Abstract
Nuisance algal growths pose a significant threat to irrigation canals for water conveyance and flood control. Copper algaecides are routinely used to control algae in moving water. In this research, we monitored an application of a new use pattern of a chelated copper algaecide to a flowing canal and evaluated efficacy using controlled bench-scale study of the field-realized exposure using an alga commonly found in flowing water (Oedogonium sp.). The highest peak copper concentration (3.17 ppm) was measured 0.80 km from the application site though it decreased significantly at each sampling interval to the 9.66 km sampling point and remained at or below 0.2 ppm at 9.66–19.31 km from the application site. Copper mass dissipated with an exponential curve (R2 = 0.9558), and less than 10% of applied copper was recovered at or beyond 9.66 km from application. Peak concentration dissipated by exponential decay (R2 = 0.9922) and was predicted to achieve background concentrations by 20.9 km from application. Scaled laboratory experiments showed control at exposure achieved through 6.44 km from application. This research demonstrates a method to improve operational efficiency of copper use in flowing water to achieve desired algal control, while decreasing overall copper use and complying with applicable discharge levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water conveyance through canal systems is critical for delivery to irrigated crops as well as flood control (Getsinger et al. 2014). There are over 62,000 miles of irrigation canals throughout the 17 conterminous Western USA (Sytsma and Parker 1999) that are the primary water source to support over 40 million acres of irrigated crops (Maupin et al. 2014). Nuisance algal growths commonly impede water flow through canal systems as well as clog discharge devices, gate valves, and irrigation intakes (Getsinger et al. 2014). Copper algaecides are one of the few USEPA registered chemistries that have been widely used in canals for decades to control algae and pose no irrigation restrictions (USEPA 2009). Recent label language on a 28.2% active copper ethanolamine complex algaecide (SePRO 2014) has specific allowable use instructions for a pulse (i.e., slug) copper application method to flowing canals. This allows for ease of application (decreased application time and equipment needs) compared with continuous drip or injection applications as well as potential increased effectiveness. Limited data exist on copper exposures achieved in moving water following pulse applications. By modeling copper concentrations down canal systems, improved predictions of distance of target algal control can be made based on the exposure, and subsequently guide application sites along a canal system to achieve desired control. Additionally, modeling the dissipation of maximum copper concentrations or peak concentration can inform water resource managers of predicted copper discharge levels at downstream sites in order to comply with regulatory standards (if applicable) to receiving waters (example: WADOE 2016).
Enhancing efficiency of algal control in canal systems is critical to support functionality of the canal and degree of management effort required. Significant amounts of copper sulfate are applied to canal systems (USEPA 2009) and may have undesirable results such as shifting to copper sulfate less-susceptible algal types (Garcia-Villada et al. 2004; Izaguirre 1992; Hanson and Stefan 1984). Chelated copper has been shown to decrease the amount of copper needed to achieve the critical burden (threshold copper amount for control of algae) compared with copper sulfate (Bishop and Rodgers 2012) and has less interaction with water chemistry that may ameliorate copper toxicity to target algae (Chakoumakos et al. 1979). Chelated copper is predicted to have an increased travel distance down a canal system and longer zone of effective algal control. Adsorption of copper to sediments and algae are critical factors that may decrease travel distance of copper. Additionally, decreases in maximum exposure concentrations have been documented as copper moves through a canal system (Clearwater et al. 2011). Dispersion processes in moving water tend to create extended copper exposures at lower peak concentrations. To account for these dynamic exposures encountered by algae at different downstream points, we used a factor exposure assessment where the copper concentration is multiplied by the exposure duration (Bishop et al. 2017). These exposure data coupled with efficacy testing according to the mass of algae present (critical burden concept; Bishop and Rodgers 2012; Bishop et al. 2015) can provide a framework to predict algaecidal efficacy in a range of canal systems based on site-specific parameters (e.g., algal biomass, type of algae, flow). This research will increase efficiency of copper use through a better understanding of exposure characteristics (e.g., formulation, duration, concentration) toward minimizing environmental load of copper while achieving management objectives.
Data on the exposure (concentration and duration) and efficacy of copper in a chelated complex applied as a slug or pulse to a canal will provide valuable information to design effective and efficient algal management programs. The overall goal of this research is to evaluate copper dissipation and exposure factor in a flowing canal in order to achieve desired algal control (degree and distance) through ease of application while complying with potential discharge regulations. Specific objectives were to: (1) measure Cu concentrations downstream of a canal system following pulse addition of a 28.2% mixed ethanolamine chelated copper algaecide; (2) model the dissipation of peak Cu concentrations in a canal based on distance from application site; (3) model the dissipation of total Cu mass following algaecide addition; and (4) measure the exposure factor achieved at a series of distances down a canal system and relation to algaecidal effectiveness.
Methods
Site characteristics and copper sampling
This research used a canal operated by the Kennewick Irrigation District and specifically used a 19.31 km section of a canal starting at 46° 06′ 23.95″ N 119° 00′09.58″ W. Ten minutes before treatment, rhodamine dye was applied in the same location as 28.2% active copper ethanolamine complex algaecide was applied. The dye’s purpose was to act as a visual indicator of algaecide location in the canal to assist in initiating copper monitoring. Rhodamine was selected as the dye because of its tendency to remain in the water column and flow at a velocity equal to or greater than the copper algaecide down the canal. The flow velocity and cross-sectional area were measured immediately before application. The algaecide used and labeled for slug application was Captain® XTR (SePRO 2014). Captain XTR was applied at 3.34 L/m3 (1 quart/cfs) or a total of 33.1 L (3615 g Cu). The algaecide was poured into the center of the canal, at a weir to promote mixing, over a 15-minute timeframe. Free copper was measured in the field using Hach Method 8506 (comparable to Standard Method 3500-Cu C; SMEWW 2005). Samples were collected from seven stations approximately every three minutes with the goal to capture the pulse of copper traveling through the canal from background to peak and back to background at each sampling point. Sampling at each point was initiated at the time dye was observed. Sampling points were located at 0.80, 3.22, 6.44, 9.66, 12.87, 16.09, and 19.31 km (0.5, 2, 4, 6, 8, 10, and 12 miles) from the application site. Algal density was measured immediately before treatment by dragging a fine mesh net along the bottom of the canal six times at each sampling point and converted to mass per area using the net swath width (0.41 m) and the diameter of the canal (4.57 m). Free floating fresh weight filamentous algal density was measured by suspending a fine mesh net for one minute in the middle of the canal at each sampling location and lightly compressing to remove excess water.
Peak and mass dissipation modeling
The highest measured copper concentration at each sampling point was defined as the peak for modeling copper dissipation in the canal. The peak copper concentration was modeled by nonlinear regression using exponential decay single three-parameter (y = y0 + a*exp(− b*x)) equation discerned with SigmaPlot version 12.5 (Systat Software, San Jose, CA). The copper mass that passed by each sampling site was calculated using left- and right-hand limits with measured copper concentrations starting and ending on the sampling event that was immediately before and after detection above background, respectively. The background copper concentration was defined as the concentration before there was a detectable increase attributed to the algaecide application. The initial applied mass of copper, 3615 g or 7.97 lbs, along with the calculated mass of copper that passed by each sampling point downstream the canal was modeled by nonlinear regression as outlined above.
Algaecidal efficacy evaluations
Constant inflow of algae from the Columbia River that feeds the canal as well as sporadic Zannichellia palustris (horned pondweed) present in sections of the canal interfered with in situ efficacy evaluation of the slug treatment based on chlorophyll a measurements and general biomass observations. To understand slug treatment efficacy in the canal, controlled scaled laboratory experiments were used to assess algaecidal effectiveness throughout a canal system. Oedogonium sp. was used in testing as it commonly grows attached in flowing waters. The exposure achieved at different locations in the canal was scaled for bench testing in the laboratory. The specific exposures were based on the calculated mass of copper passing by each canal sampling point as relative to the average mass of algae in the canal system. The volume of water relative to algal biomass measured in the canal was comparably scaled in the laboratory as well. A weighted average copper concentration above background relative to time was divided by the total volume of water that passed by the site during the time copper concentration was above background to derive the copper mass. The copper mass-to-algae ratio was calculated for each section of the canal for the bench experiment (Table 1). The average algal biomass per unit area for the entire length of canal divided by the canal volume was used to define the ratio of algae to water, 0.012 g algae/L. To account for exposure time, the duration that the copper concentration was above background was used to mimic the contact time at each sampling point. The algal mass was moved to clean water of similar background chemistry to represent the return to background levels in a flowing scenario. After 4 days, chlorophyll a was measured and analyzed by ANOVA with a Dunnett’s post hoc test (α = 0.05). Chlorophyll a analysis was modified from standard methods by freezing the sample (− 12 °C) for a minimum of 24 h, subsequently amending the sample with 5 mL buffered acetone and sonicated to lyse cells (modified from SMEWW 2005).
Results and discussion
Copper dissipation
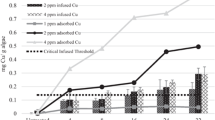
On the day of treatment, the canal had a flow of 1 m3/s (35 cfs) and a velocity of 1.65 kph (1 mph). The highest peak copper concentration (3.17 ppm) was measured 0.80 km from the application point, although decreased at each sampling interval and was 0.2 ppm at the 9.66 km sampling point. Peak concentrations remained below 0.2 ppm for the rest of the analyses and were 0.12 ppm at (19.31 km) from the application site (Figs. 1, 2). The peak copper concentration was subject to exponential decay of mg Cu/L = 0.021 + 3.83e(−0.239(km)) (R2 = 0.9922, n = 8). In other work, Clearwater et al. (2011) found use of Gemex™, a chelated copper algaecide, applied to Princhester Creek in New Zealand, for Didymosphenia geminata control also had an exponential peak copper concentration decay of mg Cu/L = 20.861e(−0.851(km)) at 3.5 km past the application site (R2 = 0.975, n = 5). With increased regulatory scrutiny in many states (WADOE 2016), there is a desire to be more efficient with copper use and decrease overall environmental load. Copper may have regulated concentrations at points of compliance, where treated water from constructed canal systems enters natural surface water (WADOE 2016). Complying with regulatory standards is important to protect aquatic life, or elicit negligible risks to receiving waters. This research can assist water resource managers in prediction of maximum copper concentration downstream of an algaecide application at points of compliance.
The exposure time, or duration the slug treatment passed by each sampling point, increased from 0.9 h at the 0.80 km sampling point to 1.58 h at the 6.44 km sampling point (Table 1). Downstream of the 6.44 km sampling point the exposure duration varied, which was likely attributed to sorption and dispersion of applied copper, as well as the differing background concentrations throughout the canal (< 0.04–0.07 mg Cu/L). The initial increase in exposure time is likely attributed to differing flow rates vertically and horizontally within the canal spreading the applied mass of copper. The flow rate would differ with highest resistance where water is closest to the canal bed and at the air–water interface, and be fastest in the middle of the water column (Subramanya 2009). Potential desorption of Captain XTR is another factor that could increase exposure time. Bishop et al. (2018) found that Lyngbya wollei treated at 0.5, 1, 2, and 4 mg Cu/L as Captain XTR for 6 h released 22.1, 21.9, 22.3, and 20.0% of sorbed copper, respectively, after transfer to untreated water for 4 days. Algal cells surfaces have a variety of sites which copper can loosely bind (Campbell 1995; Hassler et al. 2004; Kaduková and Virčíková 2005), which can be potentially released back into the water column.
The mass of copper down the canal decreased in a similar pattern as the peak copper concentration with an exponential decay equation of g Cu = − 225.3 + 3865.4e(−0.142(km)), (R2 = 0.9558, n = 8; Fig. 3). At 0.80 km from application, 3,091 g Cu was calculated to pass or 85.6% of the applied copper amount, whereas only 226.5 g of copper or 6.27% of applied copper was measured at 9.66 km (Table 1). Sorption is hypothesized as the primary factor removing applied copper out of the water column, with dispersion of copper below the analytical detection limit, 0.04 mg Cu/L (USEPA 1996), likely accounting for a portion of unmeasurable copper mass. Copper complexation with carbonates and sulfides, as well as particulate matter, likely accounted for a marginal removal of copper mass since copper was chelated, the alkalinity was less than 40 mg CaCO3/L, and the canal water was well oxygenated with low turbidity (< 10 NTU). Algal biomass (primarily Pithophora spp.) measured growing attached to the canal was 0.08 (0.06), 0.02 (0.01), 0.11 (0.10), 0.03 (0.02), 0.03 (0.02), 0.04 (0.04), and 0.68 (0.54) g/m2 (standard deviation; n = 6) at the 0.80, 3.22, 6.44, 9.66, 12.87, 16.09, and 19.31 km sampling points, respectively. Additionally, the mean Pithophora spp. biomass freely floating down the canal was 4.94 g/m3 (n = 7). Algal biomass is a significant factor influencing the short-term binding of copper (Crist et al. 1990; Levy et al. 2007). Site-specific algal biomass in canals may alter the dissipation curve and biomass are predicted to be inversely proportional to the distance copper travels.
In this research, we treated before algal biomass was causing problems to proactively offset issues. This is recommended in order to use less copper and achieve greatest distance of control rather than allowing biomass to attain nuisance levels. By treating proactively, canal capacity can be reserved for water rather than algae and prevent excessive accumulation of algae on screens reducing or preventing water flow for irrigation. Copper sulfate is often applied as a salt similar as a slug and dissolves through time. Copper sulfate is a salt that does not have chelation to aid with uptake and depends on active transport for copper internalization to toxic sites of action (Sunda 1989; Knauer et al. 1997). Other chelated coppers lack labeled use for slug treatments and treatment is based on a drip rate usually over 1–3 h depending on formulation (Applied Biochemists 2017a; Alligare 2017a). This often results in greater time spent by the applicator treating a canal and greater opportunity for error. Additionally, with drip treatments compared with slug, the amount of copper algaecide required to achieve similar control is greatly increased (Table 2). Copper sulfate is labeled for slug application (Old Bridge Chemicals, Inc 2017), but it is likely more copper must be applied to deliver comparable efficacy as observed with chelated copper.
Efficacy
Bishop and Rodgers (2012) found copper formulations elicited significantly different responses independent of total sorbed copper amounts. Copper sulfate has a sorption and depuration profile that is expected to rapidly move to the algae but not effectively internalize for algaecidal effects (Bishop 2016). The sorption kinetics observed by Bishop et al. (2017) in short exposures indicate Captain XTR to have increased ability to penetrate into nuisance algae and achieve internal control thresholds, and are not solely driven by active transport from externally sorbed copper. Interactivity of copper from copper sulfate with water chemistry (e.g., alkalinity; Meyer 1999) and algal cells (Crist et al. 1990) is predicted to rapidly remove copper mass. This would limit the travel distance down a canal, and copper sulfate may not continue to enter the cell to attain internal control thresholds (Bishop et al. 2018). Murray-Gulde et al. (2002) found copper half-lives to be lower with copper sulfate than chelated copper in some natural waters. Masuda and Boyd (1993) also found lower half-lives of copper sulfate than chelated copper under elevated pH conditions and presence of different sediment types. Therefore, algaecidal efficacy and distance of control is predicted to decrease with copper sulfate compared with chelated copper, even at shorter distances. Understanding the activity of different copper formulations can be valuable and highly applicable in achieving desired management objectives while complying with regulatory standards.
In the canal, exposure factors drastically changed between the 6.44 and 9.66 km sampling points, 0.484 and 0.0635, respectively. The scaled laboratory experiment exposure factors for the 0.80, 3.22, and 6.44 km points were 0.866, 0.675, and 0.484, respectively (Table 1). The measured chlorophyll a concentrations for the exposure factors at the 0.80, 3.22, and 6.44 km points were significantly less than the untreated controls indicating an algaecidal effect. Oedogonium sp. treated in the laboratory with the exposure factors measured at the 9.66, 12.87, and 16.09 km sampling points did not have a significant reduction in chlorophyll a content compared to untreated controls (n = 4; α = 0.05; Fig. 4). Visible observations of treated algae in the canal showed chlorotic symptoms at the 0.80, 3.22, and 6.44 km sampling points and were deemed as satisfactory control by the applicator, while algae at the 12.87, 16.09, and 19.31 km locations lack observable chlorosis. Based on the results from the field observations and the controlled laboratory study, the 0.484 exposure factor elicited desired results. The exposure factor at the 0.80 km points was approximately double what was measured at the 6.44 km location.
Previous studies have found long, lower copper concentration to be more effective than short pulse exposures (Knauer et al. 1997; Campbell et al. 2002; Angel et al. 2015). Much of this work has been done with ionic copper exposures such as from copper sulfate. Copper bound from copper sulfate can readily desorb back into solution and thereby not become internalized to elicit a response (Angel et al. 2015). Copper sulfate is subject to active transport mechanisms (Sunda and Huntsman 1998) and thereby is predicted to take a longer duration to pass the algal cell surface and require longer exposure durations to increase performance. It is predicted the reversible binding nature of adsorbed copper would be accentuated by short exposure durations (< 3 h) in canal treatments as this provides less contact time with the algal surface and rapid decrease in external aqueous concentrations. Angel et al. (2015) found rapid desorption or release of copper sulfate back into solution following a pulse exposure and overall less copper internalization. However, data comparing formulations showed that chelated copper with surfactant continued to drive internalization even after copper exposure ceased (Bishop et al. 2018). This would support findings from the current study and also reports of less efficacy observed in field canal systems following copper sulfate use. Conversely, chelated copper can have an enhanced ability to pass through the algal cell wall and membrane due to the charge properties (Stauber and Florence 1987), and this internalized copper is less likely to be released into solution (Kuyucak and Volesky 1989; Yan and Pan 2002). Surfactants and adjuvants further can increase this interaction with the algal cell and assist in internal transfer (Closson and Paul 2014; Ullah et al. 2015). Bishop et al. (2017) found pulsed exposures (1–4 h) of Captain XTR can be effective at controlling a nuisance mat-forming algae, although require a proportional increase in concentration at lower durations (i.e., exposure factor). Chelated copper provides more rapid copper internalization than copper sulfate and should be highly considered in short duration exposures such as through canal systems (Bishop 2016). The measured efficacy of short pulse (less than 3 h) exposures of Captain XTR as found in this research would not be expected when compared to results from other another study with copper sulfate (Angel et al. 2015). This finding further confirms that formulations of copper algaecides substantially differ in behavior and effectiveness.
Comparison with drip injection
Copper applications to flowing water applied by drip or slug tend to spread out through time (lower peak concentration and longer duration). There is an exposure factor (concentration times duration) that is sub-lethal to the algae. At low copper concentrations, less interaction with algae may occur due to diffusion limited transfer of copper to the cell (Stauber and Davies 2000). This is predicted to be achieved faster in an injection application due to lower peak concentration that can be attained, thereby not achieving the same distance of control. With slug applications, peak copper was over 3 ppm at 0.80 km, but less than 1 ppm by 6.44 km with the slug application. Higher peak concentrations will likely saturate binding sites to a greater extent and, depending on formulation, have increased likelihood of achieving internal threshold for control. Control was measured out through the 4-mile point with these peak copper concentrations near 1 ppm. Prolonged, lower concentrations (as in injection) are more likely to establish equilibrium conditions with algal binding sites (Gonzalez-Davila et al. 1995; Quigg et al. 2006). This would limit the driving force of copper into the cell especially in short exposures.
Conclusions
In this research, a novel use pattern (e.g., slug, pulse) for a copper-based algaecide in flowing water was evaluated. Dissipation of copper mass and peak concentration after a pulse application followed an exponential decay curve. Algaecidal efficacy correlated with exposure factor achieved based on mass of copper added and mass of algae in canal. Slug application of copper is predicted to maintain an exposure factor at a lethal concentration further down a canal than a drip application. This would operationally reduce the number of required application points for treatment thereby decreasing labor as well the amount of copper required to attain desired control. This research will assist canal managers in selecting enhanced application programs to achieve desired algal control through canal systems while understanding copper dissipation and ability to comply with applicable copper discharge regulations.
References
Alligare (2017a) ARGOS Specimen Label. http://www.alligare.com/assets/pdf/argos_product_label.pdf. Accessed 18 Dec 2017
Alligare (2017b) 8% Copper Specimen Label. http://www.alligare.com/assets/pdf/ALLIGARE_8_Copper_Label.pdf. Accessed 18 Dec 2017
American Public Health Association/Water Environment Federation (SMEWW) (2005) Standard methods for the examination of water and wastewater 21st edn, Washington DC
Angel BM, Simpson SL, Chariton AA, Stauber JL, Jolley DF (2015) Time-averaged copper concentrations from continuous exposure predicts pulsed exposure toxicity to the marine diatom, Phaeodactylum tricornutum: importance of uptake and elimination. Aquat Toxicol 164:1–9
Applied Biochemists (2017a) Cutrine®-Ultra Specimen Label https://www.lonza.com/~/media/Files/water-treatment/Cutrine_Ultra.ashx?la=en. Accessed 18 Dec 2017
Applied Biochemists (2017b) Cutrine®-Plus Specimen Label. https://www.lonza.com/~/media/Files/water-treatment/Cutrine_Plus_SpecimenLabel.ashx?la=en. Accessed 18 Dec 2017
Applied Biochemists (2017c) Clearigate® Specimen Label. https://www.lonza.com/~/media/Files/water-treatment/Clearigate.ashx?la=en. Accessed 18 Dec 2017
Bishop WM (2016) A risk-based decision information system for selecting an algal management program. Dissertation, North Carolina State University
Bishop WM, Rodgers JH Jr (2012) Responses of Lyngbya wollei to exposures of copper-based algaecides: the critical burden concept. Arch Environ Contam Toxicol 62:403–410
Bishop WM, Willis BE, Horton CT (2015) Affinity and efficacy of copper following an algicide exposure: application of the critical burden concept for Lyngbya wollei control in Lay Lake, AL. J Environ Manag 55:983–990
Bishop WM, Lynch CL, Willis BE, Cope WG (2017) Copper-based aquatic algaecide adsorption and accumulation kinetics: influence of exposure concentration and duration for controlling the cyanobacterium Lyngbya wollei. Bull Environ Contam Toxicol 99:365–371
Bishop WM, Villalon GV, Willis BE (2018) Assessing copper adsorption, internalization, and desorption following algaecide application to control Lyngbya wollei from Lake Gaston, NC/VA, USA
Campbell P (1995) Interactions between trace metals and aquatic organisms: a critique of the free ion activity model. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, Chichester, pp 45–102
Campbell PG, Errécalde O, Fortin C, Hiriart-Baer VP, Vigneault B (2002) Metal bioavailability to phytoplankton-applicability of the biotic ligand model. Comp Biochem Physiol C Toxicol Pharmacol 133:189–206
Chakoumakos C, Russo RC, Thurston RV (1979) Toxicity of copper to cutthroat trout (Salmo clarki) under different conditions of alkalinity, pH, and hardness. Environ Sci Technol 13:213–219
Clearwater SJ, Jellyman PG, Biggs BJ, Hickey CW, Blair N, Clayton JS (2011) Pulse-dose application of chelated copper to a river for Didymosphenia geminata control: effects on macroinvertebrates and fish. Environ Toxicol Chem 30:181–195
Closson KR, Paul EA (2014) Comparison of the toxicity of two chelated copper algaecides and copper sulfate to non-target fish. Bull Environ Contam Toxicol 93:660–665
Crist RH, Martin JR, Guptill PW, Eslinger JM, Crist DR (1990) Interaction of metals and protons with algae. 2. ion-exchange in adsorption and metal displacement by protons. Environ Sci Technol 24:337–342
Garcia-Villada L, Rico M, Altamirano M, Sanchez-Martin L, Lopez-Rodas V, Costas E (2004) Occurrence of copper resistant mutants in the toxic cyanobacteria Microcystis aeruginosa: characterisation and future implications in the use of copper sulphate as algaecide. Water Res 38:2207–2213
Getsinger KD, Dibble ED, Rodgers Jr JH, Spencer DF (2014) Benefits of controlling nuisance aquatic plants and algae in the United States. In: Council on agricultural science and technology. http://www.cast-science.org/download.cfm?PublicationID=282524&File=1030aa96c911b0e51e9b6b1c1c3a7f464a28TR. Accessed 18 Dec 2017
Gonzalez-Davila M, Santana-Casiano JM, Perez-Pena J, Millero FJ (1995) Binding of Cu(II) to the surface and exudates of the alga Dunaliella tertiolecta in seawater. Environ Sci Technol 29:289–301
Hanson JM, Stefan HG (1984) Side effects of 58 years of copper sulfate treatment of the Fremont Lakes, Minnesota. Water Resour Bull 20:889–899
Hassler C, Slaveykova V, Wilkinson K (2004) Discriminating between intra- and extra-cellular metals using chemical extractions. Limnol Oceanogr 2:237–247
Izaguirre G (1992) A copper-tolerant phormidium species from Lake Mathews, California, that produces. 2-methylisoborneol and geosmin. Water Sci Technol 25:217–224
Kaduková J, Virčíková E (2005) Comparison of differences between copper bioaccumulation and biosorption. Environ Int 31:227–232
Knauer K, Behra R, Sigg L (1997) Adsorption and uptake of copper by the green alga Scenedesmus subspicatus (Chlorophyta). J Phycol 33:596–601
Kuyucak N, Volesky B (1989) The mechanism of cobalt biosorption. Biotechnol Bioeng 33:823–831
Levy JL, Stauber JL, Jolley DF (2007) Sensitivity of marine microalgae to copper: the effect of biotic factors on copper adsorption and toxicity. Sci Total Environ 387:141–154
Masuda K, Boyd CE (1993) Comparative evaluation of the solubility and algal toxicity of copper sulfate and chelated copper. Aquaculture 117:287–302
Maupin MA, Kenny JF, Hutson SS, Lovelace JK, Barber NL, Linsey KS (2014) Estimated use of water in the United States in 2010. https://dx.doi.org/10.3133/cir1405. Accessed 18 December 2017
Meyer JS (1999) A mechanistic explanation for the ln(LC50) vs. ln(hardness) adjustment equation for metals. Environ Sci Technol 33:908–912
Murray-Gulde CL, Heatley JE, Schwartzman AL, Rodgers JH Jr (2002) Algicidal effectiveness of clearigate, cutrine-plus, and copper sulfate and margins of safety associated with their use. Arch Environ Contam Toxicol 43:19–27
Old Bridge Chemicals Inc (2017) Copper sulfate fine crystals. http://media.wix.com/ugd/a1ec21_bb0d35c48282416f8cef8a8c9dbde8e9.pdf. Accessed 18 Dec 2017
Quigg A, Reinfelder JR, Fisher N (2006) Copper uptake kinetics in diverse marine phytoplankton. Limnol Oceanogr 51:893–899
SePRO Corporation (2014) Captain XTR algaecide product label. SePRO Corporation, Carmel, p 46032
Stauber JL, Davies CM (2000) Use and limitations of microbial bioassays for assessing copper bioavailability in the aquatic environment. Environ Rev 8:255–301
Stauber JL, Florence TM (1987) Mechanism of toxicity of ionic copper and copper complexes to algae. Mar Biol 94:511–519
Subramanya K (2009) Velocity distribution. In: Mukherjee S (ed) Flow in open channels, 3rd edn. Tata McGraw Hill Publishing Company Limited, New Delhi, pp 5–6
Sunda WG (1989) Trace metal interactions with marine phytoplankton. Biol Oceanogr 6:411–442
Sunda WG, Huntsman SA (1998) Processes regulating cellular metal accumulation and physiological effects: phytoplankton as model systems. Sci Total Environ 219:165–181
Sytsma M, Parker M (1999) Aquatic vegetation in irrigation canals. http://pdxscholar.library.pdx.edu/centerforlakes_pub/11. Accessed 18 Dec 2017
Ullah H, Bishop W, Whitford B (2015) Pesticidal copper compositions and methods for using the same. Patent US 9,137,999 B1, Washington DC
United States Environmental Protection Agency (USEPA) (1996) Hatch Method 10272, Report 2016-16516. USEPA, Washington DC
United States Environmental Protection Agency (USEPA) (2009) Reregistration eligibility decision (RED) for coppers, Report 738-R-09-304. USEPA, Washington DC
Washington Department of Ecology (WADOE) (2016) Fact sheet for the aquatic plant and algae management NPDES general permit. https://www.ecy.wa.gov/DOE/files/c5/c514c529-657d-425e-baf7-18591e4b4577.pdf. Accessed 19 Dec 2017
Yan H, Pan G (2002) Toxicity and bioaccumulation of copper in three green microalgal species. Chemosphere 49:471–476
Acknowledgements
The authors thank SePRO Corporation for funding this research, as well as Kennewick Irrigation District for use of their canal for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Willis, B.E., Pearce, M. & Bishop, W.M. Evaluation of copper dissipation, exposure factor, and algaecidal efficacy in an irrigation canal following pulse ‘slug’ application of a chelated copper algaecide. Appl Water Sci 8, 194 (2018). https://doi.org/10.1007/s13201-018-0842-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0842-3