Abstract
Depth filtration is a conventional concept for the solid–liquid separation process through granular media, and this technique is very useful in removal of large-sized particle. However, in the case of microparticles smaller than the pore space it does not function well. Accordingly, an effective small particle removal technique is desired. In this research, a carbon fiber felt was introduced as filter media and ± 1.0 V terminal voltage was applied for activating the media to enhance in adsorbing as well as desorbing the fine particles in the filtration and back-washing. Kaolin particles in the range of 0.1–4.0 µm were used as a model of microparticles. The complete kaolin particle removal was observed in pure water, and it decreased to around 50% in the anionic surfactant-contaminated wastewater at the hydraulic loading of 283 L/m2/min. On the contrary, at the back-washing hydraulic loading of 1274 L/m2/min, the kaolin particle washout removal efficiency was found to be around 73% in pure water and it dramatically decreased and found within the range of 5–50% when the kaolin was in contact with anionic surfactant and wastewater. Although the kaolin particle washout removal rate was observed very low in wastewater, this technique would be very useful in enhancing the refreshment of the filter media for extending the production time of the filter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last half century, depth filtration process in porous bed has become more common in wastewater treatment area due to the stringent effluent quality standards (Torkzaban et al. 2010). In this method, solid particles in suspension are generally adsorbed on or retained by the media while filtration. However, the effectiveness of particles removal depends on the surface forces acting between particles and filter grains. The latter is significant when distance between particles and filter grains is in the order of nanometers (Aiken et al. 2011).
Numerous complementary research works have been performed to clearly understand the effects of media material on removal of SS by depth filtration (Sun et al. 2015). Still in recent years, depth filtration models cannot completely account for the fundamental insights concurring with a large variety of solid loading rate for wastewater filtration (Ding et al. 2015). It is well known that tiny particles separation is a very complex process and influenced by several factors such as wastewater characteristics, types of SS, and the operation mode of filtration (Bedrikovetsky et al. 2011). When filtration proceeds over time without back-washing, effluent quality may be improved but clogging of filter bed will result in an increase in head loss and a decrease in filtration flux (Loderer et al. 2012). Clogging and head loss are influenced by various factors such as particle size distribution, particle concentration, particle surface chemistry, filter media size, and media type (Coustumer et al. 2012). Filter media with an uneven media size distribution normally have a greater chance of clogging than uniform filter media. The formation of clogging depends on the size distribution of SS, fine bedload, and channel sediments. Large fine particles diameter more than 30 µm are subjected to mechanical filtration rather than surface phenomena such as the van deer Waals force. Medium-sized particles between 3 and 30 µm are retained by both filtration mechanisms. Adhesion of colloidal particles and bacteria is exclusively due to physiochemical process (Wang et al. 2012).
In general, performance of a filter is quantified by particle removal efficiency and head loss across the packed bed. Since filtration is often thought to be a unit operation at post-treatment stage in wastewater treatment process, a secure removal of SS is required to satisfy the specific standard limit. In depth filtration mechanism, large and submicron particles, colloidal, and insoluble materials are removed while passing through a tortuous path before it is able to reach the other side (Karan et al. 2013). Figure 1 shows the flow of fluid and particles through depth filter media.
It is logical that particles larger than the pore size would be easily removed by mechanical filtration that is termed as sieving, straining, or size exclusion. In addition, adsorption and attraction mechanisms are also observed in depth filtration, where particles are adsorbed by the electrokinetic force or surface affinity. Relatively large particles impact the filter medium through inertia, and relatively small particles impact through random Brownian motion. In these processes, particles are finally adsorbed on the filter medium by the van der Waals force or the interaction in the electric double layers (EDLs) of the surfaces (Mahmoud et al. 2010). However, the removal of particles smaller than the pore size and microorganisms like total coliform (TC) and general bacteria (GB) is less intuitive. In this case, an electrokinetic effect in charge-modified depth filter media would be able to remove these submicron particles, colloidal materials, and insoluble contaminants (Loo et al. 2012). Natural particles and colloids in water usually have a negative surface charge. Therefore, if filter media have an opposite surface charge against particles in water, the particle removal performances of the system will be enhanced (Keller et al. 2010; Ghernaout and Ghernaout 2012).
Considering this notion, a laboratory-scale depth filtration system was developed using a conducting carbon fiber felt made from polyacrylonitrile fibers with 5.3 µm in diameter as filter media, in which surface charge of filter media was actively controlled with a direct current (DC) power supply. In this system, wastewater filtration was performed under a positive surface charge of the filter media and back-washing was performed under a negative surface charge. These operations would enhance the particle rejection and back-washing effect through electrostatic interactions between particles and media surface (Kishimoto et al. 2010). The specific objectives of this research were to find out an optimum condition of the active surface charge control depth filter for the removal of fouling materials of the RO membrane such as organic and inorganic particles. In addition, efficacy of this system on desorption of particles from filter media at the back-washing step was also explored.
Materials and methods
Experimental setup
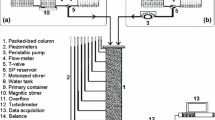
Figure 2 shows the experimental setup. The laboratory-scale experimental system was composed of a filter module, a feed pump (RP-1000, EYELA, Japan), a digital DC power supply (AD-8735D, AND, Japan), and a high-power ultrasonic cleaner (SUS-200, Shimadzu, Tokyo, Japan).
Laboratory-scale experimental setup. 1: ultrasonic cleaner, 2: influent, 3: feed water pump, 4: effluent, 5: DC power supply in filtration mode, 6: DC power supply in back-washing mode, 7: filter module, 8: acrylic tube, 9: acrylic resin cover, 10: porous stainless steel plate, 11: polytetrafluoroethylene mesh, 12: silicon rubber, 13: carbon fiber felt, and 14: bolts. a Picture of the carbon fiber felt with the magnitude of 75 times, b picture of scale with the 1000 µm/scale (magnitude of 75 times), c picture of the carbon fiber felt with the magnitude of 150 times, d picture of scale with the 1000 µm/scale (magnitude of 150 times). These pictures were taken with a digital microscope (VH-5000, Keyence, Osaka, Japan)
The filter module was constituted of a filter bed chamber made of silicon rubber with 2.5 cm height and two covers made of acrylic resin with an inlet or outlet. Five sheets of carbon fiber felt (weight per area: 250 g/m2; Kanai Juyo Kogyo, Osaka, Japan) with 3 cm in diameter and 0.5 cm in thickness were stacked in the filter module. Two porous stainless steel (ANSI304) plates with 5 mm pores were set at the upper and lower end of the filter module as a counter electrode and a conductor to the carbon fiber felt, respectively. A polytetrafluoroethylene mesh sheet with 1 mm in thickness was inserted between upper stainless steel plate sheet and carbon fiber felt as a separator. The empty volume of filter bed chamber was 17.7 cm3.
Experimental procedure and operational conditions
Before each experimental run, the filter module was washed with deionized water (DW) of electrical conductivity (EC) less than 1 µS/cm, unless and until particles were not detected in the effluent. In this study, Run 1 was conducted to observe the kaolin particle removal efficiency in DW with no electric charge to the filter bed, Run 2 was conducted to observe the effect of conductivity on kaolin particle removal performances with no electric charge, and Run 3 was conducted to observe the effect of electric charge to the filter bed on kaolin particle removal performances.
The kaolin particle removal efficiency in synthetic contaminated source water was observed in Runs 4 and 5 at pH 6.7 and 7.8, respectively. Runs 4 and 5 demonstrated the effect of synthetic contaminated source water pH on kaolin particle removal performances at around neutral pH. Run 6 was demonstrated to observe the effect of anionic surfactant on kaolin particle removal performances. The Fe and kaolin particle removal performances were observed in Runs 7 and 8 at pH 4.6 and 8.6, respectively. Runs 7 and 8 demonstrated the effect of acidic and alkaline pH on kaolin particle and Fe colloids removal performances. Moreover, the effect of anionic surfactant in Fe and kaolin particle removal performances was observed in Runs 9 and 10 at pH 3.8 and 7.9, respectively. Runs 9 and 10 were demonstrated to observe the effect of anionic surfactant on kaolin and Fe colloids removal performances at acidic and alkaline pH. Here, the objectives of doing several runs were to find out the exact parameters that influence on the kaolin particle and Fe colloids removal performances.
Tables 1 and 2 show the operational conditions and the synthetic influent characteristics of Runs 1–10, respectively. The potential of the conducting carbon felt against the counter electrode in the filter module was set to ± 1.0 V at filtration mode of Runs 3–10, though the potential was not applied at Runs 1–2 (Table 1). At the back-washing mode, the potential was reversely changed to − 1.0 V at Runs 3–10 and the potential was not applied at Runs 1–2. The terminal voltage was set ± 1.0 V, because this terminal voltage was within the potential window of water electrolysis, where electricity was used only for charging of the EDLs of filter media and the counter electrode (Loo et al. 2012). The back-washing solutions were flowed from the opposite direction of filtration mode at all experimental runs as shown in Fig. 2 to remove the clogged or retained particles in the filter media, which is defined as washout step. The filtration hydraulic loading and filtrated water volume of Runs 1–10 was 283 L/m2/min and 800 mL, respectively. On the other hand, the back-washing hydraulic loading and back-washed water volume of Runs 1–10 was 1274 L/m2/min and 1000 mL, respectively.
Table 3 shows the synthetic contaminated source water characteristics of Runs 4 and 5. Here, the influent used in Runs 4–5 was municipal wastewater of Ryukoku University diluted by five times with normal tap water. After that, it was kept in room temperature for about 2 months. As a result, organic matter was decomposed into inorganic carbon, nitrate (NO3−), and ammonium (NH4+). Consequently, NO3− and NH4+ concentrations were found to be higher than biochemical oxygen demand (BOD) and chemical oxygen demand (COD) (Table 3). The synthetic contaminated source water was filtrated using a glass fiber filter with 1-µm particle rejection (GF/B, Whatman, Japan) just before experimental runs.
In the back-washing experiments, DW was used as back-washing solution in Runs 1–2 and a sodium chloride (NaCl) solution with NaCl concentration of 5 mM (292.2 mg/L) (EC = 580 µS/cm) was used in Runs 3–10. In the preparation of the influent of Runs 1–10 a high-power ultrasonic cleaner was operated for 30 min to promote the dispersion of fine particles. The ultrasonic cleaner was continuously operated during the filtration operation too. In this experimental study, kaolin powder of 0.1–4.0 µm in diameter (Nacalai, Tesque, Kyoto, Japan), NaCl, sodium lauryl sulfate (CH3(CH2)11OSO3Na), and ferrous sulfate heptahydrate (FeSO4·7H2O) were used for the source of microparticles, electron conductor, anionic surfactant, and ferrous ion, respectively. Moreover, 50 mM (200 mg/L) sodium hydroxide (NaOH) was used as alkaline solution to increase the pH in Runs 5, 8, and 10, respectively.
Analytical methods
The pH, dissolved oxygen (DO), and EC were measured with a pH meter (B-212, Horiba, Japan), a DO meter (LDO, HQ10, Hach, USA), and an EC meter (Twin Cond B-173, Horiba, Japan), respectively. A pH controller (FD-02, Series-B, Japan) was used to adjust the pH. Turbidity and color were measured with a digital turbidity/color meter (Aqua Doctor, WA-PT-4DG, Kyoritsu Chemical-Check Lab, Japan). Concentrations of nitrite (NO2−), NO3−, and NH4+ were determined by colorimetric method (4500-NO2− B) (Rice et al. 2012), cadmium reduction method (4500-NO3− E) (Rice et al. 2012), and phenate method (4500-NH3 F) (Rice et al. 2012), respectively. Ferrous ion (Fe2+) was measured by phenanthroline method (3500-Fe B) (Rice et al. 2012). The BOD was measured by 5-day BOD test (5210 B) (Rice et al. 2012), and COD was measured by closed reflux colorimetric method (5220 D) (Rice et al. 2012). Particle size distributions of kaolin suspension and Fe colloids in influent, effluent, and back-washed water were measured with a laser diffraction particle size analyzer (SALD-300V, Shimadzu, Kyoto, Japan). Zeta potential was measured with a zeta potential meter (ZEECOM ZC-2000, Microtec, Funabashi, Japan). Total coliform (TC) and general bacteria (GB) were measured by test papers for TC and GB (Sibata, Saitama, Japan) using colony count, most-probable-number (MPN) method with thermostat incubator (CALBOX, CB-101, Sibata, Saitama, Japan). Filtrate was collected as effluent, and the mass of kaolin in the filtrate was measured as SS using glass fiber filter with 0.7-µm particle rejection (GF/F, Whatman, Japan). DW with the EC less than 1 μS/cm was used for the dilution and preparation of standard solution, as obtained from a water purification system (Autostill, WA5000, Yamato, Japan).
Results and discussion
Zeta potential of particles
Figure 3 shows the zeta potentials of particles at Runs 1–10 depending on pH and artificially spiked ingredients, namely kaolin particles, NaCl, sodium lauryl sulfate, and FeSO4·7H2O in the influent (Table 2). Here, the zeta potential of kaolin particles was found to be − 34.1 mV in DW at pH 7.4 (Run 1). But, when NaCl was added to the suspension (Runs 2 and 3), pH decreased to 5.9 and zeta potential increased to − 21.2 mV, which would be caused by protonation with the decrease in pH (Leroy et al. 2011; Kishimoto et al. 2010; Aksoy and Kaya 2011).
However, the zeta potential of kaolin suspension in synthetic contaminated source water in Run 4 and 5 was found to be − 30.5 and − 26.5 mV at pH 6.7 and 7.8, respectively. In this case, pH was increased by the addition of NaOH in Run 5. Therefore, the zeta potential of kaolin particle in synthetic contaminated source water showed reverse behavior with pH due to the effect of positively charged sodium ion (Na+) in the kaolin suspension, which accords with the experimental results of Moayedi et al. (2011).
The zeta potential of Run 6 was found to be − 47.4 mV, which was lower than that of other runs, where kaolin particle and sodium lauryl sulfate were diluted with DW at pH 6.6. Since sodium lauryl sulfate is a negatively charged anionic surfactant, the adsorption of sodium lauryl sulfate onto the kaolin particle may take part in decreasing the zeta potential regardless of pH in the solution.
In the case of Runs 7 and 8, the zeta potentials of kaolin suspension in DW with Fe ions were found to be − 5.4 and − 37.4 mV at pH 4.6 and 8.6, respectively. Since the zeta potential of ferric hydroxide ((Fe(OH)3) is reported to be positive at pH less than around 6 (Li et al. 2010). The zeta potential in Run 7 was found to be higher than that of all runs, though the value was still negative. On the contrary, when the pH of Run 7 was increased to 8.6 (Run 8), the zeta potential decreased to − 37.4 mV due to deprotonation effect (Aksoy and Kaya 2011).
The zeta potentials of Runs 9 and 10 were found to be − 42.3 and − 30.5 mV at pH 3.8 and 7.9, respectively. Here, kaolin particle, sodium lauryl sulfate, and FeSO4·7H2O were diluted in DW. The zeta potential in Run 9 was found to be lower than in Run 10. In the case of Run 9, negatively charged head of sodium lauryl sulfate (SLS) was associated with positively charged Fe colloids in Run 9. As a result, Fe colloids covered by SLS that hydrophobic tails are exposed to the outer side namely, bulk water. Then, other SLSs were associated with SLSs adsorbed on the Fe colloids by hydrophobic interaction. Consequently, the surface of the Fe colloid was covered by negatively charged heads of SLSs, which result in the low zeta potential. In Run 10, negatively charged surface of Fe colloids prevents the adsorption of SLS onto the Fe colloids. Accordingly, the zeta potential observed in Run 10 was higher than that in Run 9.
At a glance, the zeta potential characteristics of kaolin particles in pure water, synthetic contaminated source water, anionic surfactant, and Fe-contaminated water are summarized here. The zeta potential of kaolin particles and kaolin particles with Fe(OH)3 in pure water was found to increase with the decrease in pH probably due to the protonation effect (Runs 1, 2, 3, 7, and 8). However, the increment in zeta potential with the increment in pH was observed in Runs 4 and 5, where synthetic contaminated source water was added. Adsorption of positive ions/or organic ions in synthetic contaminated source water onto the kaolin surface might be responsible for such a phenomenon. However, extensive researches will be required for elucidating the mechanism in the future.
Effect of conductivity and electric charge on particle separation
Figure 4a–f shows the volume-based kaolin suspension particle size distribution of influent and effluent of Runs 1–3. Here, the conductivity in influent of Runs 1–3 was 3, 560, and 550 µS/cm, respectively (Table 2) and a terminal voltage of + 1.0 V was applied for Run 3 alone (Table 1). In addition, it is notable that the diameter of kaolin particles used in the experiments was in the range of 0.1–4.0 µm. However, the kaolin particle diameter increased to the range of 0.139–8.200 µm in the influent of Run 1 (Fig. 4a), 0.139–68.431 µm in the influent of Run 2 (Fig. 4c), and 0.139–58.126 µm in the influent of Run 3 (Fig. 4e), because of self-flocculation.
Figure 4b, d shows the particle size distribution of effluent in Runs 1 and 2. From these graphs, it is observed that the particles larger than 6.965 µm were decreased by filtration without impressed voltage. This result indicates that the particles larger than 6.965 µm were removed by the sieving mechanism and the adsorption mechanism by van der Waals forces.
Figure 4f shows the particles were completely removed by the depth filtration with the impressed voltage at + 1.0 V. Accordingly, it was inferred that smaller particles less than 6.965 µm were mainly removed by electrokinetic adsorption mechanism. On the other hand, smaller particles within the range of 0.100–0.266 µm in the effluent of Run 1 (Fig. 4b) and 0.100–0.434 µm in the effluent of Run 2 (Fig. 4d) were found to be decreased without electric charge and thought to be due to the effect of self-aggregation of small particles during the filtration.
Figure 5 shows the SS, turbidity, and color removal efficiency of Runs 1–3. The influent of Run 1 with the EC of 3 µS/cm was filtrated without application of terminal voltage, and SS removal efficiency was found to be 92.2%. But, when the influent of Run 2 with the EC of 560 µS/cm was filtrated without application of terminal voltage like Run 1, SS removal efficiency was found to be a little bit more than Run 1, and it was found to be 97.3%. Due to addition of NaCl in Run 2, the ionic strength of the solution was intensified and the thickness of the electrical double layer was decreased, which helped to enhance the particles contact with the filter media and affected in particle removal efficiency (Rica et al. 2012). Moreover, the application of terminal voltage (+ 1.0 V) in Run 3 showed 100% removal efficiency of all parameters (Fig. 5) probably due to the electrostatic attraction between the particles and the filter media (Lee et al. 2012). The overall rejection of particles in a filtration system depends on several factors such as the kaolin particle size distribution and filter media, namely filter media particle size, filter media material, filter bed length, etc. Here, the particle removal efficiency widely changed and is thought to be due to the influence of the filtration system used.
From the above discussions it can be concluded that the kaolin particles in size more than 6.965 µm in diameter were physically clogged by the pore space of the filter module and physically adsorbed on the filter media by van der Waals forces, but the kaolin particles less than 6.965 µm were mainly trapped by the electrostatic adsorption onto the filter media. Considering the 100% removal efficiency of all indices in Run 3, a terminal voltage of + 1.0 V was adopted at the following experiments (Runs 4–10) using synthetic contaminated source water, anionic surfactant, and Fe-contained water.
Kaolin particle and iron removal performances in synthetic contaminated source water and anionic surfactant-contained water through filtration
The SS, turbidity, color, and Fe removal performances were observed on Runs 4–10 at the terminal voltage of + 1.0 V with the filtration hydraulic loading of 283 L/m2/min (Table 1). Since Fe2+ was spiked in the influent of Runs 7–10 at the concentration of 10 mg/L, SS in the influent was found to be a little bit more than that of Runs 1–6 (Table 2). Figure 6a–n shows the volume-based particle size distribution of influent and effluent of Runs 4–10. The particle size distribution of influent in Runs 4, 5, and 6 (Fig. 6a, c, e) shows the similar pattern to that in influent of Run 1 (Fig. 4a), though the influent in Runs 4, 5, and 6 had the similar EC of 480, 670, and 770 µS/cm to that in Run 2 (EC = 560 µS/cm) and 3 (EC = 550 µS/cm). Figure 7 shows the SS, turbidity, color, and Fe removal efficiency of Runs 4–10. In the case of Runs 4–6, SS removal efficiency was found to be lower than that of other runs, where synthetic contaminated source water (Table 3) was used for the dilution of kaolin particles in Runs 4 and 5 and kaolin particles with sodium lauryl sulfate was diluted with DW in Run 6.
a–n Volume-based kaolin suspension particle size distribution of influent and effluent of Runs 4–10, where synthetic contaminated source water (Table 2) was used as a solvent in Runs 4 and 5. Sodium lauryl sulfate at the concentration of 100 mg/L was used in Runs 6, 9, and 10, and Fe was spiked at the concentration of 10 mg/L in Runs 7–10
The SS removal efficiency was 22.2% in Run 4 at pH 6.7, 45.7% in Run 5 at pH 7.8, and 51.1% in Run 6 at pH 6.6, respectively, though 100% removal efficiency was observed at Run 3. In Run 6, SS removal efficiency was found to be decreased due to the addition of sodium lauryl sulfate probably due to the adsorption of negatively charged sodium lauryl sulfate onto the filter media, which changed the surface charge of filter media from positive to negative. Besides, SS removal efficiency in Runs 4 and 5 was found to be much lower than in Run 6. Here, it is assumed that anionic surfactant-like compounds were contained in the wastewater, affected seriously in charge-based particle removal mechanism of the depth filtration system (Becerra et al. 2010; Gehrke et al. 2015).
Figure 6g, i shows the particle size distribution of Runs 7 and 8 in the influent of kaolin suspension with Fe. The particles in the influent were found to be in the range of 0.118–9.653 µm in Run 7 and 4.268–41.938 µm in Run 8. Although Fe was spiked at the same concentration of 10 mg/L in Runs 7 and 8, the particle size distribution showed the different pattern. This was happened due to the difference in the oxidizing behavior of Fe at acidic and alkaline pH. In the case of Run 8, Fe was completely oxidized at pH 8.6 as given in Eqs. 1 and 2 and the Fe(OH)3 flocs were accumulated in the solution, which formed larger particles in comparison with original kaolin particles.
As a result, SS removal efficiency in Run 8 was nearly equal to 100% (Fig. 7). The Fe removal efficiency of 96.4% in Run 8 supported the above discussion. The less amount of Fe removal efficiency in Run 7 was thought to be due to the partial oxidation of Fe at acidic pH of 4.6, which is supported by the low Fe removal efficiency of 29.9% as shown in Fig. 7. The un-oxidized Fe easily passed through the filter module, because both the filter module and the Fe were positively charged.
Figure 6k, m shows the particle size distribution of Runs 9 and 10 in the influent of kaolin suspension with sodium lauryl sulfate and Fe. Here, the particles were found within the range of 0.118–5.025 µm in Run 9 and 0.983–214.498 µm in Run 10. The influent particle size distribution of Runs 9 and 10 shows the similar pattern to that of Runs 7 and 8. Moreover, the influent particle size distribution of Runs 6 and 9 was found in the same range like Run 1 (Fig. 4a), though sodium lauryl sulfate was added in Runs 6 and 9. This result suggests that sodium lauryl sulfate did not take part in particle aggregation. The SS removal efficiency in Run 9 was not so high (72.2%) due to the presence of anionic surfactant as aforementioned in Run 6, and the removal efficiency of Fe was also low (47.5%) due to the acidic pH as was mentioned in Run 7.
On the contrary to Run 9, the SS removal efficiency and the Fe removal efficiency in Run 10 were 100% and 98.1%, respectively. Although sodium lauryl sulfate was present with the Fe colloids in the influent of Run 10, unlike Run 6 sodium lauryl sulfate could not influence on SS and Fe removal performances in Run 10 due to the stronger aggregation of kaolin suspension with Fe colloids at pH 7.9. Similar results were observed by Gupta et al. (2011).
From the above experimental results, it could be summarize that kaolin particle removal performances were significantly affected by the contaminated source water (Runs 4 and 5) as well as anionic surfactant-contained water (Run 6) regardless of pH. The complete kaolin particle removal was observed in the pure water (Run 3) and pure water with Fe at alkaline pH (Run 10). Similarly, Fe removal performances were found to be affected in acidic pH (Runs 7 and 9) due to the partial oxidation, and 96.4% Fe removal was observed in Run 8 and 98.1% was observed in Run 10 at slightly alkaline pH.
Evaluation of total coliform (TC) and general bacteria (GB) removal performances through filtration
Figure 8 shows the total coliform (TC) and general bacteria (GB) removal performances. This experiment was conducted to observe the TC and GB removal performances through filtration at the hydraulic loading of 283 L/m2/min with the terminal voltage of + 1.0 V. To conduct this experiment, municipal wastewater of Ryukoku University was diluted by five times with normal tap water. Then, it was used as influent for the source of TC and GB. Here, the TC and GB concentration in the influent was found to be 88,800 CFU/mL and 116,000 CFU/mL, respectively. The filtrated water volume was 2000 mL. In this experimental study, the TC and GB removal efficiency through filtration was found to be 23.7% and 30.3%, respectively. Similar removal efficiencies were observed by Michen et al. (2012) and Jenkins et al. (2011).
Effect of back-washing on Kaolin particle and iron washout from filter media
A series of back-washing experiments were demonstrated on Runs 1–10 at the back-washing hydraulic loading of 1274 L/m2/min, using a back-washing solution of DW for Runs 1–2 and 5 mM NaCl solution (EC = 580 µS/cm) for Runs 3–10. At the back-washing mode, − 1.0 V potential was applied at Runs 3–10 and the potential was not applied at Runs 1–2 (Fig. 2). Figure 9a–j shows the volume-based particle size distribution in back-washed water of Runs 1–10. As a whole, the particles diameter of the back-washed water in Runs 1–10 was found to be in the range of 0.139–68.431 µm. Here, part of particles in back-washed water of all the runs was found to be aggregated. The particle aggregation in back-washed water may occur through aeroflocs mechanism due to air intrusion while shifting the operation mode from filtration to the back-washing (Oliveira et al. 2014).
Figure 10 shows the SS, turbidity, color, and Fe washout removal performances of Runs 1–10. Here, the washout removal efficiency is defined as the ratio of the amount of particles washed away by the back-washing to the amount of particles removed at the filtration step. The SS washout removal efficiency in Run 3 was higher (72.5%) than that in Run 1 (60.0%) and slightly higher than that in Run 2 (68.2%).
The higher washout removal efficiency in Run 3 was achieved for the application of terminal voltage of − 1.0 V, which generated a repulsive fore against the kaolin particles and facilitated in washout removal process. Similar result was observed by Min et al. (2013) which supports this discussion.
Among Runs 1–10, SS washout removal efficiency in Runs 6, 9, and 10 remained low, namely 7.8%, 4.6%, and 12.5%, respectively (Fig. 10). Since sodium lauryl sulfate was added into the influent of Runs 6, 9, and 10, it is inferred to inhibit the repulsive force mechanism through the linkage between the filter medium and particles by the hydrophobic tails in the surfactant.
In the case of Runs 4 and 5, sodium lauryl sulfate was not added in the influent, but kaolin particle was diluted in the wastewater given in Table 3. Here, the SS washout removal efficiency was found to be relatively low, namely 59.5% in Run 4 and 55.0% in Run 5. In these cases, such a low washout removal efficiency is predicted due to the presence of anionic surfactant-like compounds in the wastewater, which affected in repulsion mechanism.
The SS washout removal efficiency in Runs 7 (45.9%) and 8 (50.7%) was found to be lower than that in Run 3 (72.5%), but to be higher than that in Runs 6, 9, and 10. In Runs 7 and 8, Fe was spiked into the influent without addition of sodium lauryl sulfate. As a result, SS washout removal was not so much hindered by Fe like Runs 6, 9, and 10. However, SS washout removal efficiency in Run 7 was found to be a little bit less than in Run 8. In this case, the un-oxidized Fe2+ was positively charged at pH 4.6. As a result, the application of negative potential in the back-washing step created an attracting force between the Fe2+ and the filter media instead of repulsive force, which hindered in SS washout removal efficiency in Run 7.
The Fe washout removal efficiency was measured in Runs 7–10. The Fe washout removal efficiency in Run 8 (59.7%) was to some extent higher than that in Run 7 (49.6%). Fe was completely oxidized at alkaline pH and formed hydroxide flocs in Run 8. As a result, a thin soft cake layer of Fe(OH)3 would be formed on the surface of filter media, which were washed away easily at the high back-washing hydraulic loading of 1274 L/m2/min from the surface of filter media during the back-washing stage. But, in the case of Runs 9 (6.4%) and 10 (15.1%) the Fe washout removal efficiencies were found to be comparatively very low with respect to Run 8 (59.7%). Since sodium lauryl sulfate was present in the influent of Runs 9 and 10, which influenced Fe washout removal performances in the back-washing step. This can be explained in such a way that the positively charged hydrophobic tails of sodium lauryl sulfate covered the oxidized or un-oxidized Fe and the application of negative potential in the back-washing step created an attracting force that captured the Fe and hindered in washout removal performances.
From the above discussions, it can be summarized that simple back-washing has the potentiality for the washout of physically clogged particles if the particles are deposited over the surface of the filter media like a cake layer. The washout removal efficiency by back-washing of the particles can be enhanced if a repulsive force is created between the particles and the filter media. On the other hand, the washout removal efficiency can be decreased dramatically if the particles are in contact with the anionic surfactant or anionic surfactant-like compounds, because these types of surfactants inhibit in repulsive force mechanism between the particles and the filter media.
Conclusion
In this study, an effect of active control potential on filter medium was demonstrated at the terminal voltage of + 1.0 V at the filtration operation and of − 1.0 V at the back-washing operation using kaolin particle suspensions. An electric current was not observed, when the terminal voltage was impressed, because the applied terminal voltage of ± 1.0 V was within the potential window of water electrolysis. Accordingly, the electricity was used only for charging the electrical double layers of filter media and counter electrode. This symptom denotes the zero energy consumption technique, and this operation could be highly effective in enhancing charge-based particle separation mechanism in depth filtration.
The zeta potential of kaolin suspension in pure water and synthetic contaminated source water with or without an anionic surfactant showed the negative values, but it varied in a wide range depending on the pH of the solution and iron contamination. The kaolin particle removal efficiency in filtration was observed 100% at the terminal voltage of + 1.0 V in the kaolin suspension in 5 mM sodium chloride solution at the hydraulic loading of 283 L/m2/min. However, the kaolin particle removal efficiency decreased to 22.2–45.7% in synthetic contaminated source water and 51.1% in anionic surfactant-contained water. On the contrary, the kaolin particle washout removal efficiency was observed 72.5% at the terminal voltage of − 1.0 V at the back-washing hydraulic loading of 1274 L/m2/min. But, the kaolin particle washout removal efficiency was decreased to 55.0–59.5% in wastewater and 4.6–12.5% in anionic surfactant-contaminated water. The iron colloids suspension washout removal performances were observed to be 59.7% in anionic surfactant-free water, but 6.4% in anionic surfactant-contained water.
Moreover, total coliform and general bacteria removal efficiency was found to be 23.7% and 30.3%, respectively. Therefore, the application of electric charge technique in the depth filtration would be effective in removing colloidal size particles like inorganic and organic particles in the sewage-contaminated water, though the removal of total coliform and general bacteria was not expected very much. From this experimental study, I recommend 0.283 m3/m2/min hydraulic loading for the removal of organic aggregates and iron colloids with the terminal voltage of + 1.0 V. For the washout of organic aggregates and iron colloids from the filter media, I recommend the hydraulic loading of 1.27 m3/m2/min with 5 mM sodium chloride-contained pure water with the terminal voltage of − 1.0 V.
References
Aiken GR, Hsu-Kim H, Ryan JN (2011) Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ Sci Technol 45(8):3196–3201
Aksoy YY, Kaya A (2011) A study of factors affecting on the zeta potential of kaolinite and quartz powder. Environ Earth Sci 62(4):697–705
Becerra FY, Acosta EJ, Allen DG (2010) Alkaline extraction of wastewater activated sludge biosolids. Bioresour Technol 101(18):6972–6980
Bedrikovetsky P, Siqueira FD, Furtado CA, Souza ALS (2011) Modified particle detachment model for colloidal transport in porous media. Transp Porous Media 86(2):353–383
Coustumer SL, Fletcher TD, Deletic A, Barraud S, Poelsma P (2012) The influence of design parameters on clogging of stormwater biofilters: a large-scale column study. Water Res 46(20):6743–6752
Ding B, Li C, Dong X (2015) Percolation-based model for straining-dominant deep bed filtration. Separ Purif Technol 147:82–89
Gehrke I, Geiser A, Somborn-Schulz A (2015) Innovations in nanotechnology for water treatment. Nanotechnol Sci Appl 8:1–17
Ghernaout D, Ghernaout B (2012) Sweep flocculation as a second form of charge neutralization-a review. Desalin Water Treat 44(1–3):15–28
Gupta V, Hampton MA, Stokes JR, Nguyen AV, Miller JD (2011) Particle interactions in kaolinite suspensions and corresponding aggregate structures. J Colloid Interface Sci 359(1):95–103
Jenkins MW, Tiwari SK, Darby J (2011) Bacterial, viral and turbidity removal by intermittent slow sand filtration for household use in developing countries: experimental investigation and modeling. Water Res 45(18):6227–6239
Karan S, Wang Q, Samitsu S, Fujii Y, Ichinose I (2013) Ultrathin free-standing membranes from metal hydroxide nanostrands. J Membr Sci 448:270–291
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44(6):1962–1967
Kishimoto N, Kawasaki H, Sasaki T, Sasaki S (2010) Effect of active control of electric potential of filter medium on depth filtration. Water Sci Technol 62(5):1022–1027
Lee S, Laldawngliana C, Tiwari D (2012) Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated waste waters. Chem Eng J 195–196:103–111
Leroy P, Tournassat C, Bizi M (2011) Influence of surface conductivity on the apparent zeta potential of TiO2 nanoparticles. J Colloid Interface Sci 356(2):442–453
Li Z, Deng S, Yu G, Huang J, Lim VC (2010) As(V) and As(III) removal from water by a Ce–Ti oxide adsorbent: behavior and mechanism. Chem Eng J 161(1–2):106–113
Loderer C, Wörle A, Fuchs W (2012) Influence of different mesh filter module configurations on effluent quality and long-term filtration performance. Environ Sci Technol 46(7):3144–3850
Loo S, Fane AG, Krantz FW, Lim T (2012) Emergency water supply: a review of potential technologies and selection criteria. Water Res 46(10):3125–3151
Mahmoud A, Olivier J, Vaxelaire J, Hoadley AFA (2010) Electrical field: a historical review of its application and contributions in wastewater sludge dewatering. Water Res 44(8):2381–2407
Michen B, Meder F, Rust A, Fritsch J, Aneziris C, Graule T (2012) Virus removal in ceramic depth filters based on diatomaceous earth. Water Sci Technol 46(2):1170–1177
Min F, Zhao Q, Liu L (2013) Experimental study on electrokinetic of kaolinite particles in aqueous suspensions. Physicochem Probl Miner Process 49(2):659–672
Moayedi H, Huat BBK, Kazemian S, Daneshmand S, Moazami D, Niroumand H (2011) Electrophoresis of suspended kaolinite in multivalent electrolyte solution. Int J Electrochem Sci 6:6514–6524
Oliveira C, Rodrigues RT, Rubio J (2014) Operating parameters affecting the formation of kaolin aerated flocs in water and wastewater treatment. Clean-Soil Air Water 42(7):909–916
Rica RA, Brogioli D, Ziano R, Salerno D, Mantegazza F (2012) Ions transport and adsorption mechanisms in porous electrodes during capacitive-mixing double layer expansion (CDLE). J Phys Chem 116(32):16934–16938
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC
Sun Y, Gao B, Bradford SA, Wu L, Chen H, Shi X, Wu J (2015) Transport, retention, and size perturbation of graphene oxide in saturated porous media: effects of input concentration and grain size. Water Res 64:24–33
Torkzaban S, Kim HN, Jiri Simunek J, Bradford SA (2010) Hysteresis of colloid retention and release in saturated porous media during transients in solution chemistry. Environ Sci Technol 44(5):1062–1669
Wang Y, Kim J, Baek J, Miller GW, Pennell KD (2012) Transport behavior of functionalized multi-wall carbon nanotubes in water-saturated quartz sand as a function of tube length. Water Res 46(14):4521–4531
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ashadullah, A.K.M., Kishimoto, N. Effectiveness of active control of surface charge of filter media on separation of microparticles from contaminated wastewater. Appl Water Sci 8, 231 (2018). https://doi.org/10.1007/s13201-018-0878-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0878-4