedia, India), methyl orange (HiMedia, India), brilliant blue (HiMedia, India) and phenol red (HiMedia, India), which are commonly being used in many industries like textile, paper, tanneries, food, pharmaceutical, etc., was carried out in a reaction vessel using CeO2/AC composites under LED light source. About 0.015 g of composite material was suspended into 50 ml of an aqueous dye solution with continuous stirring; then, it was kept in dark for 3 h to attain the adsorption–desorption equilibrium. A blank experiment was maintained without composite material. The initial and final concentrations of dye in an aqueous solution were determined using spectroscopic techniques with respect to the wavelength. After 3 h, dyes in an aqueous medium with suspended composite were irradiated to a light source (Philips, 9 W LED lamp) for 6-h duration. After that, aqueous dye samples were centrifuged to remove the suspended composite materials and concentration of dyes in aqueous media was determined. Photocatalytic treatment of real-time industrial wastewater, which was collected from textile industry, was determined using CeO2/AC composites under LED light source for 5-h duration. Degradation rate of organic pollutants including dyes was evaluated by the determination of the initial and final COD value of industrial wastewater (Shivaraju et al. 2017).
Results and discussions
CeO2/AC composites with different weight ratios were prepared using soft chemical and hydrothermal technique, and varied weight percent ratios of AC and CeO2 in the composites are given in Table 1.
Characterization of CeO2/AC composites
Band gap energy
As-prepared CeO2/AC composites were determined for its band gap energy using spectrophotometric techniques, and the results obtained are shown in Fig. 2. Absorption of UV–visible light may result the excitation of electrons in both atoms and molecules to higher energy levels. Each molecule undergoes electronic excitation following the absorption of light. The wavelength of light absorbed is the characteristic of its chemical structure. Hence, the absorption spectra can be used qualitatively to identify atomic and molecular species. Crystalline cerium dioxide has a band gap of 3.19 eV (Devadoss et al. 2016) and absorbs strongly in the UV region with the absorption threshold near λ = 1240/Eg = 400 nm (25,000 cm−1) (Ranjan and Rao 2000). The band gap of a material can be estimated from the absorption edge wavelength of the inter-band transition (Rao and Sahu 2001). The particle size of semiconductor material such as ceria is very sensitive to the UV absorption edge wavelength. All the samples exhibit a strong absorption at near UV region, i.e., > 360 nm. The charge transfer transition from O2− (2p) to Ce4+ (4f) orbitals in CeO2 (Rao and Sahu 2001; Wang et al. 2007) resulted in the sharp absorption bands at 282 and 308 nm in the UV range. These spectral profiles indicate the inter-band transitions in CeO2 (Zaki et al. 1997). It is observed that the intensity of the absorption is decreased as the % wt of CeO2 decreases from 5 to 0. Direct band gap (Eg) of samples is determined by fitting absorption data to direct transition Eq. (1):
where α is the optical absorption coefficient, hυ the photon energy, Eg the direct band gap and Ed a constant (Gao et al. 2001). Band gap of CeO2 samples have been measured by plotting (αhυ)2 as a function of photon energy and extrapolating linear portion of the curve to adsorption equal to zero (Babitha et al. 2015).
Powder X-ray diffraction spectroscopic study
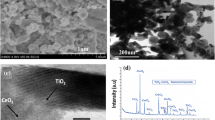
Figure 3 shows the XRD patterns of CeO2/AC composites, and CeO2 sample exhibits typical peaks corresponding to (111), (200), (220) and (311) planes, which are typical of face-centered cubic fluorite structure. All peaks can be indexed to a pure cubic fluorite structure of CeO2, which is in agreement with the JSPDS file No. 81-0792 for CeO2. Similarly, XRD peaks of AC were confirmed with the JSPDS file No. 46-0944, while new peaks formed in the composite implies the formation of CeO2/AC and it was in agreement with JSPDS file No. 26-0356. The results obtained showed that the characteristic peaks of AC have not appeared in XRD pattern of the composite that attributed to fact that the crystalline extent of ACs is much lower than the crystalline extent of CeO2 and it may lead to the shielding of the peaks of AC by CeO2 in the compositions (Gupta and Saleh 2011a, b). The XRD results clearly showed good crystalline structure of CeO2/AC prepared using hydrothermal techniques, and crystalline phases of composites apparently exhibit good photocatalytic activity and stability.
FTIR study
FTIR spectroscopy is used to identify the functional groups and study the vibrational motion of atoms and molecules (Babitha et al. 2015). In FTIR spectroscopy, each molecule only absorbs IR light of certain frequencies based on the characteristic for each molecule. Hence, it is possible to identify the molecule type (qualitative analysis) and the amount of molecule in the sample (quantitative analysis) by studying the absorption spectrum. The FTIR spectra of the samples of different compositions are shown in Fig. 4. The absorption peak found at 2000–3400 cm−1 which is assigned to OH stretching vibrations of H2O in the CeO2 (Gao et al. 2001) and absorption band at 1380 cm−1 correspond to physically adsorbed water molecules (Ho et al. 2006). The absorption bands at 848 cm−1 and 521 cm−1 are produced by CeO2, which is the typical peak for the Ce–O stretching vibrations (Niu et al. 2009; Palard et al. 2010; Khan et al. 2011). In addition, the spectra of CeO2 show all principal vibrational modes, which are in good agreement with the literature (Gao et al. 2001; Ho et al. 2006; Niu et al. 2009; Palard et al. 2010; Khan et al. 2011). It is observed that most of the peaks of CeO2/AC composite are located approximately at the following regions of the FTIR spectra: 3440 cm−1, 2932 cm−1, 2860 cm−1, 1626 cm−1, 1445 cm−1, broad band between 1300 cm−1 and 1000 cm−1, and small bands between 870 cm−1 and 450 cm−1, respectively. The surface functional groups relating to these regions were found to be hydroxyl group of water molecules adsorbed: C–H symmetric and asymmetric stretching of residual methylene groups on the surface (El-Khouly et al. 2017). Sharp bands observed at 880 cm−1 and 1445 cm−1 are assigned to bending vibration of C-H bond in 1,2,4-trisubstituted group and vibration of α-CH2 bending, respectively. The band observed at 1625 cm−1 is assigned to stretching vibration of C=O bond in carbonyl group. The absorption bands of phosphorous-containing groups (e.g., P=O, P–O–P, P=OOH, P–O–C) and C–O groups are located between 1300 and 1000 cm−1 (Puziy et al. 2002; Bandosz and Ania 2006; El-Khouly et al. 2017), respectively. The formation of various functional carbonylic and alcoholic groups can be observed at 3430 cm−1 (–OH), 1625 cm−1 (COOH) and 1082 cm−1 (C–O) (El-Khouly et al. 2017), indicating that the fictionalization process has been effectively taken place. The end region is usually associated with the residues of aliphatic groups and the out of plane deformation mode of C–H in substituted benzene rings, resulting in the carbonization of lingo cellulosic materials (Puziy et al. 2002). In the case of CeO2, the characteristic peak of Ce–O bond appears at around 450 cm−1, whereas in the case of composite it has been shifted to 461 cm−1. The shift may be due to the formation of chemical bond between cerium and AC through oxygen-containing groups (Gupta and Saleh 2011a, b; Irajiboroujeni et al. 2016; El-Khouly et al. 2017). During the synthesis of composite, strong interactions between metal oxide and ACs have formed, which may be a plausible reason for the enhanced physical and chemical properties of the synthesized composite.
SEM study
Details regarding size and surface morphology of the composites can be studied using scanning electron microscopy (SEM). The results obtained clearly indicate the rough surface area with aggregated particles in composite systems, and SEM images of CeO2/AC composites are shown in Fig. 5. SEM images showed very porous morphology due to the presence of AC that apparently shows high surface area and porosity.
BET surface area analysis
The surface area measurement by standard BET technique was taken for the prepared composite materials. The surface area of the prepared CeO2, AC and CeO2/AC composites is given in Table 2. The BET surface area of CeO2 was found to be 164.43 m2 g−1, and the table shows that the % wt of AC has an inevitable relation with the surface area of composite (Ching et al. 2005; Pan et al. 2008; Fang et al. 2008). The increase in BET surface area in composites was observed with the increase in wt% of activated carbon, but after a specific point, the decrease in BET surface area can be seen.
Photocatalytic activity
Photocatalytic activities of CeO2/AC composites were determined using 0.01 M methyl violet dye under different light sources, and the results obtained are shown in Fig. 6. The catalytic composites B, C and D showed considerable photocatalytic activities under visible light and photocatalytic activities of these composites under visible light confirmed spectrophotometric study. The mean averaged degradation efficiency of CeO2/AC (3:2) composite under different light sources was about 91%, and composite C showed the highest photocatalytic activity under visible light. Moreover, the increase in catalytic activity of composite E in dark was pointed out the part played by adsorption in the degradation of methyl violet dye. Photocatalytic activities of composite materials under different light sources were clearly indicated the synergy effect of CeO2 and AC. Such synergic effect can be observed only when CeO2 and AC form a perfect composite and having an appropriate interaction between them (Irajiboroujeni et al. 2016; El-Khouly et al. 2017). For the appropriate selection of the composite for the treatment of textile industry wastewater, variables such as pH, catalyst load and irradiation time were studied.
Photocatalytic degradation of industrial dyes
Photocatalytic degradation of industrial dyes in aqueous media was carried out under 5 W LED light source (Philips, India) for 6-h duration, and degradation rate of industrial dyes is shown in Figs. 7. The results obtained clearly showed the potential removal of industrial dyes under photocatalytic degradation processes within 6 h. The presence of stable AC within the composite material system apparently attracts more dye molecules in aqueous media and supply to the active site of photocatalysts. In addition, CeO2/AC composites such as C, D and E were showed considerable rate in the dye removal efficiency by the exhibition of adsorption as well as photocatalytic processes. Combined processes of adsorption and photocatalytic oxidation apparently enhance the overall removal efficiency of organic pollutants and dye molecules in aqueous media.
Effect of important parameters on photocatalytic degradation
Different conditions, which affect the photocatalytic degradation of industrial dyes in aqueous media, were studied. During the study, methyl violet (0.01 M) was used as model dye and parameters such as pH, catalyst load and irradiation time were considered using LED light source.
Effect of the initial pH
The effect of pH on photocatalytic degradation of methyl violet (0.01 M) dye was assessed using 0.015 g of photocatalyst (C) for 120 min under LED light source (Philips India, 6 W) by varied pHs, i.e., 2–12. The pH of the dye solution was adjusted using diluted HCl or NaOH solution. The photocatalytic degradation rate of methyl violet dye was significantly increased up to 93.6% under alkaline as well as acidic condition, and the results obtained are depicted in Fig. 8. The results clearly showed that photocatalytic degradation efficiency was strongly depending on pH of dye solution (Ghorai 2011; Nagaraju 2013; Ravishankar et al. 2014; El-Khouly et al. 2017). The increased rate of photocatalytic degradation under alkaline condition may be attributed to the increased hydroxyl ions, which further induces hydroxyl radicals. Under acidic condition, the perhydroxyl radicals can form hydrogen peroxide, which further gives rise to the hydroxyl radicals (Shivaraju et al. 2017). The hydroxyl group on the CeO2/AC composite surface undergoes the acid–base equilibrium as shown in Eqs. (2)–(3).
The plausible reason seems to be due to the redox potential of the Ce4+/Ce3+ couple, high wide band gap energy and its strong light absorption in the visible region that apparently enhances photocatalytic activities under LED (Ji et al. 2009; Li et al. 2012; Sun et al. 2012; El-Khouly et al. 2017).
Effect of photocatalyst load
Photocatalytic degradation efficiency depends on the nature of the catalyst and the amount of photocatalyst utilized. In order to determine the optimal dosage of the catalyst, photocatalytic degradation study of methyl violet was carried out for 120 min under LED light sources by varying the photocatalyst (C) load from 0.005 to 0.02 g/50 ml. The result showed a significant increase in the degradation efficiency up to 94%, and the results are shown in Fig. 9. The optimal load was found to be 0.015 g/50 ml, and there was no significant change in degradation efficiency beyond 0.015 g of catalyst load. The possible reason was the increased number of active sites on the surface of catalyst suspended for photoreaction, which in turn increases the rate of radical formation in an aqueous solution (Shivaraju et al. 2017). Above the optimal level of catalyst load (0.015 g/50 ml), photocatalytic degradation rate was significantly decreased due to the increased turbidity that apparently affects the light penetration, and the availability of hydroxides and superoxides becomes minimal in the media.
Effect of irradiation time
The effect of reaction time on photocatalytic degradation efficiency was studied under LED light sources using methyl violet (0.01 M) and 0.015 g/50 ml photocatalyst (C). As shown in Fig. 10, there was a significantly increased photocatalytic degradation efficiency that attributed to continuous production of hydroxyl radicals under light sources that apparently leads to the continuous degradation of organic pollutants in aqueous media.
Photocatalytic treatment of real-time industrial wastewater
CeO2/AC (3:2) composite-assisted photocatalytic degradation of organic pollutants including dyes in real-time textile industrial wastewater was studied, and the results obtained are shown in Fig. 11. Potential degradation of organic pollutants and dye molecules was observed under LED light and it was attributed to the synergic effect of CeO2 and AC on the composite with desired properties. Coexistence of AC (Aluyor and Badmus 2008) with CeO2 apparently enhances the overall degradation rates of pollutants in aqueous media by enhancing the contact rate between organics and photocatalyst by supplying organic molecules under adsorption. Potential photocatalytic degradation efficiency of about 91.3% was achieved within 6 h under visible light source that enables the use of natural sunlight for the treatment of water or wastewater as an alternative driving energy. The enhancement in photocatalytic activity of CeO2/AC composite can be explained based on electron transportation from composite nanoparticles to AC and adsorption ability of AC. When CeO2 nanoparticles are exposed to light with energy equivalent to or greater than its band gap, it results in the development of electron–hole pair on surface of CeO2, which has undergone redox reaction with organic substrate in water (Faisal et al. 2011; Khan et al. 2011). However, photogenerated electrons and hole in semiconductor-based photocatalysts have very short lifetime as they recombine within a short duration. For the enhancement in photocatalytic activity of semiconductors, electron–hole recombination process has to be delayed (Wang et al. 2009). The formation of composite helps in transfer of photogenerated electrons from CeO2 to AC, since AC acts as a good electron acceptor and CeO2 acts as a good electron donor. AC can adsorb oxygen as well as dye molecules and helps in the generation of additional superoxide anion radical (O.−2) by the reduction of adsorbed oxygen molecule by photogenerated electrons. It will increase the number of radicals that can react with the dye and produce hydroxyl radical (OH) to oxidize the dye. Meanwhile, hydroxyl radical was formed because of reaction photogenerated holes in CeO2 with hydroxyl anion (OH−). In short, CeO2/AC composite can reduce the electron–hole recombination by improving the injection of electrons to ACs and also increase the adsorption of O2 and the organic dye for photocatalytic reaction (Wang et al. 2009; Ji et al. 2009; Gupta and Saleh 2011a, b).
Reusability study
Reusability and regeneration of CeO2/AC composites for photocatalytic degradation applications were studied using recovered composites, and the results obtained are shown in Fig. 12. Recovery and effect of recycle experiments were studied using methyl violet (0.01 M) and 0.015 g of photocatalyst for 120 min under LED light source. In view of green technology, recycling and recovery can be foreseen as a good practice for sustainable wastewater treatment. Therefore, it was studied for reusability of composite, i.e., after a water treatment the photocatalyst could be reused. The results obtained suggested as the number of reuse cycles increased, the photodegradation efficiency of composites was reduced. This could be due to the aggregation and sedimentation of dye around composites (Shivaraju et al. 2017).
Conclusions
The present work revealed well about the preparation of the visible light-responsive CeO2/AC composites through soft chemical technique. The effect of AC loading on CeO2 was investigated by SEM, XRD, FTIR and BET surface area measurements. It was found that loading of AC has remarkable impacts on the surface, structural and textural properties of CeO2. The photocatalytic activities of composites prepared were determined using methyl violet dye, and CeO2/AC (3:2) composite showed highest photocatalytic activity (up to 94%) under different light sources. The photocatalytic degradation study of methyl violet dye revealed significant effects of pH of the media, irradiation time and catalyst load. Potential photocatalytic degradation of industrial dyes and organic pollutants in real-time textile wastewater within short duration was observed and it was attributed to performance of adsorption and photocatalytic processes within CeO2/AC (3:2) composite. Reusability studies were showed that CeO2/AC composite of wt% ratio 3:2 exhibited a higher photocatalytic performance as compared to other wt% ratios. The incorporation of AC was enhanced the overall photocatalytic properties in the CeO2/AC composite. However, it is observed that other wt% ratios showed a diminished activity. Incorporation of CeO2/AC composite-fabricated materials for water and wastewater treatment would be enable the utilization of natural sunlight as an alternative driving energy, which may be substituted the conventional treatment methods.
References
Aluyor EO, Badmus OAM (2008) COD removal from industrial wastewater using activated carbon prepared from animal horns. Afr J Biotechnol 7(21):3887–3891
Babitha KK, Sreedevi A, Priyanka KP, Sabu B, Varghese T (2015) Structural characterization and optical studies of CeO2 nanoparticles synthesized by chemical precipitation. Indian J Pure Appl Phys 53:596–603
Bandosz TJ, Ania CO (2006) Surface chemistry of activated carbons and its characterization. In: Bandosz TJ (ed) Activated carbon surfaces in environmental remediation. Elsevier, Oxford, pp 186–193
Chaudhari K, Bhatt V, Bhargava A, Seshadri S (2011) Combinational system for the treatment of textile wastewater: a future perspective. Asian J Water Environ Pollut 8:127–136
Ching JYC, Cobo F, Aubert D, Harvey HG, Airiau M, Corma A (2005) A general method for the synthesis of nanostructured large-surface-area materials through the self-assembly of functionalized nanoparticles. Chem Eur J 11:979–987
Devadoss MDMP, Karuppasamy S, Manickam M, Suresh S (2016) Structural, optical, morphological and dielectric properties of cerium oxide nanoparticles. Mater Res 19(2):478–482
El-Khouly SM, Mohamed GM, Fathy NA, Fagal GA (2017) Effect of nanosized CeO2 or ZnO loading on adsorption and catalytic properties of activated carbon. Adsorpt Sci Technol 35(9–10):774–788
Faisal M, Khan SB, Rahman MM, Jamal A, Asiri AM (2011) Smart chemical sensor and active photo-catalyst for environmental pollutants. Chem Eng J 173:178–184
Fang J, Cao Z, Zhang D, Shen X, Ding W, Shi L (2008) Preparation and CO conversion activity of ceria nanotubes by carbon nanotubes templating method. J Rare Earth 26(2):153–157
Foo KY, Hameed BH (2010) Insights into the modelling of adsorption isotherm system. Chem Eng J 156(1):2–10
Gao J, Guan F, Ma Y, Yang W, Kang JW, Deng HL, Qi YL (2001) Preparation of CeO2 nanoparticles and its application to ion-selective electrodes based on acetyl cellulose. Rare Metals (Beijing) 20(4):217–220
Ghorai TK (2011) Photocatalytic degradation of 4-chlorophenol by CuMoO4 doped TiO2 nanoparticles synthesized by chemical route. Open J Phys Chem 1:28–36
Gosetti F, Gianotti V, Angioi S, Polati S, Marengo E, Gennaro MC (2004) Oxidative degradation of food dye E133 Brilliant Blue FCF liquid chromatography–electrospray mass spectrometry identification of the degradation pathway. J Chroma A 1054(1–2):379–387
Gupta VK, Saleh TA (2011a) Synthesis of carbon nanotube- metal oxides composites; adsorption and photodegradation. In: Bianco S (ed) From research to applications. In Tech, London. ISBN 978-953-307-500-6
Gupta VK, Saleh TA (2011b) Functionalization of tungsten oxide into MWCNT and its application for sunlight induced degradation of Rhodamine B. J Coll Interface Sci 362:337–344
Ho C, Yu JC, Kwang T, Mark AC, Lai S (2006) morphology-controllable synthesis of mesoporous CeO2 nano- and microstructures. Chem Mater 17(17):4514–4522
Ince NH, Tezcanh G (1999) Treatability of textile dye-bath effluents by advanced oxidation: preparation for reuse. Water Sci Techol 40:183–190
Irajiboroujeni M, Bahrevar MA, Youzbashi AA, Khanlarkhani A (2016) Synthesis and characterization of MWCNTS/CeO2 nanocomposite as A photocatalyst. Int J Adv Sci Eng Technol 2:112–116
Ji P, Zhang J, Chen F, Anpo M (2009) Study of adsorption and degradation of acid orange 7 on the surface of CeO2 under visible light irradiation. Appl Catal B 85:148–154
Kannan N, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons - a comparative study. Dyes Pigment 51:25–40
Khan SB, Faisal M, Rahman MM, Jamal A (2011) Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications. Sci Total Environ 409(15):2987–2992
Li H, Wang G, Zhang F (2012) Surfactant-assisted synthesis of CeO2 nanoparticles and their application in wastewater treatment. RSC Adv 2(32):12413–12423
Nagaraju G (2013) Ionothermal synthesis of TiO2 nanoparticles: photocatalytic hydrogen generation. Mater Lett 109:27–30
Niu F, Zhang D, Shi L (2009) Facile synthesis, characterization and low-temperature catalytic performance of Au/CeO2 nanorods. Mater Lett 63(24–25):2132–2135
Palard M, Balencie J, Maguer A, Hochepied JF (2010) Effect of hydrothermal ripening on the photoluminescence properties of pure and doped cerium oxide nanoparticles. Mater Chem Phys 120:79–88
Pan C, Zhang D, Shi L (2008) CTAB assisted hydrothermal synthesis, controlled conversion and CO oxidation properties of CeO2 nanoplates, nanotubes, and nanorods. J Solid State Chem 181(6):1298–1306
Puziy AM, Poddubnaya OI, Alonso AM (2002) Synthetic carbons activated with phosphoric acid: I. Surface chemistry and ion binding properties. Carbon 40:1493–1505
Rai HS, Bhattacharyya MS, Singh J, Bansal TK, Vats P, Banerjee UC (2005) Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Environ Sci Technol 35:219–238
Ranjan SH, Rao GR (2000) Characterization of combustion synthesized zirconia powder by UV-vis, IR and other techniques. Bull Mater Sci 23(5):349
Rao GR, Sahu HR (2001) XRD and UV-Vis diffuse reflectance analysis of CeO2–ZrO2 solid solutions synthesized by combustion method. Proc Indian Acad Sci Chem Sci 113(5&6):651–658
Ravishankar TN, Manjunatha K, Ramakrishnappa T, Nagaraju G, Kumar D, Sarakar S, Anandakumar BS, Chandrappa GT, Reddy V, Dupont J (2014) Comparison of the photocatalytic degradation of trypan blue by undoped and silver-doped zinc oxide nanoparticles. Mater Sci Semi Conf Proc 26:7–17
Roy R, Roy RA, Roy DM (1986) Alternative perspectives on “quasi-crystallinity”: non-uniformity and nanocomposites. Mater Lett 4(8–9):323–328
Schmidt D, Shah D, Giannelis EP (2002) New advances in polymer/layered silicate nanocomposites. Curr Opin Solid State Mater 6(3):205–212
Shivaraju HP (2011) Removal of organic pollutants in the municipal sewage water by TiO2 based heterogeneous photocatalysis. Intl J Environ Sci 1(5):911–923
Shivaraju HP, Midhun G, Anil Kumar KM, Pallavi S, Pallavi N, Behzad S (2017) Degradation of selected industrial dyes using Mg-doped TiO2 polyscales under natural sun light as an alternative driving energy. Appl Water Sci 7(7):3937–3948
Shivaraju HP, Egumbo H, Madhusudan P, AnilKumar KM, Midhun G (2018) Preparation of affordable and multifunctional clay-based ceramic filter matrix for treatment of drinking water. Environ Technol 1:1–11
Solmaz A, Ustun SK, Birgul GE, Yonar A (2009) Advanced oxidation of textile dyeing effluents: comparison of Fe+2/H2O2, Fe+3/H2O2, O3 and chemical coagulation processes. Fresen Environ Bull 18:1424–1433
Sun C, Li H, Chen L (2012) Nanostructured ceria-based materials: synthesis, properties, and applications. Energy Environ Sci 5:8475–8505
Wang Z, Quan Z, Lin J (2007) Remarkable changes in the optical properties of CeO2 nanocrystals induced by lanthanide ions doping. J Inorg Chem 46(13):5237–5242
Wang K, Pyrgiotakis G, Sigmund W (2009) Photocatalytic carbon-nanotube TiO2 composites. Adv Mater 21:2233–2239
Wojnarovits L, Takacs E (2008) Irradiation treatment of Azo dye containing wastewater: an overview. Radiat Phys Chem 77(3):225–244
Zaki MI, Hussein GAM, Mansour SAA, Ismail HM, Mekhemer GAH (1997) Ceria on silica and alumina catalysts: dispersion and surface acid-base properties as probed by X-ray diffractometry, UV-Vis diffuse reflectance and in situ IR absorption studies. Colloids Surf A Physicochem Eng Asp 127(1–3):47–56
Acknowledgements
The authors thank JSS Science and Technology University, Mysuru, for providing XRD and SEM facilities in the research work. The authors would also like to thank DST-India for INSPIRED Ph.D. fellowship to first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gopi, M., Harikaranahalli Puttaiah, S., Shahmoradi, B. et al. Preparation and characterization of cost-effective AC/CeO2 nanocomposites for the degradation of selected industrial dyes. Appl Water Sci 10, 25 (2020). https://doi.org/10.1007/s13201-019-1105-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-1105-7