Abstract
Powdered adsorbent prepared from Albizia lebbeck pods as agricultural waste has been used for the adsorption of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions. The powdered adsorbent was characterized by X-ray diffraction, Fourier transform infrared spectroscopy and Brunauer–Emmett–Teller. Effects of various parameters like contact time, solution pH, initial concentration dosage and temperature were investigated on a batch adsorption system. Equilibrium and kinetic experiments were carried out at the optimum pH of 6, 8 and 10 at 29 °C using particle size of 250 μm for Cd(II), Pb(II), Zn(II) and Cu(II) ions. Changes in free energy, enthalpy and entropy were also evaluated. The adsorption data fitted well with the Langmuir isotherm model with correlation coefficient (\(R^{2} > 0.94\)), whereas the adsorption kinetics followed the pseudo-second-order kinetics. The thermodynamic parameters proved that adsorption of metal ions is endothermic and non-spontaneous at low temperatures, while spontaneity occurred at higher temperatures. This study shows that powdered Albizia lebbeck pods prove to be a promising inexpensive adsorbent for metal ion removal from aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial activities in developed, underdeveloped and developing countries in recent times have faced a lot of problems due to improper disposal of wastes. These have resulted in emerging contaminants in water bodies causing severe health implications even at low concentrations. Effluents from industries such as tanning, mining, textile, fertilizer, pharmaceutical and inputs from other anthropogenic activities have contributed to the increase in contaminated waters all over the globe. Therefore, it is very important to do check and balancing of water quality, especially when their heavy metal ion concentrations are higher than the permissible limits in order to make them fit for human consumption and other purposes.
Several analytical methods have been pointed out and used for the removal of heavy metals from aqueous solutions. These include chemical precipitation, ion exchange, ultra-filtration, reverse osmosis, electrodialysis and adsorption (Reddy et al. 2014; Zare et al. 2015; Banerjee et al. 2016). These methods have some setbacks due to the inherent operational problems they possess. For instance, microbial electrochemical technology has been employed for the wastewater treatment (Zhang and Angelidaki 2014; Escapa et al. 2014, 2016) but was also reported to be expensive as reported by (Zhang and Angelidaki 2016). However, the method that has been described by numerous researchers to be highly selective, efficient, easy to operate and cost-effective is adsorption. Adsorption has been an alternative technique for heavy metal removal at low concentrations, and it depends on the adsorbent surface area, surface morphology, pore size distribution, polarity and functional groups attached to the adsorbent surface (Ali et al. 2016). The high cost of activated carbon in the markets has motivated researches to use low-cost biomass as adsorbents for the removal of heavy metals from wastewater. Therefore, there is a need to find alternative adsorbents of comparable effectiveness with lower costs for the removal of heavy metals. Several authors have used raw adsorbents for the removal of toxic heavy metal ions from aqueous solutions and industrial wastewater (Tasar et al. 2014; Ali and Saeed 2015; Asif and Chen 2017; Asuquo et al. 2018) and these were found to be promising.
Albizia lebbeck (Linn.) Benth is widespread tree grown in Senegal, Kenya, Angola, South Africa and in some parts of West African countries. It is called a woman’s tongue in English. It is planted along roadsides and in gardens. The pods of the tree do not have much importance to the environment and thus litter the environment especially during the dry season and often constitute an environmental nuisance. This calls for research efforts in using this waste as an adsorbent for wastewater treatment. In the present work, raw Albizia lebbeck pods which are highly abundant in Nigeria area were used. This raw material is low cost with high ease of preparation. Batch experiments, kinetics, isotherms and thermodynamic studies of the lead, cadmium, copper and zinc on this adsorbent were carried out.
Materials and methods
All the chemicals used were of analytical grade (reagents ≥ 98.5%) supplied by Sigma-Aldrich (UK).
Preparation of adsorbent
Albizia lebbeck pods were washed with deionized water in order to remove dust and other debris. They were then oven-dried for 24 h at 378 K for the reduction in moisture content. The dried biomass was ground and sieved to particles (250 μm). The sieved powder was stored and used for batch experiments.
Adsorbent characterization
The crystalline structure of Albizia lebbeck pod was determined by X-ray diffraction (XRD) analysis via XRD 6000 (Shimadzu, Japan), with CuKα radiation, and their diffraction patterns were recorded between diffraction angles of 0° and 90°. The functional groups that could influence the adsorption method were determined using Fourier transform infrared (FTIR) (a PerkinElmer 2000 FTIR spectrometer). The BET surface area, average pore volume and size distribution of the raw adsorbent were detected from the plot of the volume adsorbed (cm3/g STP) against relative pressure (P/Po).
Metal solutions
The solutions of lead, cadmium, zinc and copper ions were prepared by dissolving certain amounts of Pb, Cd, Zn and Cu salts in deionized water into volumetric flasks. Initial concentrations of the metal ions were prepared by diluting the stock solutions. The desired pH of the solutions was adjusted using 0.5 M HCl and 0.5 M NaOH solutions, respectively.
Adsorption experiments
Batch adsorption techniques were carried out by varying contact time, solution pH, initial concentration, adsorbent load and temperature on the dried Albizia lebbeck pod powder. Adsorption experiments were carried out by adding accurate amounts of the adsorbent to 40 cm3 of the wastewater in conical flasks placed on an orbital shaker in order to attain the equilibrium time. The solution pH (2–12), adsorbent dosage (0.4–1.2 g), initial metal ion concentrations (10–30 mg/dm3) and the solution temperature (30–80 °C) were varied to study their effects on the metal ion removal efficiency. The analysis of the metal ion concentrations was done after filtering with Whatman 42 filter paper.
The metal uptake and the percentage removal were calculated according to the following respective equations:
where \(C_{\text{o}}\) (mg/dm3) is the initial concentration; \(C_{\text{e}}\) (mg/dm3) is the equilibrium concentration; \(Q_{\text{e}}\) (mg/g) is the adsorption capacity; and \(m\) (g) is the weight of the adsorbent.
Adsorption isotherms
Adsorption isotherms were investigated for 10–30 mg/dm3 initial metal ion concentrations using 0.2 g of the raw adsorbent added to 40 cm3 of the metal ion concentrations and shaken for equilibrium time at 150 rpm. Two isotherm models, namely Freundlich and Langmuir, were employed.
Adsorption kinetics
About 40 cm3 of each aqueous solution was added to 0.2 g of the adsorbent at room temperature and shaken vigorously at respective contact times. The obtained residual metal ion concentrations were used to calculate the pseudo-first-order and pseudo-second-order adsorption kinetics.
Adsorption thermodynamics
Adsorption thermodynamic parameters were calculated from the temperature variations at a constant adsorbent weight and initial concentration.
Results and discussion
Characterization of the adsorbent
The FTIR spectroscopy was used to identify the active groups and bonds involved in adsorption of metals onto the adsorbent. FTIR spectrum in Fig. 1 displays peaks of different functional groups attributable to the adsorption of the metal ions. As seen in the spectrum of Albizia lebbeck pods, a broad and intense peak at around 3300 cm−1 corresponds to –OH group of cellulose, pectin and lignin stretching vibration. The peak at 2929 cm−1 represents the asymmetric and symmetric –CH2 stretching vibrations. The peaks between 1730 and 1407 cm−1 represent –C=O stretching, asymmetric and symmetric vibration of carboxylic groups, acid or ester and asymmetric and symmetric vibration of carboxylic groups which appeared at 1632 cm−1 and 1418 cm−1. The peaks from 1240 to 750 cm−1 are assigned to stretching vibration of alcohol and the stretching vibrations of C–O–C. Thus, the presence of abundant hydroxyl groups in the adsorbent abundance may be involved in the formation of coordination bonds with the metal ions.
The powdered Albizia lebbeck pod adsorbent shows several diffraction peaks of oxalate at major reflections at 2θ = 15.1°, 24.2°, 30.5°, 38.1° and 49.7°. The information from this analysis provides supporting evidence to the effects of pH on the chemical interaction of the adsorbent with the existing hydroxyl and oxalate groups in the formation of metal oxalate complexes (Fig. 2).
Figure 3 represents the N2 adsorption–desorption isotherms obtained at − 196 °C of the powdered Albizia lebbeck pod adsorbent. It displays the type IV isotherm. The calculated specific area and pore volume were 34.07 m2/g and 0.027 cc/g, respectively.
Influence of adsorption time
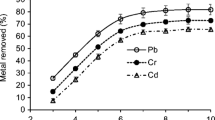
The effect of contact time on the adsorbent and metal ions is shown in Fig. 4. The rate of adsorption with respect to residence time on the surface of Albizia lebbeck pod shows that the percentage removal of the metal ions firstly increased with increasing contact time; that is, the adsorption processes is fast until they reached their optimum times. This is attributed to the availability of large surface areas of the adsorbent. At these points, the equilibrium times were attained. After the plateau, the surface pores of the adsorbent became enclosed and desorption began. The slow rate of adsorption at this stage also known as the desorption process may be due to the agglomeration of metal ions on the surface of the adsorbent.
Effect of solution pH
The pH of an aqueous solution is described as a significant parameter that influences the adsorption process. During this process, the functional groups, surface charges, degree of ionization and solubility of the adsorbent are frequently responsible for the binding of metal ions onto the adsorbent. In Fig. 5, the optimum pH values ranged from 6 to 8 for the studied metal ions. It was observed that maximum removal of metal ions occurred at acidic medium and a further increase in pH above their optimum caused the formation of precipitates. At lower pH, the adsorbent is protonated and the protons compete with the metal ions, resulting in less adsorption. The interaction between these metal ions increased with pH, and at higher pH, the presence of negatively charged functional groups such as hydroxyl and oxalate ions ensure the metal oxalate complex formation, thus influencing the adsorption of metal ions onto the adsorbent. The electrostatic interaction between the positively charged metal ions and the negatively charged oxalate ions, a bidentate ligand, is responsible for the formation of the metal oxalate complexes.

A rare interpretation of the effect of pH on the adsorption process via sorption edge, in which the concentration of the adsorbate is kept constant while the pH is varied, is displayed in Fig. 6. The adsorption coefficient, \(K_{\text{D}}\), is evaluated using the equation:
where \(C_{\text{w}}\) (mol/dm3) the concentration in the solution at equilibrium, while adsorption capacity \(C_{\text{s}}\) (mol/m3) is computed as follows:
where M the mass of adsorbent loaded in the aqueous solution (g) and A is the specific area of the adsorbent (m2/g). The plot of \(K_{\text{D}}\) versus pH gave similar curves to the plot of percentage removal against pH, but in contrast, the plots, in this case, gave sharper curves.
Effect of initial concentration
Figure 7 shows the effect of the initial concentration of the metal ions (10–30 mg/dm3) on their adsorption on the adsorbent. The results obtained show that as the initial heavy metal ion concentration increased, at 25 °C, constant pH and constant adsorbent mass for the respective optimum times, the % removal of the metal ions decreased. The higher initial metal ion concentrations provide a strong driving force between the liquid/solid phases (Ali et al. 2016). At lower concentrations, the ratios of available binding sites to the initial metal ion concentrations were larger, while at higher concentrations, the saturation of the adsorption sites occurred. This behaviour is attributed to less availability of surface-active sites.
Effect of dosage
The influence of adsorbent dose of Albizia lebbeck pod powder was investigated in the range of 0.4–1.2 g for the initial metal ion concentrations. The plots of percentage removal of metal ions against dosage are presented in Fig. 8. It is worth noting that the % removals of the studied metal ions increased as the adsorbent dose increased. This could be due to the increased adsorbent surface area, pore size and volume, and the availability of vacant sites. The higher adsorbent dosage gave rise to higher removal of metal ions, providing an important driving force to overcome all mass transfer resistances of the metal ions between the aqueous and solid phases.
Effect of temperature and thermodynamics of adsorption
The effect of temperature parameter on the uptake of metal ions by Albizia lebbeck pod describes the relationship between temperature and the concentrations of the metal ions adsorbed onto the adsorbent at optimum contact time. As illustrated in Fig. 9, the adsorption of the metal ions on the adsorbent increased as the temperature increased from 30 to 80 °C. The increase in adsorption of metal ions could be the result of an increase in the mobility of the metal ions as a result of acquired energy in the system. This indicated that the interaction of metal ions with active sites at the surface of the adsorbent is an endothermic process, which could also be considered as a chemical adsorption process.
In order to describe the spontaneity of the adsorption process by thermodynamic parameters, temperature was varied from 303 to 353 K. Thermodynamic parameters of adsorption such as change in standard Gibbs free energy (\(\Delta G^{\uptheta}\)), change in standard enthalpy (\(\Delta H^{\uptheta}\)) and change in standard entropy (\(\Delta S^{\uptheta}\)) were computed by from the following equations:
where \(\Delta G^{\uptheta}\) is the change in standard free energy (kJmol−1), R is the universal gas constant (8.314 Jmol−1K−1), \(K_{\text{e}}\) is the thermodynamic equilibrium constant and T is the absolute temperature (K). The values of \(\Delta H^{\uptheta}\) and \(\Delta S^{\uptheta}\) were determined from the slope and the intercept from the plot of \(\ln K_{\text{e}}\) against \(\frac{1}{T}\) (K−1). The recorded values of \(\Delta H^{\uptheta}\) and \(\Delta S^{\uptheta}\) are presented in Table 1. The positive values of \(\Delta H^{\uptheta}\) indicate that the adsorption was endothermic in nature, while the positive values of \(\Delta S^{\uptheta}\) show the affinity of the adsorbent for the metal ions, indicating an increase in sorbate concentration in the solid–liquid interface. The thermodynamic parameters show that the adsorption of metal ions is non-spontaneous at low temperature, while spontaneity occurs at high-temperature values. This confirms the increasing randomness at the solid solution interface during sorption. Furthermore, the activation energy (\(E_{\text{a}}\)) is determined from the straight-line plot of \(\ln K_{\text{e}}\) against \(\frac{1}{T}\) using the Arrhenius equation:
The calculated \(E_{\text{a}}\) from the slopes (− \(\frac{{E_{\text{a}} }}{R}\)) from this plot was found to be between 33.161 and 25.685 kJ/mol, indicating chemisorption adsorption (Fig. 10).
Adsorption isotherm
Langmuir isotherm
The Langmuir adsorption isotherm assumes that monolayer adsorption exists at all surface sites that is homogeneity, with the ability of no interaction of adsorbed molecules with the neighbouring adsorption sites. The linear Langmuir equation is represented as follows:
where \(C_{\text{e}}\) is the equilibrium concentration (mg/dm3), \(q_{\text{e}}\) is the amount (mg/g) adsorbed at equilibrium time, and \(Q_{m} \;{\text{and}}\;b\) are Langmuir constants related to maximum adsorption capacity (mg/g) and energy of adsorption related to the heat of adsorption (L/mg), respectively. The Langmuir parameters were computed from the slopes and intercepts of the linear plot of \(C_{\text{e}}\) as the abscissa and \(C_{\text{e}} /q_{\text{e}}\) as the ordinate as displayed in Fig. 12.
The Langmuir isotherm parameters were used to calculate the affinity between the adsorbent and adsorbate via dimensionless separation factor, \(R_{\text{L}}\), as determined using the following equation:
where \(b\) is the Langmuir constant and \(C_{\text{o}}\) is the initial concentration of metal ions. The \(R_{\text{L}}\) values promulgate whether the adsorption is irreversible (\((R_{\text{L}} = 0)\), favourable \((0 < R_{\text{L}} < 1)\) or linear or unfavourable \((R_{\text{L}} = 1\;{\text{or}}\;R_{\text{L}} > 1)\). The values of \(R_{\text{L}}\) calculated for different initial concentrations are given in Table 3.
Freundlich isotherm
The empirical Freundlich isotherm model is applied to describe the adsorption on a non-uniform (heterogeneous) surface with the interaction between adsorbed molecules in the reversible and non-ideal adsorption process. The Freundlich adsorption isotherm linear form is given as:
where \(K_{\text{F}}\) and \(n\) are Freundlich constants representing coefficient and intensity, respectively. The experimental values for \(n\) and \(K_{\text{F}}\) based on the slope and the intercept, respectively, are determined by plotting \(\ln C_{\text{e}}\) against \(\ln q_{\text{e}}\) as shown in Fig. 11.
The Langmuir isotherm showed better fit to the experimental data with higher correlation coefficients for all the metal ions; as given in Table 2, the maximum adsorption capacities of the adsorbent were found to be between 8.68 and 7.17 mg/g. The \(R_{\text{L}}\) in the range of 0–1 decreased with increasing initial metal ion concentration which indicates favourable uptake of metal ions.
The n values measure the favourability of the adsorption process. The n values for the metal ions correspond to the favourable adsorption process of \(0 < n < 10\). The correlation coefficient of Freundlich isotherm was found to be far away from unity when compared to the Langmuir isotherm model. Table 3 shows that the equilibrium data for the adsorption of metal ions onto the powdered Albizia lebbeck pod fitted the Langmuir more than the Freundlich isotherm model (Figs. 12, 13).
Adsorption kinetics
The adsorption kinetics and rate constants were determined from kinetic models including the pseudo-first-order and pseudo-second-order models. The pseudo-first-order and pseudo-second-order adsorption kinetics based on equilibrium adsorption are represented as follows:
where \(q_{\text{e}}\) and \(q_{t}\) are the amounts of metal ions adsorbed onto the powdered Albizia lebbeck pods (mg/g) at equilibrium and at a time, t, respectively. \(k_{1}\) and \(k_{2}\) are the rate constants for pseudo-first-order and pseudo-second-order kinetics, respectively. The first-order constant (min−1) was determined in linear form by plotting \(\ln \left( {q_{\text{e}} - q_{t} } \right)\) against t. A plot of \(\frac{t}{{q_{t} }}\) against t was used to determine pseudo-second-order constant (mg/g min).
Adsorption kinetics provides information on the reaction pathways and the mechanisms of the adsorption of sorbate by the sorbent. Table 4 describes the kinetics of the adsorptions of studied metal ions on powdered Albizia lebbeck pods by using pseudo-first and pseudo-second-order models. For the pseudo-first-order model, the low \(k_{1}\) suggests that a slow adsorption process takes place, while for the pseudo-second-order model, \(k_{2}\) values were high indicating an increase in adsorption rates. The \(R^{2}\) values for the adsorption kinetics of the metal ions were higher for the pseudo-second-order model compared to those of the pseudo-first-order model. Thus, it appears that pseudo-second-order model is a better model fitting the kinetics of the adsorption of the metal ions employed in this study (Figs. 14, 15).
Furthermore, in order to select a better model that fits the experimental data, Chi-square (\(\chi^{2}\)), the mean square error (MSE), correlation coefficient (R2) and the validation by the normalized standard deviation (q) listed in Table 4 were used to justify the favoured adsorption kinetic model. The smaller the \(\chi^{2}\) value, the closer the agreement in the fit between the experimental data and linearized forms of the kinetic equations using the following relationships.
where \(N\) is the number of data points, \(q_{\text{e,exp}}\) is the experimental adsorption capacity and \(q_{\text{e,cal}}\) is the calculated adsorption capacity.
Conclusion
The present study has demonstrated that Albizia lebbeck pods powdered can be used as a natural, promising, economic and environmentally friendly adsorbent for Pb(II), Cd(II), Zn(II) and Cu(II) from aqueous solutions. BET, XRD and FTIR were used to analyse for the surface area, phase identification and functional groups that are responsible for the metal ion removal from aqueous solutions. The batch adsorption method is dependent on the contact time, dosage, solution pH, initial metal ion concentrations and temperature. The experimental data in the adsorption process indicated good correlations with the pseudo-second-order kinetic model and Langmuir isotherm. The thermodynamic study showed that the process is highly endothermic in nature, with positive values of enthalpy changes (\(\Delta H^{\uptheta}\)) and changes in the entropy (\(\Delta S^{\uptheta}\)). Based on this study, the readily available Albizia lebbeck pods which are nuisance to the environment could be used as a natural adsorbent, due to its high removal efficiencies of the metal ions involved in this study.
References
Ali A, Saeed K (2015) Decontamination of Cr(VI) and Mn(II) from aqueous media by untreated and chemically treated banana peel: a comparative study. Desalin Water Treat 35(11):3586–3591
Ali RM, Hamad HA, Hussein MM, Malash GF (2016) Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol Eng 91:317–332
Asif Z, Chen Z (2017) Removal of arsenic from drinking water using rice husk. Appl Water Sci 7(3):1449–1458
Asuquo ED, Martin AD, Nzerem P (2018) Evaluation of Cd(II) ion removal from aqueous solution by a low-cost adsorbent prepared from white yam (Dioscorea rotundata) waste using batch sorption. ChemEngineering 2:35
Banerjee S, Mukherjee S, LaminKa-ot A, Joshi SR, Mandal T, Halder G (2016) Biosorptive uptake of Fe2+, Cu2+ and As5+ by activated biochar derived from Colocasia esculenta: isotherm, kinetics, thermodynamics, and cost estimation. J Adv Res 7(5):597–610
Escapa A, San-Martin MI, Moran A (2014) Potential use of microbial electrolysis cells in domestic wastewater treatment plants for energy recovery. Front Energy Res 2:19
Escapa A, Mateos R, Martinez EJ, Blanes J (2016) Microbial electrolysis cells: an emerging technology for wastewater treatment and energy recovery: from laboratory to pilot plant and beyond. Renew Sustain Energy Rev 55:942–956
Reddy NA, Lakshmipathy R, Sarada NC (2014) Application of Citrullus lanatus rind as biosorbent for removal of trivalent chromium from aqueous solution. Alex Eng J 53(4):969–975
Tasar S, Kaya F, Ozer A (2014) Biosorption of lead(II) ions from aqueous solution by peanut shells: equilibrium, thermodynamic and kinetic studies. J Environ Chem Eng 2:1018–1026
Zare H, Heydarzade H, Rahimnejad M, Tardast A, Seyfi M, Peyghambarzadeh SM (2015) Dried activated sludge as an appropriate biosorbent for removal of copper (II) ions. Arabian J Chem 8(6):858–864
Zhang Y, Angelidaki I (2014) Microbial electrolysis cells turning to be versatile technology: recent advances and future challenges. Water Res 56:11–25
Zhang Y, Angelidaki I (2016) Microbial electrochemical systems and technologies: it is time to report the capital costs. Environ Sci Technol 50:5432–5433
Acknowledgements
The authors acknowledge Shuaib, Damola Taye, a Ph.D. student in the Department of Chemistry, Illinois Institute of Technology, Chicago, USA, and Department of the Chemistry Federal University of Technology, Minna, for supporting this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mustapha, S., Shuaib, D.T., Ndamitso, M.M. et al. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions using Albizia lebbeck pods. Appl Water Sci 9, 142 (2019). https://doi.org/10.1007/s13201-019-1021-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-1021-x