Abstract
Unorganized development all over the world has resulted in the deterioration of the environment. The Mithi river located in Mumbai area is also one of the victims of this unorganized development. It has become a dumping site for industrial effluents, which contains various toxic materials including heavy metals such as mercury, chromium, arsenic. Physicochemical analysis of the Mithi river water samples has confirmed the deterioration of river water. Various parameters of Mithi river water such as electrical conductivity (EC), total dissolved salts (TDS), salinity, chemical oxygen demand (COD) and biological oxygen demand (BOD) were found to be higher than the normal values prescribed for the river water. In the present study, mercury-resistant bacteria were isolated from Mithi river and identified by biochemical and molecular analysis. The molecular identification of the mercury-resistant bacteria was done by 16S rDNA identification, which were identified as Bacillus sp. strain CSB_B078, Klebsiella pneumoniae strain FY2, Klebsiella pneumoniae isolate 23, Enterobacter sp. strain Amic_7, Enterobacter sp. strain 08, Acinetobacter seohaensis strain S34, Acinetobacter sp. 815B5_12ER2A. Mercury-resistant bacteria were isolated from both low- and high-salinity sites of Mithi river. The minimum inhibitory concentration of bacterial isolates against mercury was determined. Bacterial isolates could tolerate and grow in the presence of 700 ppm mercury and could also tolerate a high salinity of 35 ppt of NaCl. Resistance of bacterial isolates to high concentration of mercury and high-salinity stress suggests that the bacterial isolates could be an efficient candidate for bioremediation of mercury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is an essential component of the earth living system which forms a major portion of all living beings. Clean potable water is a prime requirement for humans and other living organisms for their better health and life (Vasudevan and Oturan 2014). In recent period, developing countries are being subjected to high risk of increasing water pollution due to rapid and unorganized industrialization, increased human population and domestic and agricultural activities. Water pollution is one of the major causes leading to various life-threatening diseases in humans. Heavy metals are among the highly toxic pollutants which is contaminating the water bodies and affecting the living organisms. Globally, heavy metal pollution is becoming the most serious environment problem (Santos et al. 2017; Waseem et al. 2014).
River is an important natural resource and played a crucial role in human civilization. Many reports have confirmed the severe contamination of major as well as minor rivers with heavy metals and other toxic chemicals in India (Siddiqui and Pandey 2019; Patel et al. 2018; Singh and Giri 2018). Mercury contamination in river water poses serious health issues to human beings and to other water living bodies such as plants and fishes (Officioso et al. 2016; Dash and Das 2015). Bioaccumulation and magnification of mercury in food trophic chain (Rua-Ibarz et al. 2016) lead to serious health risks to human beings, which includes acute necrotizing bronchitis and pneumonitis that may lead to failure of respiratory system and even death. Long-term exposure to mercury affects human brain functions and is also responsible for disorders in central nervous system (Holmes et al. 2009). Other human health problems associated with mercury pollution include proteinuria or nephritic disorder (Rafique et al. 2015). Various effects of mercury toxicity depend upon its chemical form and the route followed for mercury exposure (Rice et al. 2014). Mercury in its methylated form can impair reproduction and interrupt the expression of hormones like oestradiol and testosterone (Tartu et al. 2013).
Sources of mercury pollution in river water may include both natural and anthropogenic activities (Chaudhary et al. 2019; Carocci et al. 2014). Its use in various industries such as paper industry, textile factories, paints, cosmetics, preservatives, thermometers, manometers, energy-efficient fluorescent light bulbs, and to a limited extent, mercury batteries, fertilizer industry, mining facilities and tanneries, causes its release as a waste that pollute river water and ultimately the environment (Srinivas et al. 2017; Baul et al. 2013; Yue et al. 2014). Mithi river flowing in Mumbai area has become a receiving point for various pollutants including toxic heavy metals (Singare et al. 2012a, b; Pushkar et al. 2015). Various studies have reported the presence of mercury at different sites of Mithi River (Nagarsekar and Kakde 2014; Singare et al. 2012a, b). Annual average concentration of mercury in Mithi river was reported to be 32.06 ppm (Singare et al. 2015), which is much higher than the permissible limit (0.001 ppm) set by Central Pollution Control Board (CPCB) of India and World Health Organization (WHO).
Bacteria are adaptive to extreme environmental stress condition (Liu et al. 2016). The presence of high concentrations of mercury in Mithi river may induce tolerance in bacteria towards mercury. Various studies have reported that bacteria develop resistance towards heavy metals (Pepi et al. 2013; Dash and Das 2016). Further, bacteria are highly ubiquitous in nature and can bioremediate heavy metals using their integral mechanisms (Mahbub et al. 2016). Bacteria can also work in combination with other microorganisms for effective removal of mercury (Santos-Gandelman et al. 2014). Hence, it was speculated in the current investigation that Mithi river may contain mercury-resistant bacteria which could be isolated and further used to bioremediate mercury pollution from various water resources.
In the current study, physicochemical characteristic of Mithi river water was determined to understand the current status of Mithi river pollution. Mercury-resistant bacteria were isolated and characterized to determine their identity. Bacterial isolates were further studied to determine their tolerance to varying concentration of mercury. Salinity tolerance of the bacterial isolates was determined to understand the mercury remediation capability of bacterial isolates in the presence of varying salt concentration. Salinity tolerance study of bacterial isolates will help in understanding their efficacy to remediate mercury in the presence of varying salt concentration present in Mithi river. Thus, the objective of current study was to determine an efficient candidate which can be used for bioremediation of mercury from river water.
Material and methods
Sample collection
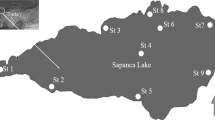
Mithi River originates from the over flow of Vihar Lake; it flows for a distance of 18 km before meeting Arabian Sea at Mahim Creek. Salinity of Mithi river is low all along its stretch except at estuary where the salinity is higher due to the mixing of sea water. The water samples were collected from high-salinity stretch of Western Express Highway (19°03′02.5″N 72°50′29.5″E) and Kalanagar (19°03′07.6″N 72°50′53.4″E) and low-salinity stretch of Bandra-Kurla Complex (19°03′29.7″N 72°52′11.0″E) and Krantinagar (19°05′06.7″N 72°52′42.9″E). Water samples were collected in sterile 100-ml screw cap glass bottle covered with silver foil and were stored at 4 °C till further analysis. Sampling site are given in the Fig. 1.
Physicochemical analysis of water samples
Water samples collected from four sites of Mithi river were analysed for various parameters such as pH, electrical conductivity (EC), salinity, total dissolved solid (TDS), chemical oxygen demand (COD) and biological oxygen demand (BOD). Physical parameters such as pH, EC, salinity and TDS were analysed using Multiparameter (Labman Multiparameter, water quality meter). Other parameters such as COD and BOD were analysed by following the standard protocols of American Public Health Association (APHA 1989).
Sample processing
Water samples were processed prior to the isolation of mercury-resistant bacteria. Water samples were filtered using Whatman filter paper no. 1 to separate suspended solid particles and further serially diluted till 10−4 dilution using sterile 0.85% saline. Diluted water samples were used for the isolation of mercury-resistant bacteria.
Isolation of mercury-resistant bacteria
Isolation of mercury-resistant bacteria from Mithi river water sample was performed under sterile condition in laminar air-flow hood. Mercury-resistant bacteria were isolated on nutrient agar medium containing 50, 100 and 150 ppm (parts per million) of mercury, respectively. Mithi river water samples diluted to 10−2, 10−3 and 10−4 dilution using sterile 0.85% saline were spread-plated on nutrient agar plates containing 50, 100 and 150 ppm of mercury, respectively. High concentration of mercury in Mithi river (Singare et al. 2012a, b; Pushkar et al. 2015) suggested the possibility that the river may harbour a high number of mercury-resistant bacteria. Hence, the river water was diluted in the above-mentioned dilution before spread-plating it on nutrient agar plates containing mercury so as to get the isolated colonies of mercury-resistant bacteria (Zeroual et al 2001; Pushkar et al. 2015). Nutrient agar plates were incubated at 37 °C for 24 h. After the incubation period, morphologically different colonies were selected from nutrient agar plates for further study. Isolated bacterial colonies selected from nutrient agar plates were further inoculated in nutrient broth and incubated at 37 °C on shaker incubator with 150 rpm (revolutions per minute) for 24 h. After the incubation period of 24 h, a loop full of bacterial culture from nutrient broth was further subjected to isolation on nutrient agar plate and incubated at 37 °C for 24 h. The above procedure was repeated 2–3 times to get the purified isolated bacterial colonies comprising of single type of bacteria. Purity of bacterial colonies was confirmed by Gram staining technique. Purified bacterial isolates were stored in glycerol at − 20 °C till further study.
Biochemical characterization
Mercury-resistant bacteria isolated in the current study were analysed for their biochemical characteristics. Bacterial isolates were analysed for their ability to ferment glucose, lactose, maltose, xylose and sucrose. Bacterial isolates were also tested for indole, methyl red, Voges–Proskauer, Simmons citrate tests (IMViC test). Other biochemical tests included in study were triple sugar iron (TSI), motility, gelatinase and catalase. Bacterial isolates were also plated on selective and differential media such as mannitol salt agar, eosin methylene blue agar, MacConkey agar and Cetrimide agar to study their growth pattern on these media. Biochemical characterization of bacterial isolates helped in determining the genus of mercury-resistant bacterial isolates.
16S rDNA identification
Identification of mercury-resistant bacteria isolated in the current study was also done by using molecular technique of 16S rDNA sequencing. 16S rDNA is a conserved gene present in all bacteria, and it can be sequenced to know the identity of unknown bacteria. Universal primers were used to amplify 16S rDNA sequence using PCR (polymerase chain reaction). 500-bp region of 16S rDNA was amplified using forward primer 347F-5′GGAGGCAGCAGTAAGGAAT-3′ and reverse primer 803R-5′CTACCGGGGTATCTAATCC-3′ (Wu et al. 2014). The 50 µl reaction mixture containing 25 µl of ready-to-use Hi‐Chrom PCR Master Mix (Himedia) and 1 µl of 20 pmol/µl of each forward and reverse primer and 15 ng of template DNA was used for PCR. The final volume of PCR mixture was made to 50 µl using sterile DNAase free water. PCR was performed as follows: initial denaturation at 94 °C for 5 min, denaturation at 94 °C for 30 s, primer annealing at 50 °C for 35 s, extension at 72 °C for 45 s and a final extension at 72 °C for 10 min. PCR was performed using Veriti Thermal Cycler, and the amplified gene product was subjected to agarose gel electrophoresis to confirm the amplification of 16S rDNA and its molecular size. Further, the sequencing of amplified PCR product was done in SciGenom Labs, Cochin, and the identity of bacteria was determined by performing BLAST of the sequence obtained.
Minimum inhibitory concentration (MIC)
Minimum inhibitory concentration (MIC) is defined as the lowest concentration of the heavy metal at which no visible growth of microorganism is observed after incubation period of 24 h (Andrews 2002; Yoshida et al. 2006). In the current investigation, MIC of mercury-resistant bacterial isolates was determined by using nutrient broth medium containing mercury in a range of 50 ppm to 1000 ppm. Log phase culture of mercury-resistant bacterial isolates was inoculated in the above nutrient broth medium and incubated at 37 °C on a shaker incubator with 150 rpm for 24 h. After the incubation period of 24 h, nutrient broth tubes were observed for the presence and absence of bacterial growth.
Salinity tolerance
Salinity of the surrounding environment plays an important role in physiological activity of bacterial and its survival (Jadhav et al. 2010; Díaz et al. 2002). Mercury-resistant bacteria isolated in the current study were analysed for their ability to tolerate salinity by using nutrient broth medium containing varying concentration of salt (sodium chloride) in a range of 5 to 35 ppt (parts per thousand). Log phase culture of the mercury-resistant bacterial isolates was inoculated in the above-mentioned nutrient broth medium and incubated at 37 °C on a shaker incubator with 150 rpm for 24 h. After the incubation period of 24 h, nutrient broth with varying salt concentrations was observed for the presence and absence of bacterial growth.
Results and discussion
Physicochemical analysis of water samples
The physicochemical characterization of various parameters of Mithi river water is presented in Table 1. Values of pH of water from four sites of Mithi river were found within the acceptable limits. The pH of Mithi river water was slightly alkaline at all the sites, indicating that there is no acid or alkali contamination in river water. Electrical conductivity (EC) of Mithi river water was higher than the permissible limit at all four sites. Water from WE highway site showed highest EC in comparison with the water of other sites. High EC in water samples from all four sites of Mithi river may be due to the presence of large amount of dissolved ions (Dey et al. 2016). Pollution of river water by discharge of sewage and industrial waste increases the EC of river water. High level of dissolved ion imparts mineral taste to the water and also affects plants and organisms (Mohabansi et al. 2011). Total dissolved solids in Mithi river water were also higher than the standard value at all sites of river except at Krantinagar. High TDS in river water is mainly due to the presence of carbonates, bicarbonates, chlorides, phosphates and nitrates of calcium, magnesium, potassium and manganese, organic matter salts and other solid particles. Pollution of Mithi river by industrial discharge could be the reason for high TDS as the above constituents are significantly present in Industrial effluent (Mohabansi et al. 2011). The presence of high amount of TDS in river water also makes it unfit for consumption (Patil and Patil 2010).
Salinity of Mithi river water in upper stretch of river, i.e. Krantinagar, was 0.47 ppt which is within the acceptable limits. Salinity of river water at the other three sites of river was significantly higher than the acceptable limits. High amount of dissolved minerals increases the salinity of river water, which could also be one of the reasons for increased salinity of Mithi river water at these sites. High salinity of river water makes it unfit for irrigation as well as for consumption (Ajao et al. 2017). The COD and BOD analysis of Mithi river water gave important information about the status of organic pollutants in water system. COD and BOD values for Mithi river water were above the standard values at all four sites of river. Highest COD was at Krantinagar and lowest at Western Express Highway. Highest BOD was observed at Krantinagar and lowest at Kalanagar. High BOD and COD values of river water indicate the high level of organic pollutants in river water, which leads to depletion of dissolved oxygen (DO). Decreased level of oxygen in river water affects the aquatic life as DO is the prime requirement for organisms living in water (Ajao et al. 2017).
Physicochemical characterization of Mithi river water at four sites of river indicates that level of organic pollution is high in the upper stretch of river. Probable source of organic pollution at these sites could be the domestic waste discharge from highly populated human settlements at these sites along the Mithi river. Higher EC, TDS and salinity were observed in the lower stretch of the river water. Mithi river pollution by inorganic waste discharge by industries situated along river side could be the reasons for high EC, TDS and salinity of river water as observed in the current study.
Isolation of mercury-resistant bacteria
Mercury-resistant bacteria were isolated from four sites (Western Express Highway, Kalanagar, Bandra-Kurla Complex and Krantinagar) of Mithi river. Table 2 shows the number of colony-forming units (CFU) of bacterial isolates observed on nutrient agar medium containing 50, 100 and 150 ppm of the mercury. Growth of bacterial isolates from river water samples could be observed in the presence of 150 ppm of mercury. Growth of high number of CFU of bacteria in the presence of high concentration of mercury indicates that the bacteria either possess intrinsic resistance or have developed the resistance due to the exposure to high concentration of mercury present in river water (Singare et al. 2012a, b; Barkay and Olson 1986). A less number of CFU of mercury-resistant bacteria were observed in 10−4 diluted river water when compared to 10−2 diluted river water from all the sites of Mithi river. This may be due to reduction in the pool of mercury-resistant bacteria when the river water is diluted. Also it was observed that high number of bacteria could grow in nutrient medium containing low concentration of mercury when compared to bacterial growth in nutrient medium containing high concentration of mercury. Less bacterial growth in nutrient medium containing high concentration of mercury suggests that Mithi river water harbours a low number of bacteria that are resistant to high concentration of mercury. Also there is presence of high number of bacteria in Mithi river water which are resistant to low concentration of mercury. Thus, Mithi river harbours mercury-resistant bacteria having differential capacity to tolerate mercury.
Further, it was also observed that there were a less number of mercury-resistant bacteria as depicted by CFU count of river water collected from Bandra-Kurla Complex site of the river. Earlier investigations have reported that there is low level of mercury in Mithi river water of Bandra-Kurla Complex (BKC) site when compared to the water from other sites of river (Singare et al. 2012). Due to the presence of low levels of mercury at BKC site, less number of bacteria could have develop the resistance towards mercury. Mercury-resistant bacterial CFU count as observed in current study also indicates over all high load of mercury-resistant microbes in Mithi river water.
Seven mercury-resistant bacterial isolates from four different sites of Mithi river were selected for further study. Bacterial isolates were selected on the basis of morphological differences as observed in colony characters of bacteria and their ability to grow at a high concentration of mercury.
Biochemical characterization
Various biochemical tests were performed to determine the identity of mercury-resistant bacteria isolated in the current study. Table 3 shows the ability of mercury-resistant bacterial isolate to ferment various types of sugar. Isolated bacteria were analysed for biochemical characters in order to determine their approximate genus. Bacterial isolate, WHg3b could utilize and grow in the presence of glucose, sucrose and maltose without any gas production. Other bacterial isolates such as HgKN1 and HgKN2 could ferment all types of sugars with gas production, except glucose. Both of these bacterial isolates could utilize all four types of sugars with gas production except glucose. HgKN3 could only ferment and grow in the presence of sucrose. HgKN4 could ferment lactose maltose and sucrose along with the production of gas. HgS4a and HgS4b were not able to ferment glucose, lactose, maltose, sucrose and xylose. All bacterial isolates which could ferment sugar along with gas production may belong to the Enterobacteriaceae family.
Other biochemical tests performed include IMViC (indole, methyl red, Voges–Proskauer and citrate) test, motility, catalase, TSI and gelatinase. Growth pattern of the bacterial isolates was observed by growing them on selective and differential medium such as MacConkey agar medium, eosin methylene blue agar medium (EMB), Cetrimide agar medium and mannitol salt agar medium. Observation of the above-mentioned tests is depicted in Table 4. WHg3b could utilize some sugar in TSI and produce gas. HgKN1 and HgKN2 show similar biochemical characteristics. They were methyl red positive which confirm that these isolates could produce stable acids. These bacterial isolates could also utilize citrate as carbon source and were motile in nature. They could produce catalase enzyme under oxidative stress and were also strong acid producer with gas by fermenting all three sugars in TSI. Considering all the biochemical tests and their growth on EMB and MacConkey agar medium, it could be said that HgKN1 and HgKN2 isolates may belong to Enterobacteriaceae family. Bacterial isolates HgKN3 and HgKN4 also showed results for biochemical tests similar to the bacterial isolates HgKN1 and HgKN2 but could not produce stable acid as they were methyl red negative. HgS4a and HgS4b were able to utilize citrate as carbon source and produce catalase enzyme which shows that they can survive under oxidative stress by neutralizing the free radicals but were unable to utilize glucose, sucrose and lactose of TSI. Also the two isolates could not produce any gas. On the basis of biochemical characters, it can be said that probably these two isolates belong to Acinetobacter genus.
Mithi river water sampling sites for isolation of mercury-resistant bacteria (Singare, 2015)
16S rDNA identification
Mercury-resistant bacteria isolated in current study were also identified by 16S rDNA identification technique. PCR-amplified 16S rDNA sample of the bacterial isolates was observed on agarose gel electrophoresis. It showed the presence of a single band of 500 bp size on agarose gel (Fig. 2). Further, the PCR-amplified 16S rDNA samples of bacterial isolates were sequenced and the sequence was analysed by BLASTn to identify the bacteria. 16S rDNA sequences of the isolated bacteria were submitted to NCBI (National Centre for Biotechnology Information) GenBank. Table 5 shows bacterial identity and NCBI accession number of 16S rDNA sequence of bacterial isolates. 16S rDNA identification technique showed that mercury-resistant bacteria isolated in current study belonged to genus Bacillus, Klebsiella, Enterobacter and Acinetobacter. Earlier reports suggest that Enterobacter sp. (Sinha and Khare 2012) and Bacillus sp. (Pushkar et al. 2019; Green-Ruiz 2006; Sinha et al. 2012; vanDijl and Hecker 2013) of bacteria could effectively bioremediate mercury. It was also observed that the bacteria isolated in the current study could tolerate higher concentration of mercury in comparison to earlier reported mercury-resistant bacteria (Dash et al. 2014) which suggests that the mercury-resistant bacteria isolated in the current study could serve to effectively remediate mercury pollution from river water.
Phylogenetic analysis of the isolated bacteria was done by using CLUSTALW program by using neighbour-joining tree analysis of 500 bp 16S rDNA gene of the bacterial isolates. Phylogenetic tree was prepared to find the evolutionary distance or relationship among all mercury-resistant bacterial isolates as shown in Fig. 3, which shows the relatedness of bacterial isolates on the basis of nucleotide sequences. It also shows seven external nodes, which indicate the point of divergence, and the presence of two clusters, which indicates the evolutionary closeness. Evolutionarily related bacteria may possess a similar mechanism of mercury tolerance and its remediation.
Minimum Inhibitory concentration
Bacteria isolated from Mithi river were grown in nutrient broth containing varying concentrations of mercury to determine the minimum inhibitory concentration (MIC). It was observed that bacterial isolates were able to grow in nutrient broth containing higher concentrations of mercury. Table 6 gives the minimum inhibitory concentration of seven mercury-resistant bacteria. Out of seven bacterial isolates, MIC for six bacterial isolates was 700 ppm of mercury, while for one bacterial isolate MIC was 500 ppm of mercury. The high MIC of the isolated bacteria indicates that bacterial isolates could tolerate high concentration of mercury. Acinetobacter sp. and Klebsiella sp. were reported in study by Jan et al. (2016) was capable of tolerating 148 ppm of mercury, while the Acinetobacter sp. and Klebsiella sp. isolated in current study could tolerate 700 ppm of mercury. All bacterial isolates have shown similar MIC except for the bacteria isolated from Kalanagar (WHg3b). WHg3b showed MIC of 500 ppm which is lower than rest of the bacterial isolates. The similar level of MIC of the isolated bacteria may be due to the taxonomical closeness of the isolates (Møller et al. 2011). Also it was observed in current study that WHg3b was Gram positive in nature and other bacterial isolates were Gram negative in nature, which suggests that Gram-negative isolates may have higher tolerance to mercury as compared to Gram-positive isolates. Earlier investigations by Figueiredo et al. (2016) also corroborate with the above inference.
Bacteria isolated from the Mithi river were resistant to high concentration of mercury, which they may have acquired through horizontal transfer of mercury-resistant gene between the bacteria under mercury stress (Møller et al. 2010). As reported by Dash and Das 2014, bacteria may develop resistance to mercury through horizontal transfer of mer operon which consists of different genes such as merA, merB, merT and merP (Osborn et al. 1997) which encodes for enzymes that helps in mercury reduction and volatilization. merB gene of mer operon encodes for organomercurial lyase, which helps in conversion of highly toxic organomercurial compounds such as methylmercury and phenyl mercuric acetate into almost nontoxic volatile elemental mercury after its reduction by reductase enzyme (Dash and Das 2012). Besides the reduction as reported by François et al. (2012), the bacteria may have the capability to bioadsorb the mercury on bacterial surface through exopolysaccharide. Further proteomic and metabolomics study of mercury-resistant bacteria isolated in the current study will help in understanding the mechanism employed by these bacteria for exhibiting resistance to high concentration of mercury.
Salinity tolerance
Salinity of the surrounding environment is one of the important factors that affects growth and survival of bacteria. It affects bacterial cell membrane, which is important for maintaining osmotic pressure and preventing bacterial cell damage. Earlier investigations suggest that salinity affects metal tolerance and remediation capacity of bacteria (Dash et al. 2014). Mercury-resistant bacteria isolated in present study exhibit tolerance to a wide range of salinity, though the bacterial growth was comparatively less at higher salinity when compared to its growth at lower salinity (Table 7). All seven bacterial isolates could grow in medium containing up to 35 ppt of salt.
Conclusion
Physicochemical analysis confirms the critical condition of Mithi river and recommends to take immediate measures to improve its water quality. Mithi river harbours mercury-resistant bacteria belonging to genus Bacillus, Enterobacter, Klebsiella and Acinetobacter, which can be used for bioremediation of mercury from river water. The isolated bacteria were capable of withstanding high concentration of mercury. Salinity tolerance of the isolated bacteria over a wide range confers them additional advantage for their application in bioremediation. Bacillus and Enterobacter sp. were earlier reported to be highly efficient in mercury remediation which supports their application for effective remediation of mercury from river water. Further investigation is suggested for their in situ application for the purpose of mercury remediation from the polluted environment.
References
Ajao AT, Adebayo GB, Yakubu SE (2017) Bioremediation of textile industrial effluent using mixed culture of Pseudomonas aeruginosa and Bacillus subtilis immobilized on agaragar in a bioreactor. J Microbiol Biotechnol Res 1(3):50–56
American Public Health Association (1989) Standard methods for the examination of water and wastewater, 17th edn. American Public Health Association, Washington
Andrews JM (2002) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 49(6):1049
Barkay TAMAR, Olson BH (1986) Phenotypic and genotypic adaptation of aerobic heterotrophic sediment bacterial communities to mercury stress. Appl Environ Microbiol 52(2):403–406
Baul TSB, Kundu S, Mitra S, Höpfl H, Tiekink ER, Linden A (2013) The influence of counter ion and ligand methyl substitution on the solid-state structures and photophysical properties of mercury(II) complexes with (E)-N-(pyridin-2-ylmethylidene) arylamines. Dalton Trans 42(5):1905–1920
Carocci A, Rovito N, Sinicropi MS, Genchi G (2014) Mercury toxicity and neurodegenerative effects. Rev Environ Contam Toxicol 229:1–18
Chaudhary M, Walker TR (2019) River Ganga pollution: causes and failed management plans (correspondence on Dwivedi et al. 2018. Ganga water pollution: a potential health threat to inhabitants of Ganga basin. Environment International 117, 327–338). Environ Int 126:202–206
Dash HR, Das S (2012) Bioremediation of mercury and the importance of bacterial mer genes. Int Biodeterior Biodegrad 75:207–213
Dash HR, Das S (2014) Bioremediation potential of mercury by Bacillus species isolated from marine environment and wastes of steel industry. Bioremediat J 18(3):204–212
Dash HR, Das S (2015) Bioremediation of inorganic mercury through volatilization and biosorption by transgenic Bacillus cereus BW-03 (p PW-05). Int Biodeterior Biodegrad 103:179–185
Dash HR, Das S (2016) Diversity, community structure, and bioremediation potential of mercury-resistant marine bacteria of estuarine and coastal environments of Odisha, India. Environ Sci Pollut Res 23(7):6960–6971
Dash HR, Mangwani N, Das S (2014) Characterization and potential application in mercury bioremediation of highly mercury-resistant marine bacterium Bacillus thuringiensis PW-05. Environ Sci Pollut Res 21(4):2642–2653
Dey U, Chatterjee S, Mondal NK (2016) Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol Rep 10:1–7
Díaz MP, Boyd KG, Grigson SJ, Burgess JG (2002) Biodegradation of crude oil across a wide range of salinities by an extremely halotolerant bacterial consortium MPD-M, immobilized onto polypropylene fibers. Biotechnol Bioeng 79(2):145–153
Figueiredo NL, Canário J, O’Driscoll NJ, Duarte A, Carvalho C (2016) Aerobic mercury-resistant bacteria alter mercury speciation and retention in the Tagus Estuary (Portugal). Ecotoxicol Environ Saf 124:60–67
François F, Lombard C, Guigner JM, Soreau P, Brian-Jaisson F, Martino G, Peduzzi J (2012) Isolation and characterization of environmental bacteria capable of extracellular biosorption of mercury. Appl Environ Microbiol 78(4):1097–1106
Green-Ruiz C (2006) Mercury(II) removal from aqueous solutions by nonviable Bacillus sp. from a tropical estuary. Bioresour Technol 97(15):1907–1911
Holmes P, James KAF, Levy LS (2009) Is low-level environmental mercury exposure of concern to human health? Sci Total Environ 408(2):171–182
Jadhav JP, Phugare SS, Dhanve RS, Jadhav SB (2010) Rapid biodegradation and decolorization of Direct Orange 39 (Orange TGLL) by an isolated bacterium Pseudomonas aeruginosa strain BCH. Biodegradation 21(3):453–463
Jan AT, Azam M, Choi I, Ali A, Haq QMR (2016) Analysis for the presence of determinants involved in the transport of mercury across bacterial membrane from polluted water bodies of India. Braz J Microbiol 47(1):55–62
Liu B, Bian C, Huang H, Yin Z, Shi Q, Deng X (2016) Complete genome sequence of a marine bacterium, Pseudomonas pseudoalcaligenes strain S1, with high mercury resistance and bioaccumulation capacity. Genome Announc 4(3):e00381-16
Mahbub KR, Krishnan K, Megharaj M, Naidu R (2016) Bioremediation potential of a highly mercury resistant bacterial strain Sphingobium SA2 isolated from contaminated soil. Chemosphere 144:330–337
Mohabansi NP, Tekade PV, Bawankar SV (2011) Physico-chemical and microbiological analysis of textile industry effluent of Wardha region. Water Res Dev 1:40–44
Møller AK, Barkay T, Al-Soud WA, Sørensen SJ, Skov H, Kroer N (2010) Diversity and characterization of mercury-resistant bacteria in snow, freshwater and sea-ice brine from the High Arctic. FEMS Microbiol Ecol 75(3):390–401
Nagarsekar AS, Kakde UB (2014) Studies on heavy metal contamination in Mithi river, Mumbai. Online Int Interdiscip Res J 4:202–216
Officioso A, Tortora F, Manna C (2016) Nutritional aspects of food toxicology: mercury toxicity and protective effects of olive oil hydroxytyrosol. J Nutr Food Sci 6(539):2
Osborn AM, Bruce KD, Strike P, Ritchie DA (1997) Distribution, diversity and evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiol Rev 19(4):239–262
Patel P, Raju NJ, Reddy BSR, Suresh U, Sankar DB, Reddy TVK (2018) Heavy metal contamination in river water and sediments of the Swarnamukhi River Basin, India: risk assessment and environmental implications. Environ Geochem Health 40(2):609–623
Patil VT, Patil PR (2010) Physicochemical analysis of selected groundwater samples of Amalner town in Jalgaon District, Maharashtra, India. J Chem 7(1):111–116
Pepi M, Focardi S, Tarabelli A, Volterrani M, Focardi SE (2013). Bacterial strains resistant to inorganic and organic forms of mercury isolated from polluted sediments of the Orbetello Lagoon, Italy, and their possible use in bioremediation processes. In: E3S web of conferences, 1: EDP Sciences
Pushkar BK, Sevak PI, Singh A (2015) Isolation & characterization of potential microbe for bio-remediating heavy metal from Mithi river. Ann Appl Bio-Sci 2(2):A20–A27
Pushkar B, Sevak P, Sounderajan S (2019) Assessment of the bioremediation efficacy of the mercury resistant bacterium isolated from the Mithi River. Water Sci Technol Water Supply 19(1):191–199
Rafique A, Amin A, Latif Z (2015) Screening and characterization of mercury-resistant nitrogen fixing bacteria and their use as biofertilizers and for mercury bioremediation. Pak J Zool 47(5):1271–1277
Rice KM, Walker EM, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Pub Health 47(2):74–83
Rua-Ibarz A, Bolea-Fernandez E, Maage A, Frantzen S, Valdersnes S, Vanhaecke F (2016) Assessment of Hg pollution released from a WWII Submarine Wreck (U-864) by Hg isotopic analysis of sediments and cancer pagurus tissues. Environ Sci Technol 50(19):10361–10369
Santos R, Joyeux A, Besnard A, Blanchard C, Halkett C, Bony S, Devaux A (2017) An integrative approach to assess ecological risks of surface water contamination for fish populations. Environ Pollut 220:588–596
Santos-Gandelman JF, Giambiagi-deMarval M, Muricy G, Barkay T, Laport MS (2014) Mercury and methylmercury detoxification potential by sponge-associated bacteria. Antonie Van Leeuwenhoek 106(3):585–590
Siddiqui E, Pandey J (2019) Assessment of heavy metal pollution in water and surface sediment and evaluation of ecological risks associated with sediment contamination in the Ganga River: a basin-scale study. Environ Sci Pollut Res 26(11):10926–10940
Singare PU (2015) Studies on polycyclic aromatic hydrocarbons in surface sediments of Mithi river near Mumbai, India: assessment of sources, toxicity risk and biological impact. Mar Pollut Bull 101(1):232–242
Singare PU, Mishra RM, Trivedi MP (2012a) Heavy metal pollution in Mithi river of Mumbai. Front Sci 2(3):28–36
Singare PU, Mishra RM, Trivedi MP (2012b) Sediment contamination due to toxic heavy metals in Mithi river of Mumbai. Adv Anal Chem 2(3):14–24
Singare PU, Ferns SEL, Agharia ER (2015) Studies on toxic heavy metals in sediment ecosystem of Mahim Creek near Mumbai, India. Int Lett Chem Phys Astron 43:62–70
Singh, A. K., & Giri, S. (2018) Subarnarekha River: the Gold Streak of India. In: The Indian Rivers. Springer, Singapore, pp 273–285
Sinha A, Khare SK (2012) Mercury bioremediation by mercury accumulating Enterobacter sp. cells and its alginate immobilized application. Biodegradation 23(1):25–34
Sinha A, Pant KK, Khare SK (2012) Studies on mercury bioremediation by alginate immobilized mercury tolerant Bacillus cereus cells. Int Biodeterior Biodegrad 71:1–8
Srinivas R, Singh AP, Sharma R (2017) A scenario based impact assessment of trace metals on ecosystem of river Ganges using multivariate analysis coupled with fuzzy decision-making approach. Water Resour Manag 31(13):4165–4185
Tartu S, Goutte A, Bustamante P, Angelier F, Moe B, Clément-Chastel C, Chastel O (2013) To breed or not to breed: endocrine response to mercury contamination by an Arctic seabird. Biol Lett 9(4):20130317
vanDijl J, Hecker M (2013) Bacillus subtilis: from soil bacterium to super-secreting cell factory. Microb Cell Fact 12(1):3
Vasudevan S, Oturan MA (2014) Electrochemistry: as cause and cure in water pollution—an overview. Environ Chem Lett 12(1):97–108
Waseem A, Arshad J, Iqbal F, Sajjad A, Mehmood Z, Murtaza G (2014) Pollution status of Pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. BioMed Res Int 2014:1–29
Wu J, Lin I, Hayes RB, Ahn J (2014) Comparison of DNA extraction methods for human oral microbiome research. Br J Med Med Res 4(10):1980–1991
Yoshida N, Ikeda R, Okuno T (2006) Identification and characterization of heavy metal-resistant unicellular alga isolated from soil and its potential for phytoremediation. Biores Technol 97(15):1843–1849
Yue CY, Lei XW, Liu RQ, Zhang HP, Zhai XR, Li WP, Zhou M, Zhao ZF, Ma YX, Yang YD (2014) Syntheses, crystal structures, and photocatalytic properties of a series of mercury thioantimonates directed by transition metal complexes. Cryst Growth Des 14(5):2411–2421
Zeroual Y, Moutaouakkil A, Blaghen M (2001) Volatilization of mercury by immobilized bacteria (Klebsiella pneumoniae) in different support by using fluidized bed bioreactor. Curr Microbiol 43(5):322–327
Acknowledgements
The authors are thankful to the MMR-EIS (Mumbai Metropolitan Region-Environment Improvement Society) for the financial support to the research project. The authors would also like to acknowledge Dr. Dilip Boralkar (Former Member Secretary, MPCB (Maharashtra Pollution Control Board) for his constant assistance during the research work.
Funding
The project was funded by MMR-EIS (Mumbai Metropolitan Region-Environment Improvement Society), Maharashtra, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
Not applicable as no genetic modification experiment in the study.
Informed consent
Not applicable (no involvement of human or animal for experimental purpose).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pushkar, B., Sevak, P. & Singh, A. Bioremediation treatment process through mercury-resistant bacteria isolated from Mithi river. Appl Water Sci 9, 117 (2019). https://doi.org/10.1007/s13201-019-0998-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-0998-5