Abstract
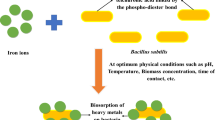
Previous studies showed that Bacillus subtilis possessed high physiological activity in industrial waste treatment as a biosorbent for recovery metals, and in this study, the sorption capacity for both La3+ and Sm3+ was demonstrated. The effects of lanthanide concentration and contact time were tested to acid and alkali pre-treated cells. Very high levels of removal, reaching up to 99% were obtained for both lanthanide ions. Langmuir isotherm model was applied to describe the adsorption isotherm and indicated a better correlation with experimental data R2 = 0.84 for La3+ using sodium hydroxide pre-treated free cells and R2 = 0.73 for Sm3+ to acid pre-treated B. subtilis cells as biosorbents. Results of this study indicated that chemically modified B. subtilis cells are a very good candidate for the removal of light rare-earth elements from aquatic environments. The process is feasible, reliable, and eco-friendly.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Biosorption is a new emerging biotechnology that has the potential to be more effective, inexpensive, and environmental-friendly than conventional methods utilized in industrial waste treatment. Because of their high sorption capacities and low production costs, all kinds of microbial cells, including yeast, bacteria, algae, fungi, and protozoa, have been used as biosorbent materials (Volesky 2007; Gupta et al. 2018).
The biosorption mechanisms include adsorption, uptake, reduction, methylation, and oxidation. In this way, biosorption is similar to an ion-exchange mechanism; the rare-earth elements can bind to oxygen donor atom groups present in the cell wall (cells) of microbial species (Palmieri et al. 2000), such as carboxylic or phosphoric groups. The different affinities between all kind of biosorbents and the lanthanides species can provide the basis for the separation and purification of rare-earth elements using this bioprocess (Diniz and Volesky 2005; Giese et al. 2019).
Recovery of the rare-earth elements is interesting due to the high economic value along with various industrial applications (Table 1); however, conventional technologies as precipitation, liquid–liquid extraction, solid–liquid extraction, and ion exchange present high processing costs and difficulties associated with separating and obtaining a high purity of these elements. In this way, the biosorption represents a biotechnological innovation as well as an excellent cost-effective tool for the recovery of rare-earth metals from aqueous solutions (Das and Das 2013).
In recent years, several scientists have employed Bacillus subtilis bacteria for the removal of different heavy metals from aqueous solutions. For instance, Sukumar et al. (2014) used free B. subtilis cells for the adsorption of chromium ions from aiming application on soils around the electroplating industry that are often polluted with metals. Hossain and Anantharaman (2006) employed the suspension free B. subtilis cells for the biosorption of lead ions from aqueous solutions. The ability of Bacillus subtilis immobilized into chitosan beads to remove copper ions from aqueous solution was studied due to a high cells density to provide a greater opportunity for reuse and recovery (Liu et al. 2013). Recently, Coimbra et al. (2019) have described that B. subtilis immobilized on calcium alginate gel is capable to biosorb light rare-earth elements. All studies recommended using B. subtilis cells as biosorbent for heavy metal removal.
Bacillus subtilis cell wall structure is well known and consists primarily of peptidoglycan and teichoic acid. Peptidoglycan is a polymer of acetylmuramic and acetylglucosamine acids that display mainly carboxylic and hydroxyl functional groups. Teichoic acid is a polymer of copyranosyl glycerol phosphate that comprises mainly phosphate and hydroxyl groups (Liu et al. 2013). A single binding site was found for rare-earth elements on B. subtilis at low pH, which is responsible by a preferential biosorption of heavy rare-earth elements (Tm, Yb, Lu). The modeling sorption suggested carboxylic groups as active adsorption groups to this species (Martínez et al. 2014).
The main objective of this investigation was to spotlight on the biosorption of lanthanum and samarium ions (La3+ and Sm3+) from aqueous solutions by pre-treated suspension free B. subtilis cells and to evaluate the effects of different parameters on the adsorption process aiming to evaluate the preferences between light and medium rare-earth elements.
Experimental
Materials
Stock solutions of La3+ and Sm3+ (1 mol/L) were prepared by dissolving lanthanum and samarium oxides (Pacific Industrial Development Corporation, Weihai, China) in a solution of deionized water–nitric acid, and diluted to the concentrations required for the experiments outlined below. The initial pH of each working solution was adjusted with HNO3 and NaOH (1 mol/L) solutions at the start of the experiment.
Microorganism and media
Bacillus subtilis screened from soil and belonging to Instituto de Microbiologia Paulo Góes (IMPG/UFRJ) was stored at TSA at 4 °C. The microorganism was transferred to 500 mL shake flasks, containing 200 mL of basal medium (TSB 30 g/L and yeast extract 5 g/L). The culture was incubated at 30 °C for 48 h at 150 rpm. Cells were harvested by centrifugation (15 min; 1500g) at room temperature. The cells were washed with deionized water and were pre-treated with 50 mL sodium hydroxide (SH) solution (1.0 mol/L) or chloridric acid (CA) solution (1.0 mol/L) for 30 min at room temperature in 125-mL Erlenmeyer flasks in a rotary shaker at 100 rpm at 30 °C. After centrifugation (1500g/15 min), the sedimented pre-treated cells recovered were used as the biosorbent material.
Biosorption experiments
The effect of contact time on the biosorption of the lanthanides was studied on the pre-treated B. subtilis cells [1.0 g (dry wt. cell)/L] added to 50 mL of ionic solutions of La3+ (10 mg/L) or Sm3+ (10 mg/L) at pH 3.0 in 125 mL Erlenmeyer flasks. The flasks were kept at contact times of 5, 10, 20, 30, 40, 50, and 60 min. For the studies on the initial concentration and Langmuir adsorption isotherm model (Langmuir 1918), the following initial concentrations of the rare-earth elements ([REE] initial) were separately evaluated: 10, 25, 40, 50, and 75 mg/L, at contact time of 20 min. The biosorption experiments were carried out in duplicate, in single or binary systems, and the average results are presented. The samples were centrifuged (15 min; 1500g), and the clear supernatant was analyzed for the lanthanide ions concentration. The La3+ and Sm3+ were determined by inductively coupled plasma atomic emission spectrometry (ICP-OES Perkin Elmer, OPTIMA3000, USA).
The percentage of biosorption, corresponded to lanthanide ions removed (%), was estimated as described in Eq. (1).
where \( {\text{lanthanide}} \)1 and \( {\text{lanthanide}} \)2 stand for the initial and final lanthanide concentration, respectively, expressed in mg/L.
Langmuir isotherm model
The Langmuir (1918) isotherm is used for monolayer sorption on the surface of the particle. The linear expression of the Langmuir model is given by Eq. (2):
where Qe and Qmax are the equilibrium and maximum rare-earth elements biosorption capacity, respectively, Ce is the equilibrium solution concentrations, and Ka is the equilibrium constant.
Results and discussion
Bacillus subtilis has a well-studied gram-positive cell wall. The studies have shown that most metal binding occurs after initial metal complexation and neutralization of the chemically active sites, mainly by a first stoichiometric interaction of metal and chemical groups following by deposition of more metal by chemical precipitation (Hossain and Anantharaman 2006; Al-Homaidan et al. 2014). The oxygen-containing (–COOH, –OH) and (–NH2) were the main functional groups related metal biosorption in Bacillus species (Liu et al. 2019). Metal sorption capacity of biosorbent can be altered by pre-treatment, which could modify the surface characteristics either by removing or masking the groups or by exposing more metal-binding sites (Singh et al. 2014). The acid or alkali treatment contributes to removing some leachable materials (organic compounds, carbonate-based materials) that could influence acid-base properties (Oliveira et al. 2011).
In the present study, the effect of pre-treatment on lanthanide ions uptake capacity of biosorbent for the different pre-treatment methods used has been studied. Living B. subtilis cells were chemically modified by acid (HCl 1.0 mol/L) or alkali pre-treatments (NaOH 1.0 mol/L). The initial concentration of lanthanide ions provides an important driving force to overcome all mass transfer resistance of metal ion between the aqueous and solid phases. Hence, the effect of initial La3+ and Sm3+ concentration was studied to both pre-treated cells from B. subtilis in single and binary systems.
The results obtained in Fig. 1 indicated that the initial concentration of rare-earth elements in the aqueous solution influenced the rate of adsorption by both pre-treated B. subtilis cells. In a single system, to alkali pre-treated B. subtilis cells, it was observed that there was an increase in the percentage of Sm3+ adsorption with increased Sm3+ concentration from 15 to 30 mg/L and maximum Sm3+ adsorption (100%) was observed with a concentration of 30 mg/L. La3+ biosorption presented higher preference by B. subtilis cells binding sites in lower initial lanthanide ions concentration (< 20 mg/L) and maximum La3+ adsorption (97%) was observed with a concentration of 50 mg/L. Anyway, the higher biosorption yields in smallest concentrations could be attributed to the possible higher interaction between lanthanide ions and the binding sites on the biosorbent surface and saturation of all binding sites with metal ions (Al-Homaidan et al. 2014).
On the other hand, the acid pre-treatment of B. subtilis cells resulted in a decrease in the percentage of La3+ and Sm3+ adsorption with increased lanthanides concentration. The biosorption capacity of La3+ decreased from 31 to 8% as well the biosorption capacity of Sm3+ decreased from 22 to 17% in the range of initial lanthanides ions concentrations of 15–100 mg/L. The higher initial concentration of lanthanide ions increased the active groups required by the adsorbent to promote the biosorption. It appears that acid pre-treatment decreased the number of binding adsorption sites in B. subtilis cell wall. To Saccharomyces cerevisiae yeast, the esterification of carboxyl and methylation of amino groups present in the cell wall significantly decreased the biosorption capacity of copper ions, which suggests that both carboxylic and amine groups play an important role in biosorption of copper (Jianlong 2002).
Martínez et al. (2014) evaluated the rare-earth element binding constants for untreated B. subtilis cells. A single binding site for the pH range of 2.5–4.5 was found, which showed to have a lower affinity for light elements (e.g., La, Ce, Pr, Nd) and a higher affinity for heavy elements (e.g., Tm, Yb, Lu). This finding was confirmed in the present work, where either B. subtilis biomass pre-treated with acid or base showed the preference in biosorption of La3+ over Sm3+.
The batch biosorption of binary system La3+–Sm3+ was also evaluated. The biosorption capacity behavior was similar to single systems reported previously. According to Fig. 2, in the lowest concentrations (15 mg/L), the La3+ (92%) was preferably biosorbed than Sm3+ (71%) by sodium hydroxide pre-treated B. subtilis cells. The increase in lanthanide ions concentration caused an increase in biosorption, which reached 99% at 85 mg/L (La3+ + Sm3+). The acid pre-treatment of B. subtilis cells resulted in a decrease in the percentage of La3+ and Sm3+ adsorption. However, this percentage had increased with the increase in initial lanthanide ions concentrations.
The sorption studies at different contact times help in determining the sorption capacities of biosorbent at varying time intervals. The effect of contact time was evaluated as one of the important parameters affecting the biosorption efficiency. Figure 3 shows the biosorption efficiency of Bacillus sp. at concentrations of 15 mg/L as a function of contact time. In the first 20 min, La3+ < Sm3+ uptake preference was observed; and after that Sm3+ < La3+ uptake increases with a rise in contact time up to 60 min, with values of biosorption in the range of 50–100% to La3+ and 76–82% to Sm3+. The fast initial metal biosorption rate was attributed to the surface binding, and the following slower sorption was attributed to the interior penetration. García et al. (2016) have observed this behavior for the biosorption of heavy metals (Cd, Cr, Mn, and Pb) by aqueous dead cells of Bacillus sp. (strain C13 and C16) isolated from the activated sludge.
Different kinds of functional groups, with different affinities to metal ions, are usually present on the cells surface. After acid or alkali pre-treatment, these functional groups will be rearranged, and the efficiency of biosorption can be affected. According to these results, the acid pre-treatment of B. subtilis have caused a decrease of 1.85-fold in biosorption capacity of La3+, which was reduced from 100 to 54% in 60 min. This decrease was in the order of 3.9-fold to biosorption capacity of Sm3+, which was reduced from 82 to 21% in 60 min. The equilibrium conditions not been attained with this pre-treated biosorbent.
In comparison, for chromium removal by acid and alkali pre-treated algal cells, Singh et al. (2014) have observed that all of the pre-treated samples showed similar trends of removal. A rapid chromium biosorption took place in the first 30 min, showing a rapid removal of 65% at 15 min. To Pleurotus florida fungi biomass, all the pre-treatment methods improved the biosorption of cadmium in comparison with live cells (Das et al. 2007). Physical, alkali, acid, and organical pre-treatments have demonstrated to be effective in increasing the adsorption capacity by this biosorbent.
Several kinetic models are available to understand the behavior of biosorbents and also to examine the rate of the controlling mechanism of the adsorption process. The Langmuir model has been giving a better fit than the other adsorption models, e.g., the Freundlich model, for the biosorption of rare-earth elements (Vijayaraghavan and Yun 2008). The Langmuir adsorption model is based on the assumption that the maximum adsorption corresponds to a saturated monolayer of solute molecules on the adsorbent surface, with no lateral interaction between the sorbed molecules (Langmuir 1918).
The plots of linear Langmuir isotherm model describe the relationship between the adsorbed amounts of La3+ (Fig. 4) and Sm3+ (Fig. 5) on pre-treated B. subtilis cells against the concentration of lanthanide ions remaining in the solution. Langmuir isotherm model fitted the experimental data appropriately. Langmuir model was also the adsorption isotherm model fitted for Eu3+ biosorption by Bacillus thuringiensis biomass, where the adsorption capacity of Eu3+ achieves as high as 160 mg/g (Pan et al. 2017). To U4+ biosorption by Bacillus amyloliquefaciens, the maximum uptake capacity was 179.5 mg/g at pH 6.0 by Langmuir model (Liu et al. 2019).
By comparing the R2 of the Langmuir model for La3+ with that obtained from the Sm3+, it can be noted that the Langmuir isotherm model best fitted the equilibrium data for La3+ adsorption. Also, could be observed that Langmuir model indicated a better correlation with experimental data R2 = 0.84 for La3+ using alkali pre-treated living cells as biosorbent and R2 = 0.73 for Sm3+ when the acid pre-treated B. subtilis cells were used as biosorbent. The results from Langmuir models also gave the facts that the B. subtilis cells had homogeneous surface. The same analysis was found recently to Bacillus badius for Cd2+ biosorption (Vishan et al. 2019) and to Bacillus xiamenensis for Pb2+ (Mohapatra et al. 2019) where Langmuir isotherm fitting depicted the monolayer adsorption behavior. Namely, it explains the adsorption procedure of La3+ and Sm3+ onto the B. subtilis cells as a homogeneous adsorbent.
Conclusion
Biosorption has received great attention during the last years, due to the potential use of microorganisms for cleaning metal-polluted water or wastewater streams. Biosorption, utilizing the ability of microbial to recover rare-earth elements from diluted solutions is considered as a more competitive, effective and economically attractive treatment method than hydrometallurgical conventional methods.
According to the results of the present experiment, it is evident that the biomass of B. subtilis pre-treated chemically was able to remove lanthanide ions from aqueous solution. It may be advantageous to use this bacterial biomass after chemical pre-treatment especially with NaOH. The results from this study also demonstrated strategies to improve the separation of lanthanides and highlighted on the biosorption mechanism of B. subtilis by the two lanthanides studied. Thus, the bacterial biomass of B. subtilis may be applied as potent biosorbent for recovery and separate lanthanide ions from leachate liquors.
References
Al-Homaidan AA, Al-Houri HJ, Al-Hazzani AA, Elgaaly G, Moubayed NMS (2014) Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arab J Chem 7:57–62
Charalampides G, Vatalis KI, Apostoplos B, Ploutarch-Nikolas B (2015) Rare-earth elements: industrial applications and economic dependency of Europe. Procedia Econ Finance 24:126–135
Coimbra NV, Gonçalves FS, Nascimento M, Giese EC (2019) Study of adsorption isotherm models on rare-earth elements biosorption for separation purposes. Int Sch Sci Res Innov 13:200–203
Das N, Das D (2013) Recovery of rare-earth metals through biosorption: an overview. J Rare Earths 31:933–943
Das N, Charumathi D, Vimala R (2007) Effect of pretreatment on Cd(II) biosorption by mycelial biomass of Pleurotus florida. Afr J Biotechnol 6:2555–2558
Diniz V, Volesky B (2005) Biosorption of La, Eu and Yb using Sargassum biomass. Water Res 39:239–247
García R, Campos J, Cruz A, Calderón ME, Raynald ME, Buitrón G (2016) Biosorption of Cd, Cr, Mn, and Pb from aqueous solutions by Bacillus sp. strains isolated from industrial waste activate sludge. TIP 19:5–14
Giese EC (2018) Rare-earth elements: therapeutic and diagnostic applications in modern medicine. Clin Med Rep 2:1–2
Giese EC, Barbosa-Dekker AM, Dekker RFH (2019) Biosorption of lanthanum and samarium by viable and autoclaved mycelium of Botryosphaeria rhodina MAMB-05. Biotechnol Prog 36:e2783
Gupta NK, Sengupta A, Gupta A, Sonawane JR, Sahoo H (2018) Biosorption—an alternative method for nuclear waste management: a critical review. J Environ Chem Eng 6:2159–2175
Hossain SKM, Anantharaman N (2006) Studies on bacterial growth and lead(IV) biosorption using Bacillus subtilis. Ind J Chem Technol 13:591–596
Jianlong W (2002) Biosorption of copper(II) by chemically modified biomass of Saccharomyces cerevisiae. Process Biochem 37:441–444
Langmuir I (1918) Adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Liu YG, Liao T, He ZB, Li TT, Wang H, Hu XJ, Guo YM, He Y (2013) Biosorption of copper(II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. Trans Nonferr Met Soc China 23:1804–1814
Liu L, Liu J, Liu X, Dai C, Zhang Z, Song W, Chu Y (2019) Kinetic and equilibrium of U(VI) biosorption onto the resistant bacterium Bacillus amyloliquefaciens. J Environ Radioact 203:117–124
Martínez RE, Pourret O, Takahashi Y (2014) Modeling of rare-earth element sorption to the Gram positive Bacillus subtilis bacteria surface. J Colloid Interface Sci 413:106–111
Mohapatra RK, Parhi PK, Pandey S, Bindhani BK, Thatoi H, Panda CR (2019) Active and passive biosorption of Pb(II) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: kinetics and isotherm studies. J Environ Manag 247:121–134
Oliveira RC, Jouannin C, Guibal E, Garcia O Jr (2011) Samarium(III) and praseodymium(III) biosorption on Sargassum sp.: batch study. Process Biochem 46:736–744
Palmieri M, Garcia O Jr, Melnikov P (2000) Neodymium biosorption from acidic solutions in batch system. Process Biochem 36:441–444
Pan X, Wu W, Chen Z, Rao W, Guan X (2017) Biosorption and extraction of europium by Bacillus thuringiensis strain. Inorg Chem Commun 75:21–24
Singh SK, Dixit K, Sudaram S (2014) Effect of acidic and basic pretreatment of wild algal biomass on Cr(VI) biosorption. IOSR-JESTFT 8:38–41
Sukumar C, Janaki V, Kamala-Kannan S, Shanti K (2014) Biosorption of chromium(VI) using Bacillus subtilis SS-1 isolated from soil samples of electroplating industry. Clean Technol Environ Policy 16:405–413
Vijayaraghavan K, Yun Y-S (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Vishan I, Saha B, Sivaprasakam S, Kalamdhad A (2019) Evaluation of Cd(II) biosorption in aqueous solution by using lyophilized biomass of novel bacterial strain Bacillus badius AK: biosorption kinetics, thermodynamics and mechanism. Environ Technol Innov 14:100323
Volesky B (2007) Biosorption and me. Water Res 41:4017–4029
Acknowledgements
The authors are grateful to the Brazilian National Council for Scientific and Technological Development (CNPq—Brazil) for research support. CS Jordão thanks the PIBIC/CNPq/CETEM for the undergraduate fellowship granted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Giese, E.C., Jordão, C.S. Biosorption of lanthanum and samarium by chemically modified free Bacillus subtilis cells. Appl Water Sci 9, 182 (2019). https://doi.org/10.1007/s13201-019-1052-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-1052-3